Abstract
Allogeneic BM transplantation from an HLA-identical sibling donor leads to long-term survival in the majority of patients (> 80%). Therefore, survival is no longer the sole concern and attention has to be paid to decreasing the incidence and severity of long-term complications. For patients without a sibling donor, transplantation from a well-matched unrelated donor can be considered after failure of a previous course of immunosuppressive therapy. After transplantation from an HLA-identical sibling donor or from an unrelated one, the use of peripheral blood stem cells must be strongly discouraged because they have been systematically associated with an increased incidence of chronic GVHD compared with the use of BM as a stem cell source, leading to an unacceptably higher risk of treatment-related mortality in this setting. For as yet unknown reasons, the age limit after which transplantation results are less satisfactory remains 40 years of age.
Introduction
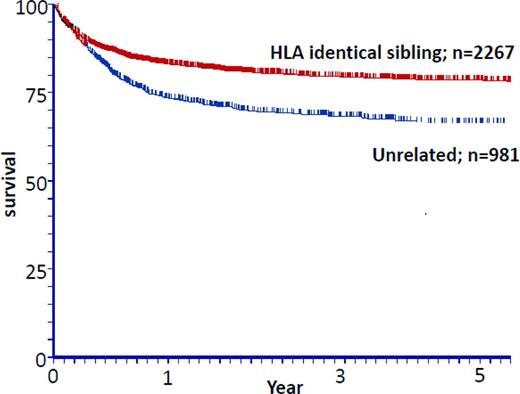
BM transplantation (BMT) from a HLA-identical related donor is the treatment of choice for young patients with severe aplastic anemia (SAA). Cyclophosphamide (CY) with anti-thymoglobulin (ATG) as a conditioning regimen and the association of cyclosporine (CsA) plus methotrexate (MTX) as GVHD prophylaxis represent an effective treatment, with a rate of engraftment of 95% and overall survival near 90%.1 All of these aspects, BM source, conditioning with CY + ATG, and GVHD prophylaxis by CsA + MTX represent the gold standard in transplantation for acquired aplastic anemia in patients receiving transplantations from an HLA-identical sibling donors. All deviations from this standard lead to poorer outcomes. Given these excellent results, survival is thus no more the sole concern in this situation and prevention/early detection of late complications after BMT is the main objective of allogeneic BMT in this setting. For patients who lack an HLA-identical sibling donor, immunosuppressive therapy (IST) remains the treatment of choice1 (also see review by Young elsewhere in this publication2 ). However, 30% to 40% of the patients will eventually relapse or be refractory to IST and will thus be considered for transplantation using an alternative donor. A molecularly HLA-matched unrelated donor (UD) is in this situation the best alternative possibility. A curve illustrating the respective outcome for patients receiving transplantations either from a sibling donor or from an unrelated one is shown in Figure 1. The use of other alternative source of stem cells, including cord blood and a haploidentical familial donor, have been reviewed recently3 and will be discussed by Kulasekararaj and Marsh elsewhere in this publication4 (these types of transplantation are usually regarded as a third-line treatment).
Acquired SAA transplantations 1999-2009: the effect of donor type and matched sibling donors versus UDs. Unpublished data from the EBMT courtesy Prof A. Bacigalupo.
Acquired SAA transplantations 1999-2009: the effect of donor type and matched sibling donors versus UDs. Unpublished data from the EBMT courtesy Prof A. Bacigalupo.
Transplantation from HLA-identical sibling donors
Allogeneic hematopoietic SCT (HSCT) is the most likely curative treatment option for SAA and should be the upfront therapeutic option of choice for young patients. The outcome of allogeneic HSCT (and of immunosuppressive therapy) has improved remarkably during the last decade because of improvements in all aspects of transplantation, including supportive care.1 Accordingly, the difference in projected survival between patients treated with HSCT and IST is increasing with time in favor of HSCT over IST. Presently, most centers offer upfront allogeneic HSCT for SAA patients with an available matched sibling donor up to the age of 40 to 50 years.
