Abstract
Lymphoplasmacytic lymphoma (LPL) is a distinct B-cell lymphoproliferative disorder primarily characterized by bone marrow infiltration of lymphoplasmacytic cells. When LPL produces a serum monoclonal immunoglobulin of the IgM class, it is termed Waldenström macroglobulinemia (WM). The differential diagnosis between LPL and other types of morphologically similar B-cell tumors that may also have plasmacytic differentiation and/or secretion of IgM paraproteins is not always clear-cut based solely on the pathologic and phenotypic features of the tumor. Although the current treatments for LPL/WM are initially effective in inducing responses in most patients, they are not curative and show decreasing efficacy with repeated administrations, ultimately resulting in the selection of a chemoresistant clone. Next-generation sequencing studies have identified somatic mutations of MYD88, a key component of the Toll-like receptor signaling machinery, in ∼90% of LPL/WM. Deregulated MYD88 signaling promoted by mutations sustains tumor cell survival in LPL/WM, demonstrating that they are gain-of-function driver events in this lymphoma. This review discusses the molecular and biological mechanisms underlying MYD88 mutations in LPL/WM, the role of MYD88 mutations as molecular biomarker for the refinement of diagnosis and the improvement classification of LPL/WM, and novel targeted therapeutic strategies for LPL/WM based on the pharmacological manipulation of MYD88 signaling to which this lymphoma is addicted.
Learning Objectives
To understand MYD88 signaling and its implications for mature B-cell physiology function
To understand how mature B-cell tumors can pirate MYD88 signaling to gain proliferative advantage, mainly focusing on the seminal paradigm of MYD88 mutations in LPL/WM
To understand the clinical implications of MYD88 mutations for LPL/WM classification, diagnosis, and targeted treatment
Introduction
The World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues recognizes lymphoplasmacytic lymphoma (LPL) as a tumor of mature B lymphocytes characterized by a typically indolent clinical course and a long median survival of ∼10 years.1 With an annual overall incidence rate of ∼3 cases per one million persons, LPL accounts for <2% of non-Hodgkin lymphomas in the United States.2
In LPL, tumor cells exhibit a cytological spectrum of lymphoplasmacytic differentiation that ranges from small B cells to plasma cells. Between these extremes lies a sizable, if not predominant, fraction of cells with intermediate features, which are therefore designated lymphoplasmacytoid or lymphoplasmacytic cells.1 Tumor cells usually infiltrate the bone marrow and, to a lesser extent (15%–30% of cases), the lymph nodes, liver, and spleen.1 When LPL involves the bone marrow and is associated with a monoclonal immunoglobulin of the IgM class in the serum, it is termed Waldenström macroglobulinemia (WM).1
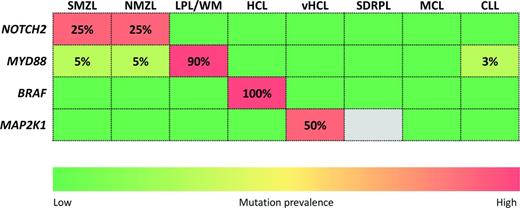
Although LPL is characteristically associated with an IgM monoclonal immunoglobulin, it does not always lead to WM. This is because ∼5% of LPL cases either produce a monoclonal immunoglobulin that is not of the IgM class (but instead of IgA or IgG classes) or do not produce monoclonal immunoglobulins at all (nonsecretory variant).1 Similarly, LPL is not the sole B-cell tumor secreting an IgM monoclonal immunoglobulin, because paraproteins of this class can be produced by other types of morphologically similar B-cell neoplasias that may also have plasmacytic differentiation [eg, splenic marginal zone B-cell lymphoma (SMZL), nodal marginal zone lymphoma (NMZL), and chronic lymphocytic leukemia (CLL)] or, in rare cases, by bona fide plasma cell neoplasms, such as IgM-secreting multiple myeloma (MM).1 Sometimes, the differential diagnosis between LPL and one of these mimicking entities (especially SMZL) is not clear-cut based solely on the pathologic and phenotypic features of the tumor. In this context, the availability of typical disease-specific markers is very useful.
