Abstract
A 75-year-old male with metastatic pancreatic cancer is undergoing chemotherapy with gemcitabine. A portal vein thrombosis was incidentally found on surveillance CT scan. He does not report any new abdominal pain or ascites. Should anticoagulation be used to treat asymptomatic portal vein thrombosis?
Learning Objective
To understand the risks of recurrent thrombosis and bleeding in patients with incidental splanchnic vein thrombosis
Discussion
The use of imaging studies to assess for response to treatment or to screen for cancer recurrence has led to increased diagnoses of incidental thrombosis. Studies of incidental pulmonary embolism suggest a similar risk of recurrent thrombosis and mortality as patients with symptomatic thrombosis.1 Although current guidelines recommend treatment of incidental pulmonary embolism and deep vein thrombosis in cancer patients,2-4 individualized risk assessment is recommended for those with incidental splanchnic vein thrombosis (SVT).3,4 Studies of symptomatic SVT that include cancer and noncancer patients suggest benefit with anticoagulant therapy with thrombus resolution in 37%–61% of patients5,6 and recurrent thrombotic events in 4%–24% of patients. However, hemorrhages occur in 2.6%–33% of symptomatic SVT patients.6-9 Therefore, it is uncertain whether anticoagulation (AC) offers net benefit. We completed a systematic rapid review to evaluate the role of AC in the treatment of hematologic and solid tumor cancer patients with incidental SVT.
We conducted a Medline search using the terms “incidental,” “thrombosis,” and “cancer” with appropriate synonyms. Key words denoting vein location (splanchnic, abdominal, portal, hepatic, splenic, mesenteric) were added. The search yielded 285 unique articles, of which 253 were excluded after title and abstract review. Of the remaining 32 articles, 8 were excluded due to lack of SVT treatment information, 5 reported only surgical management, 4 did not report outcomes, 3 did not describe incidental SVT, and 5 had no original data. References of review articles and conference presentations yielded an additional 6 manuscripts: a case control study,10 2 case report/series,11,12 a registry report,13 3 prospective cohort studies,14-16 and 6 retrospective cohort studies17-22 comprised the 13 included studies (Tables 1 and 2). Myeloproliferative neoplasms were diagnosed in 92% of patients (230/250) with hematologic malignancy.10-19,22 Positive JAK2 V617F testing was reported in 78% of patients (116/148) with myeloproliferative neoplasms in 4 studies.13,14,16,22
Management and outcomes of SVT in studies including cancer patients and incidental SVT
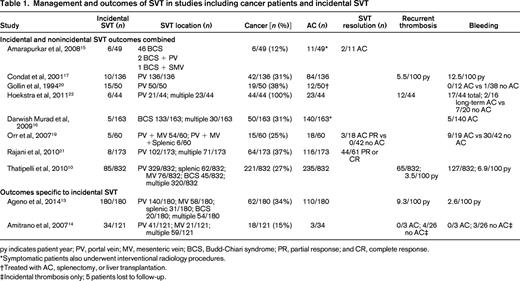
py indicates patient year; PV, portal vein; MV, mesenteric vein; BCS, Budd-Chiari syndrome; PR, partial response; and CR, complete response.
*Symptomatic patients also underwent interventional radiology procedures.
†Treated with AC, splenectomy, or liver transplantation.
‡Incidental thrombosis only; 5 patients lost to follow-up.
Management and outcomes of cancer patients with incidental SVT
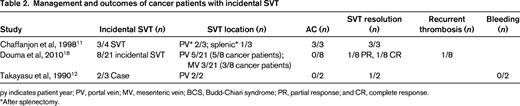
py indicates patient year; PV, portal vein; MV, mesenteric vein; BCS, Budd-Chiari syndrome; PR, partial response; and CR, complete response.
*After splenectomy.
Eight studies reported outcomes combined for cancer and noncancer patients as well as symptomatic and incidental SVT (Table 1).10,15-17,19-22 A total of 354 incidental thrombotic events were reported in 1808 patients, of whom 541 had cancer. SVT improvement or resolution was noted in 54% (49/90) of patients treated with AC.15,19,21 The incidence rate of recurrent thrombosis (3.5-5.5/100 patient-years) was lower than the risk of bleeding (6.9-12.5/100 patient-years).10,17 Condat et al reported that AC reduced the risk of recurrent thrombosis by 2/3, but AC did not influence the recurrence-free survival in another retrospective cohort study.10,17 AC increased the risk of hemorrhage in 1 study (hazard ratio = 1.9, 95% confidence interval, 1.3-2.9),10 but had no effect on risk or severity of hemorrhage in another study.17 Combining the 3 studies that reported bleeding outcomes with and without AC, a similar incidence of bleeding was reported in patients receiving AC compared with those not treated with AC [11/47 (23%) AC vs 38/100 (38%) no AC; P = .07].19,20,22 Overall, the studies of cancer and noncancer patients that included symptomatic and incidental SVT suggest a benefit of AC in thrombus resolution, but offer conflicting results regarding recurrent thrombosis and the risk of hemorrhage.