Transplantation in patients younger than 40 years
Conditioning regimen with CY + ATG.
Being a non-neoplastic hematologic disorder, the main goal of transplantation in aplastic anemia is to achieve successful engraftment with no acute or chronic GVHD. Despite significant progress in conditioning regimens, graft failure remains a significant concern in this disease, particularly in previously transfused patients. Therefore, adequate lymphoablative and immuno-ablative components of the preparative regimen are important. The addition of ATG to CY given at a total dose of 200 mg/kg has been demonstrated to promote both excellent engraftment and long-term outcome. In a nonrandomized trial, this combination resulted in a lower incidence of GVHD and improved survival compared with historical controls who received CY-only conditioning.5,6 Currently, this combination is considered the standard conditioning regimen for SAA in the setting of matched related transplantation. It is noteworthy that one prospective randomized trial involving 134 patients did not show a significant benefit from the addition of ATG,7 which nevertheless improved the long-term survival rate by 10% (thus raising the power of the trial to detect a statistical significance). Prevention of graft failure can be accomplished by the use of other more intense conventional conditioning, including radiation-based regimens. However, this must be strongly discouraged due to higher transplantation-related long-term morbidity and mortality. Use of other conditioning regimens in older patients will be discussed below.
Use of BM as the source of stem cell.
Nonmanipulated BM must be used as the stem cell source for all patients with aplastic anemia, because the use of peripheral blood stem cells (PBSCs) is associated with increased risk of chronic GVHD. Despite earlier engraftment with the use of PBSCs, a joint European Group for Blood and Marrow Transplantation (EBMT)/Center for International Blood and Marrow Transplant Research (CIBMTR) retrospective analysis suggests inferior outcome with the use of PBSCs in this disease, particularly in young patients.8 Survival advantage for BM graft was also confirmed in a more recent EBMT report in all age groups.9 This does not support the current trend of increasing use of PBSCs as a graft source in this disease. Adequate BM stem cell dose is expected to be associated with improved outcome. At least 3 × 108 mononuclear cells/kg BM stem cell dose or 2 × 106 CD34+ cells/kg should be given because a lower stem cell dose increases the risk of graft failure.
Posttransplantation GVHD prophylaxis with CsA + MTX.
Adequate posttransplantation immunosuppression is important not only for the prevention of GVHD, but also to secure adequate suppression of the host immune system and prevention of graft rejection. The combination of CsA and short-course MTX should be considered the standard posttransplantation immunosuppression. In a prospective randomized trial comparing CsA + MTX with CsA alone, the 1-year transplantation-related mortality rates for patients given CsA + MTX or CsA alone were 3% and 15%, respectively.10 The 5-year probability of survival was 94% in the CsA + MTX group and 78% for those in the CsA-alone group. Few reports have been published about the use of other immunosuppressive agents, including the use of mycophenolate mofetil, particularly in patients with renal impairment. Slow and linear taper of CsA should be carried out with careful follow-up of chimerism and blood counts to avoid graft rejection.
Current issues in transplantation from sibling donors
Transplantation in patients older than 40 years.