In ∼40% of cases, LPL/WM is preceded by a monoclonal gammopathy of undetermined significance (MGUS) producing an IgM monoclonal immunoglobulin, which may be therefore considered as a “premalignant” precursor condition of LPL/WM.3,4 Indeed, long-term follow-up of patients with MGUS of the IgM class indicates that they are at increased risk of evolution into overt LPL/WM (or one of its relatives among the B-cell-proliferative disorders) at an annual rate of 1.5%.4
Although LPL/WM shows an indolent disease course, it still remains an incurable disorder. The mainstay of treatment for LPL/WM is an anti-CD20 antibody in combination with chemotherapy or proteasome inhibitor.5,6 Although the current treatments for LPL/WM are initially effective in inducing responses in most patients, they are not curative and show decreasing efficacy with repeated administrations, ultimately resulting in the selection of a chemoresistant clone or transformation into an aggressive lymphoma. In addition, chemotherapy-based regimens are associated with short- and long-term toxic effects. In contrast, future treatment of LPL/WM with less toxic and chemotherapy-free approaches based on the pharmacological inhibition of the pathways critical to the disease could result in its eradication.
This review discusses the molecular mechanisms underlying LPL/WM, novel molecular biomarkers for the refinement of diagnosis and the improvement of future classifications of LPL/WM, and novel targeted therapeutic strategies for LPL/WM based on the pharmacological manipulation of the pathways to which this lymphoma is addicted.
MYD88 and the Toll-like receptor pathway
Mammalian cells counteract the invasion of pathogens by setting up an innate immune response mediated by members of the superfamily of Toll-like receptors (TLRs).7 To date, 10 human TLRs (TLR1-TLR10) have been identified, each one recognizing different pathogen-associated molecular patterns such as lipopeptides (TLR2), lipopolysaccharide (TLR4), flagellin (TLR5), bacterial DNA (TLR9), and viral double- or single-stranded RNAs (TLR3 and TLR7/8, respectively). TLRs are type I transmembrane proteins and comprise an extracellular domain that mediates the recognition of pathogen-associated molecular patterns, a transmembrane region, and an intracellular Toll-IL-1 receptor (TIR) domain that activates downstream signaling pathways through the engagement of intracellular adaptor proteins.7
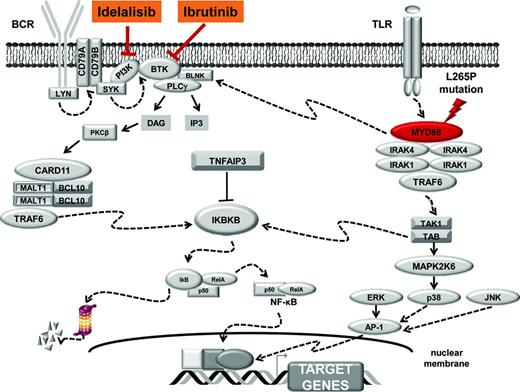
Five adaptor proteins of the TLR pathway are currently recognized and include the myeloid differentiation factor 88 (MYD88), which is essential for the signaling of most TLRs.8 MYD88 has a modular structure with a death domain (DD) at the N terminus, an intermediate linker domain (ID), and a TIR domain at the C terminus.8 The DD domain allows MYD88 oligomerization and its interaction with the respective DD of the serine-threonine kinases IRAK1-IRAK4, resulting in a multimeric complex. This complex propagates the signal and leads to activation of a series of cascades and transcription factors, such as the nuclear factor κB (NF-κB) and the activator protein 1 (AP-1) (Figure 1). The ID domain of MYD88 is a short region that, together with the DD, is involved in the interaction between MYD88 and IRAK4. The TIR domain of MYD88 is crucial for signal transduction because it mediates contacts with the intracellular TIR domains of the TLRs upon signaling activation.7,8
MYD88 signaling. After stimulation of the TLRs by pathogen-associated molecular pattern, MYD88 is recruited to the activated receptor complex as a homodimer via its TIR domain and forms complexes with IRAK1 and IRAK4. IRAK1 is then phosphorylated by IRAK4, separates from MYD88, and interacts with the tumor necrosis factor (TNF) R-associated factor 6 (TRAF6) E3 ubiquitin ligase. Active TRAF6 catalyzes polyubiquitination of a complex comprising transforming growth factor β (TGF-β)-activated kinase 1 (TAK1) and TAK1-binding proteins (TAB1, TAB2, and TAB3), which in turns phosphorylate both the inhibitor of kappaB kinase beta (IKBKB) and the mitogen-activated protein kinase 6 (MAP2K6). Active IKBKB phosphorylates IκBα, leading to its degradation by the proteasome and the release of NF-κB. On the other side, MAP2K6 activates MAPK including c-Jun N-terminal kinase 1/2 (JNK1/2), p38 and extracellular signal-regulated kinase 1/2 (ERK1/2) to stimulate AP-1 activity. Active NF-κB and AP-1 translocate into the nucleus to turn on the expression of their target genes. Molecular targets of ibrutinib and idelalisib are highlighted.