Two studies reported outcomes specific to incidental SVT in cancer and noncancer patients.13,14 A total of 214 events were reported in 301 patients, of whom 80 had cancer. AC was used to treat 53% (113/214) of incidental SVT. In contrast to the studies combining incidental and symptomatic SVT, the incidence of recurrent thrombosis was higher (9.3/100 patient-years) than the incidence of bleeding (2.6/100 patient-years) in patients with only incidental SVT.13 A prospective cohort study noted recurrent thrombosis and bleeding only in patients with incidental SVT not treated with AC.14 These studies suggest a benefit of AC in cancer and noncancer patients with incidental SVT.
Three studies described outcomes specific to cancer patients with incidental SVT.11,12,18 Of the 13 asymptomatic thrombosis cases, 9 (69%) occurred in the portal vein, 3 (23%) in the mesenteric vein, and 1 (8%) in the splenic vein (Table 2). Solid tumors were noted in 69% of cases (9/13) and hematologic malignancies in the remaining 3 cases. AC was used to treat only 3/13 (23%) of patients. Complete thrombus resolution was found in the 3 patients treated with AC11 and in 2 of 9 patients not treated with AC.12,18 A subsequent pulmonary embolism occurred in one patient not treated with AC.18 Reporting bias, lack of standardized repeat imaging, and the small number of cases limit the subgroup analysis for incidental SVT in cancer patients.
Published information suggests that a physician's decision whether to treat incidental SVT in cancer patients is influenced by the extent of thrombosis, location of involved veins, patient prognosis, and presence of varices.10 Physicians may withhold AC therapy due to concern of variceal bleeding, yet the presence of SVT can increase portal hypertension and risk of variceal hemorrhage. Overall, we conclude that AC may improve thrombus resolution and decrease the risk of thrombotic recurrence without convincingly increasing the risk of hemorrhage if patients and dosing regimens are selected carefully. We therefore suggest that cancer patients with incidental SVT be treated with therapeutic low-molecular-weight heparin if there are no contraindications such as active bleeding (grade 2C recommendation). The recommendation is weak as a result of the observational nature of the data and extrapolation. There is insufficient evidence in the literature to make recommendations regarding duration of AC, so the length of treatment must be individualized based on the extent of thrombosis and the status of the malignancy. Physicians may want to consider endoscopic treatment of known varices in patients with portal hypertension before initiation of AC (grade 2C recommendation). Future research should delineate outcomes of SVT based on symptoms at diagnosis and the presence of cancer. Controlled prospective studies are needed to outline definitively the risks and benefits of AC in incidental SVT.
Summary
Treatment with low-molecular-weight heparin therapy was initiated and follow-up CT scan showed resolution of the portal vein thrombosis. No bleeding or recurrent thrombosis occurred. The patient died 6 months later due to metastatic pancreatic cancer.
Disclosures
Conflict-of-interest disclosures: L.B.K. declares no competing financial interests. W.A. has received research funding and honoraria from Bayer Healthcare, BMS, Pfizer, Daiichi Sankyo, and Boehringer Ingelheim. A.G. has received research funding from Bristol-Myers Squibb, Pfizer, and Eisai; has consulted for Bayer, Bristol-Myer Squibb, Daiichi Sankyo, LEO Pharma, Pfizer; and has received honoraria from Avivia, Bayer, Boehringher Ingelheim, Bristol-Myer Squibb, LEO Pharma, Pfizer, and Sanofi Aventis. Off-label drug use: None disclosed.
Correspondence
Lisa Baumann Kreuziger, BloodCenter of Wisconsin; Division of Hematology and Oncology, Medical College of Wisconsin, 8701 Watertown Plank Rd., Milwaukee, WI 53226; Phone: 414-937-6826; Fax: 414-937-6580; e-mail: Lisa.BaumannKreuziger@bcw.edu.