For SAA, the effect of age on transplantation-outcomes is mixed, with some reports suggesting an adverse impact and others showing results comparable to those observed in younger patients10-12 (for review, see Maury and Aljurf11 ). Therefore, in older patients, the best first-line treatment option for SAA is debatable and has commonly led to the practice of offering IST as first-line treatment to older patients despite the availability of a matched sibling.13 Transplantation is offered after failure of 1 to 2 courses of IST. As with transplantation, survival after IST is associated with age. Older patients who respond to IST have a 5-year survival rate of ∼ 50%, which is considerably lower than the 90% seen in younger patients.14 For the nonresponder with a matched sibling donor, IST delays transplantation and exposes the patient to risks of more pretransplantation transfusions, including allogeneic sensitization and iron overload, prolonged neutropenia and infections, and possibly poor performance scores. The dilemma of offering a matched sibling transplantation to an older patient who is at higher risk of GVHD and therefore higher morbidity and mortality rate must be weighed carefully against the benefits of IST, which may produce a sustained remission but is associated with late clonal abnormalities such as myelodysplastic syndrome and acute myeloid leukemia. In a CIBMTR analysis, the age at transplantation beyond which outcomes differ were analyzed and risk factors that may be modified to improve survival after HLA-matched sibling donor transplantation for SAA including older patients were identified.15 Patients older than 40 years were more likely to have had IST, poor performance scores, and a longer interval from diagnosis to transplantation. Neutrophil recovery was similar in all age groups, but patients older than 40 years had a lower likelihood of platelet recovery compared with patients younger than 20 years but not compared with those 20 to 40 years of age. Compared with patients younger than 20 years, mortality risks were higher in patients older than 40 years and those 20 to 40 years of age. Risks were also higher in patients with poor performance scores and when the interval from diagnosis to transplantation was longer than 3 months, suggesting earlier referral when this treatment option is being considered.16,17
In patients older than 40 years, conditioning regimens other than CY + ATG have also been considered. For patients between the ages of 30 and 50 years who are potential transplantation candidates, the best conditioning regimen is not known. Patients who are older than 40 years and who are medically fit enough for BMT may receive a conditioning regimen using CY 1200 mg/m2, fludarabine 120 mg/m2, and either ATG or alemtuzumab.18,19 This approach needs confirmation in larger studies, ideally from an international randomized study.
Chronic GVHD and long-term complications.
Studies conducted by the EBMT and the CIBMTR found that the use of PBSCs was associated with an increased risk of chronic GVHD and lower 5-year overall survival compared with BM grafts. Although several studies have confirmed that HSCT after conditioning with CY + ATG is associated with excellent overall survival rates,12 the long-term follow up of those patients is still limited, with the exception of the Seattle and Hospital Saint Louis cohorts.13,20,21 Long-term survivors after HSCT are exposed to late complications, including abnormal immune reconstitution, nonmalignant organ or tissue dysfunction, delayed infections, and secondary cancers.
At the Fred Hutchinson Cancer Research Center, Deeg et al evaluated 212 patients with aplastic anemia receiving transplantations who survived more than 2 years and who have been followed for up to 26 years.16 They analyzed hematopoietic function, chronic GVHD, skin disease, cataracts, lung disease, skeletal problems, posttransplant malignancy, depression, pregnancy/fatherhood, and the return to work or school, as well as patient self-assessment of physical and psychosocial health, social interactions, memory and concentration, and overall severity of symptoms. Survival probabilities at 20 years were 89% for patients without (n = 125) and 69% for patients with chronic GVHD (n = 586). All patients had normal hematopoietic parameters. Skin problems occurred in 14%, cataracts in 12%, lung disease in 24%, and bone and joint problems in 18% of patients. Eleven patients (12%) developed a solid tumor malignancy and 19% of patients experienced depression. Chronic GVHD was the dominant risk factor for late complications. Seventeen patients died at 2.5 to 20.4 years after transplantation; 13 of these had chronic GVHD and related complications. At 2 years, 83% of patients had returned to school or work; the proportion increased to 90% by 20 years. At least half of the patients preserved or regained the ability to become pregnant or father children. Patients rated their quality of life as excellent and symptoms as minimal or mild.