MYD88 signaling. After stimulation of the TLRs by pathogen-associated molecular pattern, MYD88 is recruited to the activated receptor complex as a homodimer via its TIR domain and forms complexes with IRAK1 and IRAK4. IRAK1 is then phosphorylated by IRAK4, separates from MYD88, and interacts with the tumor necrosis factor (TNF) R-associated factor 6 (TRAF6) E3 ubiquitin ligase. Active TRAF6 catalyzes polyubiquitination of a complex comprising transforming growth factor β (TGF-β)-activated kinase 1 (TAK1) and TAK1-binding proteins (TAB1, TAB2, and TAB3), which in turns phosphorylate both the inhibitor of kappaB kinase beta (IKBKB) and the mitogen-activated protein kinase 6 (MAP2K6). Active IKBKB phosphorylates IκBα, leading to its degradation by the proteasome and the release of NF-κB. On the other side, MAP2K6 activates MAPK including c-Jun N-terminal kinase 1/2 (JNK1/2), p38 and extracellular signal-regulated kinase 1/2 (ERK1/2) to stimulate AP-1 activity. Active NF-κB and AP-1 translocate into the nucleus to turn on the expression of their target genes. Molecular targets of ibrutinib and idelalisib are highlighted.
MYD88 in normal B-cells
Information on the role of MYD88 signaling in normal B-cell function mainly come from the study of the B-cell repertoire in patients harboring germline autosomal recessive mutations that inactivate MYD88.9,10 MYD88-defective patients show a normal development of B cells and response to immunization, suggesting that MYD88 in humans is dispensable for physiological B-cell differentiation. The sole B-lymphocyte abnormality documented in MYD88-deficient patients is the accumulation of autoreactive cells, indicating that the MYD88-signaling pathway functions in selection steps of the B-cell repertoire, leading to removal of threatening autoreactive clones.9,10
MYD88 mutations in mature B-cell tumors
Next-generation sequencing studies have identified somatic MYD88 mutations in a variety of mature B-cell tumors. Among indolent B-cell malignancies, MYD88 mutations tend to cluster with LPL/WM, where they occur in ∼90% of cases.11-15 Conversely, MYD88 mutations are rare or absent in B-cell tumors mimicking LPL or WM and sharing plasmacytic differentiation or secreting an IgM monoclonal immunoglobulin, including SMZL (∼5%), CLL (∼3%), and IgM MM (0%).11,12,14-19 MYD88 mutations are also present in a significant fraction of IgM-secreting MGUS, where they are currently detected in ∼60%-80% of cases using conventional sequencing,11,12,14,15,20-22 thus pointing to MYD88 mutations as an early genetic event in the development of LPL/WM.