At the Hospital Saint Louis (Paris, France), we analyzed the long-term follow-up of all consecutive patients in our center who received a BMT from an HLA-identical related donor for SAA after conditioning with CY + ATG over a 20-year period.17 We found low mortality due to GVHD in only one patient. This represents a major improvement in the treatment of a disease for which we do not need a GVL effect. Until recently, GVHD represented the leading cause of death in patients with SAA after BMT.18 In our cohort, GVHD prophylaxis with CsA + MTX was associated with a lower risk of acute GVHD, and all but 3 chronic GVHD were limited. The only factor associated with an increased risk of chronic GVHD was a higher dose of infused CD3 cells in the graft. Avascular osteonecrosis was the most frequently encountered complication after CY + ATG conditioning (20% at 72 months). The frequency of avascular necrosis ranged from 4% to 15% in different studies depending on the conditioning regimen and characteristics of transplantation (HLA-identical sibling or UD).19 A higher risk of avascular necrosis has already been described in patients with SAA compared with patients with other diagnoses who undergo HSCT.20 We found a higher risk in older patients and in those with acute GVHD (probably related to corticosteroids). The other major nonmalignant complication was endocrine dysfunction, which until now has largely been underestimated. The incidence of such complications reached 20% at 72 months, split between dyslipidemia, thyroid dysfunction (hypothyroidism), diabetes, and gonadal dysfunction. Long-term metabolic abnormalities (diabetes, dyslipidemia, and hypertension) emerged as significant late complications25 and, therefore, need specific management. These complications are of major importance because very late cardiovascular complications are increasingly reported.26,27 In our cohort, only one patient suffered myocardial infarction; this may merely reflect the relatively short follow-up period for this particular complication. An important observation of this study is the extremely low rate of secondary malignancy: only one patient who developed Hodgkin lymphoma and died. However, it should be noted that 2 patients who presented with basal cell carcinoma and 2 others diagnosed with cervical carcinoma in situ were not considered to have developed secondary malignancy. Indeed, these cancers are usually excluded from such an analysis mainly because their surgical excision may not always have been reported. It is noteworthy that none of our patients developed squamous cell carcinoma of the oral cavity that we had previously reported in irradiated patients with chronic GVHD.11
IST versus allogeneic HSCT.
Because the chance of having a matched sibling donor in Europe and in the United States is approximately 25% to 30%, the discussion to proceed to transplantation or to use IST as a first-line therapy is a rare, but potentially difficult, clinical dilemma. Results and current standard are reviewed elsewhere in this publication by Young and others.12,14 The choice between the 2 treatment options is mainly based on patient's age and on disease severity, with HSCT from a sibling donor being the preferred treatment in children and young adults with severe disease.12,14
Transplantation from UDs
The outcome of UD transplantations for patients with SAA has improved in the last decade.28-30 Some years ago, overall survival rates ranged from 30% to 40%; now, survival rates in the range of 70% to 80% can be expected in selected patients. The improvement in HLA-typing techniques that allows better selection of UDs has probably had a major role, but also significant changes in the conditioning regimen have occurred, including the use of fludarabine (FLU)–based conditioning, the addition of low-dose (2 Gy) total body irradiation (TBI) in adults,31-33 and the use of alemtuzumab as an alternative to ATG.19,34,35 Overall, survival is currently in the 70% range.30 Results of UD transplantations have improved to such an extent that treatment recommendations should be adapted: transplantation from UDs is now considered after failure to respond to one course of immunosuppressive therapy, and in children, UD BMTs have even been considered as first-line treatment for SAA by some.15 Centers match for A, B, C, and DRB1 at the allelic level, looking for 8/8 matched donors36 or for DQ looking for a 10/10 match. In a recent EBMT analysis, we showed an effect of mismatching only for patients prepared with FLU + CY and ATG (FCA), but not for patients receiving FCA supplemented with low-dose TBI 2 Gy (FCA-TBI).30 In general, an 8/8 (A, B, C, and DRB1) matched donor would be ideal, but a 7/8 (or 9/10) match may be acceptable if low-dose TBI is used.30 The combination of FCA is used most often for the conditioning regimen in UD HSCT for acquired AA, although alemtuzumab is an emerging alternative option to ATG (FCC). Low-dose TBI, 2 or 3 Gy, was added after the Japanese and US studies.30,32,37 The dose of CY was originally set at 300 mg/m2 × 4. This was associated with a significant risk of rejection, so the current indication is to increase the dose of CY to 120 mg/kg. The working party SAA of the EBMT currently recommends FLU 30 mg/m2 × 4, CY 30 mg/kg × 4, and ATG. TBI (2 Gy) can be added for patients older than 14 years,30,38 but can also be considered for children sensitized after numerous blood transfusions.