Among aggressive B-cell malignancies, MYD88 mutations have been identified in ∼30% of activated B-cell-like diffuse large B-cell lymphoma (ABC-DLBCL). Notably, the prevalence of MYD88 mutations varies greatly according to the anatomical site primarily involved by ABC-DLBCL, with the highest prevalence (∼50%-75%) observed in ABC-DLBCL arising in immune-privileged extranodal compartments such as the primary central nervous system and the testis.23-25
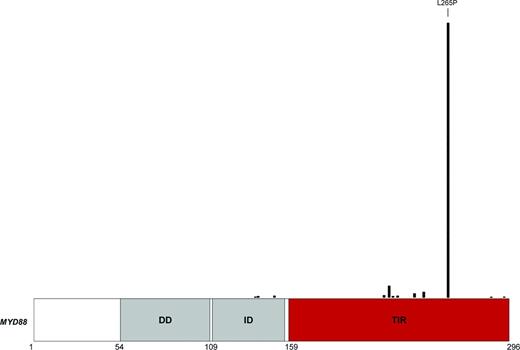
Although many different MYD88 mutations exist, the most prevalent is the L265P missense substitution. In particular, L265P is the sole MYD88 variant observed in LPL/WM, IgM-secreting MGUS, and SMZL, and it accounts for most (∼75%-80%) of the MYD88 substitutions described in ABC-DLBCL and CLL (Figure 2).11-25 Most MYD88 mutations are heterozygous, although in a fraction of cases, they may gain homozygosity through somatically acquired uniparental disomy.11
Distribution of mutations on the MYD88 protein. The MYD88 protein is represented with its functional domains, including the DD at the N terminus, the ID, and the TIR domain at the C terminus. MYD88 mutations reported by COSMIC in B-cell tumors are mapped on the protein.
Distribution of mutations on the MYD88 protein. The MYD88 protein is represented with its functional domains, including the DD at the N terminus, the ID, and the TIR domain at the C terminus. MYD88 mutations reported by COSMIC in B-cell tumors are mapped on the protein.
The L265P mutation, as well as almost all the other MYD88 mutations occurring in B-cell tumors, cluster in the evolutionary conserved beta-beta loop of the TIR domain (Figure 2), suggesting that they are selected to change the structure of MYD88 to allow spontaneous homodimerization and recruitment of IRAK1 and IRAK4. Consistently, in B-cell tumors, mutant MYD88 results in uncontrolled formation of the MYD88/IRAK complex, which translates into the recruitment of TRAF6, constitutive phosphorylation of TAK1, and, ultimately, the elevation of NF-κB activity.11,23,26 In LPL, mutant MYD88 promotes NF-κB also by binding and activating the Bruton's tyrosine kinase (BTK) (Figure 1).27 Based on these observations, a model can be envisaged in which MYD88 mutations trigger NF-κB via dual but independent pathways, namely signaling through IRAK1-4 and signaling through BTK.27 In ABC-DLBCL and LPL/WM, MYD88 mutations also result into the deregulation of JAK-STAT3 as an additional important downstream effects.23,26 At the cellular level, the signaling promoted by MYD88 mutations sustains tumor cell survival in both LPL/WM and ABC-DLBCL, documenting that they are gain-of-function driver events in these lymphoma types.11,22,27
When introduced into normal mature B cells, the MYD88 L265P mutation is sufficient to trigger NF-κB signaling and rapid cell proliferation. Proliferation and NF-κB activation triggered by the MYD88 L265P mutation are, however, short lasting and rapidly counteracted by a negative feedback loop that involves the induction of TNFAIP3, the master negative regulator of NF-κB.28 This observation may explain why MYD88 mutations frequently co-occur with TNFAIP3 disruption in B-cell tumors that are addicted to this oncogenic mechanism, such as LPL/WM and ABC-DLBCL.23,29
MYD88 mutations as a diagnostic biomarker
Among small B-cell tumors, the close association between MYD88 mutations and LPL/WM makes of this genetic lesion a helpful complement for the diagnosis of this condition. LPL/WM shares clinicopathologic features with other indolent lymphoid malignancies and sometimes the differential diagnosis between LPL/WM and one of these mimicking entities is difficult, laborious, and not easily reproducible based solely on the pathologic and phenotypic features of the tumor. In this context, assessment of the MYD88 mutation status may help to discriminate LPL/WM from other similar disorders in which MYD88 mutations are rare or absent, such as SMZL, CLL with plasmacytic differentiation, and IgM-secreting MM.
Two strategies can be applied to investigate MYD88 mutations. Most of the current literature is based on Sanger sequencing of MYD88 exon 5, the hotspot where the L265P variant maps.11,13,14,20 Although Sanger sequencing may be considered the gold standard for MYD88 mutation detection, it has a relatively low sensitivity. This technical limitation could lead to underestimate the mutation in those situations in which only a small percentage (<20%) of the assayed cells are tumor cells, and this is a particular concern for bone marrow aspirates from patients with low tumor burden. Consistent with this notion, in LPL/WM patients investigated for the MYD88 L265P mutation by Sanger sequencing, the rate of false-negative results can be up to 30%–50% if an unsorted bone marrow specimen is used as DNA source.14 One option for overcoming this limitation is purification of tumor cells before sequencing.11,20 However, CD19+ sorting is a cumbersome procedure that is not applicable in the routine diagnostic setting.
The high recurrence of one single variant (ie, L265P) among MYD88 mutations has prompted the design of allele-specific PCR-based strategies for its rapid and sensitive detection.12,14,15,22 These assays allow the reliable identification of all LPL/WM cases harboring the MYD88 L265P mutation, even when it is represented in a small fraction (down to 1%) of the cells in the specimen. Using this approach, in LPL/WM patients investigated for the MYD88 L265P mutation, the rate of false-negative results decreases down to ∼0%.12,14,15,22
MYD88 mutations as a prognostic biomarker
The prognostic impact of the MYD88 L265P mutation has been investigated in both LPL/WM and IgM-secreting MGUS. In LPL/WM, the MYD88 L265P mutation has a protective effect.30 Indeed, LPL/WM patients harboring the MYD88 L265P mutation show clues of slower disease kinetics and longer survival compared with wild-type cases.30 This information may suggest an impact of the MYD88 mutation status on the clinical presentation and course of LPL/WM. Otherwise, because the MYD88 mutation occurs in >90% of LPL/WM cases, the presence of this mutation may be simply indicative of a more accurate diagnosis of this condition.
At variance with LPL/WM, in IgM-secreting MGUS, the MYD88 L265P mutation apparently behaves as an adverse risk factor.21 Indeed, subjects harboring the MYD88 L265P mutation show a 5-fold increased risk of progression to overt LPL/WM compared with wild-type cases.21 When interpreting the discrepant clinical course of MYD88 mutated LPL/WM and IgM-secreting MGUS, one should consider that, due to limitations in the sensitivity of mutation analysis, MYD88 mutations are preferentially detected among IgM-secreting MGUS presenting with higher tumor burden, higher bone marrow infiltration, and higher levels of serum monoclonal component.12,14,15 Because tumor burden is per se a well-known risk factor for progression among IgM-secreting MGUS, it might well be that the identification of the MYD88 L265P mutation is a surrogate of tumor burden in predicting disease progression.
MYD88 mutations as a therapeutic target
Consistent with the notion that LPL/WM is addicted to MYD88 signaling, genetic and/or pharmacologic knockdown of MYD88 or its downstream targets such as BTK, IRAK1/IRAK4, and TAK1 kills LPL/WM cells through the suppression of NF-κB.11,26,27 Overall, these data, along with pivotal evidences from phase I studies,31 support the clinical investigation of the BTK inhibitor ibrutinib as targeted treatment of LPL/WM.
The activity of ibrutinib relies, among the others, on the inhibition of NF-κB. MYD88 mutations trigger NF-κB via dual, but independent pathways, namely signaling through BTK and signaling through IRAK1-IRAK4.27 In MYD88-mutated LPL/WM, ibrutinib-mediated inhibition of BTK does not affect the activation state of IRAK1 and the consequent up-regulation of NF-κB. Conversely, pharmacological inhibition of IRAK1 does not affect the constitutive BTK activation promoted by the mutant MYD88.27 Therefore, the combined use of IRAK and BTK inhibitors results in augmented inhibition of NF-κB signaling and more robust LPL/WM cell killing. These observations provide the rationale for the development of MYD88 inhibitors with the potential to more proximally block both BTK and IRAK or, conversely, for the use of a combination of BTK and IRAK inhibitors in LPL/WM patients.