Using the FCA regimen, prophylaxis of GVHD should be CSA with low-dose MTX. CSA is continued for 9 months, followed by a tapering of the dose over 3 months if chimerism is satisfactory. Using these conditioning regimens, the risk of extensive chronic GVHD is 3% for BM and 20% for peripheral blood. A study from the CIBMTR of 296 SAA patients receiving transplantations from UD compared 225 patients receiving PBSCs and 71 receiving BM cells. Hematological recovery was similar between the 2 groups; there was more acute GVHD with PBSCs but no difference in chronic GVHD.39 A recent EBMT analysis of 451 UD transplantations performed with BM stem cells show a 10-year survival of 67% compared with 48% for 153 UD PBSC transplantations (P < .0001; A. Bacigalupo, on behalf of the EBMT SAAWP, unpublished data, September 2013). Therefore, BM is the favored stem cell source. However, PBSCs continue to be used by many centers because of the convenience and lack of skill of operators, resulting in a low stem cell dose obtained from BM harvests, which increases the risk of graft rejection.
The most relevant predictor of outcome is the interval between diagnosis and transplantation, with a relative risk of death of 4.4 if the transplantation was delayed beyond 2 years. All of the rejection-related deaths were in patients grafted beyond 2 years. For patients grafted within 2 years, the actuarial 5-year survival is overall 87% and 92% for patients grafted beyond 2004.38 Using the FCA regimen, the cumulative incidence of graft failure was 17% in the EBMT study.38 Patients with a longer interval from diagnosis to transplantation (> 2 years) had a trend for a higher risk of graft failure (22%) compared with patients grafted < 1 year (12%) or between 1 and 2 years from diagnosis (14%; P = .3). The median number of infused nucleated cells was 4.5 versus 4.24 × 108/kg for patients with or without sustained engraftment. Graft failure was seen in 1/10 (10%) peripheral blood transplantations compared with 15/88 (17%) in BM transplantation recipients. For the FCC regimen, graft failure occurred in 12 patients, 9.5% for matched sibling donor and 14.5% for UD HSCT. Mixed donor chimerism after FCC occurs frequently. Full donor chimerism in unfractionated blood occurred in only 42%, and no patient achieved CD3 full donor chimerism. Stable mixed T-cell chimerism was associated with the absence of chronic GVHD and sustained myeloid engraftment.17 In the EBMT study,38 the cumulative incidence of EBV lymphoproliferative disorder was 4%. There are 2 different strategies to preventing EBV-lymphoproliferative disorder: (1) to treat preemptively patients showing an increase in EBV-DNA above a given cutoff and (2) to use prophylactic rituximab.38 Prophylactic rituximab may also reduce the risk of GVHD. Using the FCC regimen, EBV viremia was seen in 8% of patients and lymphoproliferative disorder in 4%. CMV reactivation occurred in 18% of patients, but with only once case (2%) of CMV disease.19 Using a conditioning regimen of FCA or FCA-TBI, the actuarial 10-year survival was overall 75% and 83% for patients grafted beyond 2004.30 For the FCC regimen, the 2-year overall survival is 83%. Combining UD and matched sibling donor HSCT using FCC, it was shown for the first time that the comorbidity index affects overall survival and resulted in an encouraging survival of 70% for patients older than 50 years.19 For children, outcomes after UD HSCT are similar to those of matched sibling donor HSCT,40 resulting in the move to consider UD HSCT as first-line therapy for SAA in the absence of a matched sibling donor.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Gérard Socié, MD, PhD, Hospital Saint Louis, 1 Avenue Claude Vellefaux, Paris CEDEX 10, 75475 France; Phone: 33-1-42499824; Fax: 33-1-42499639; e-mail: gerard.socie@sls.aphp.fr.