In addition to enhanced NF-κB activity, MYD88 mutations also promote malignant B-cell growth by inducing autocrine and paracrine signaling of cytokines through activation of the JAK-STAT pathway. TAK1 inhibition is able to decrease both NF-κB and cytokine signaling in LPL/WM cells, suggesting that inhibition of TAK1 may be effective therapeutically against MYD88-mutated tumors due to its ability to suppress several mechanisms critical to the survival of malignant cells.26
MYD88 mutations as a biomarker for response assessment
The availability of a disease-monitoring strategy capable of detecting residual tumor cells below the sensitivity threshold of the current biochemical and pathologic methods is compelling in LPL/WM treatment, especially given the possibility of achieving unprecedentedly high-quality responses through modern therapeutic approaches.5 However, minimal residual disease assessment strategies based on flow cytometry or the molecular detection of IGHV-D-J clonotypic rearrangements have never been implemented in LPL/WM.32
The L265P mutation of MYD88 is an ideal biomarker for minimal residual disease assessment in LPL/WM because it is clonally represented in LPL/WM,11 it occurs in almost all cases of LPL/WM,11-15 and highly sensitive and allele-specific PCR strategies have been designed and validated for the quantitative monitoring of its clonal abundance in both bone marrow and peripheral blood.15,22 Although limited to a small number of selected LPL/WM patients, pivotal experiences document a strong correlation between the decrease of tumor cell infiltration in the bone marrow upon treatment and changes in the abundance of the L265P mutation of MYD88, including undetectable mutation in cases attaining a complete response to therapy.15,22 Therefore, allele-specific PCR for the L265P mutation of MYD88 may represent an inexpensive, sensitive, and easily performed procedure for minimal residual disease monitoring in LPL/WM. Prospective validation of this procedure is needed before its inclusion in the consensus response criteria for LPL/WM.
Conclusions and perspectives
The L265P mutation of MYD88 is one of the most relevant discoveries of lymphoma genomics because of its huge potentialities as diagnostic and therapeutic target for LPL/WM. From a diagnostic standpoint, the high sensitivity (∼90%) and specificity (∼95%) of MYD88 mutations for LPL/WM will allow a more accurate diagnosis of this condition and exclusion of other lymphoproliferative disorders with overlapping features (Figure 3).11-18,33,34 From a therapeutic standpoint, preclinical models established a robust background platform for the development of chemotherapy-free approaches for the management of LPL/WM based on inhibition of the MYD88 signaling to which this disease is addicted, and clinical trials are currently testing these approaches. The BTK inhibitor ibrutinib is toxic for LPL/WM cells and induces rapid reductions in serum IgM in most LPL/WM patients.35 Recent studies also support a role for the PI3Kδ inhibitor idelealisib as therapeutic strategy in LPL/WM.36,37
Signaling pathway mutations of diagnostic relevance in small B-cell lymphoproliferative disorders. The rows in the heat map represent genes. The columns in the heat map represent diseases: SMZL, NMZL, LPL/WM; hairy cell leukemia (HCL), variant hairy cell leukemia (vHCL), splenic diffuse red pulp lymphoma (SDRPL), CLL, and MCL. The heat map is color coded according to the prevalence of gene mutations in each disease entity. Numbers in the cells indicate the prevalence of mutations.
Signaling pathway mutations of diagnostic relevance in small B-cell lymphoproliferative disorders. The rows in the heat map represent genes. The columns in the heat map represent diseases: SMZL, NMZL, LPL/WM; hairy cell leukemia (HCL), variant hairy cell leukemia (vHCL), splenic diffuse red pulp lymphoma (SDRPL), CLL, and MCL. The heat map is color coded according to the prevalence of gene mutations in each disease entity. Numbers in the cells indicate the prevalence of mutations.
LPL/WM is a multigenic disease and, aside from MYD88, several additional genes and pathways are concurrently mutated in the same patient. The chemokine receptor CXCR4 is the second most commonly mutated gene in LPL/WM (∼30% of cases).38,39 Nearly all patients with CXCR4 mutations also carry the MYD88 L265P mutation. Biologically, CXCR4 mutant LPL/WM cells have enhanced signaling downstream CXCR4 (including BTK activation), as well as increased cell migration, adhesion, survival, and resistance to BTK or PI3Kδ inhibition.38,39 Clinically, the presence of CXCR4 mutations negatively affects responses in LPL/WM patients undergoing ibrutinib treatment.35 In this context, combination strategies targeting both MYD88 signaling and CXCR4 may be advisable and, indeed, preclinical investigations indicate that targeting CXCR4 with its antagonist plerixafor may be a strategy to overcome resistance to BTK inhibitors in LPL/WM.38
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Davide Rossi, MD, PhD, Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Via Solaroli 17, 28100 Novara, Italy. Phone: 39-0321-660698; Fax: 39-0321-620421; e-mail: rossidav@med.unipmn.it.