Abstract
Target-specific oral anticoagulants are approved for use for the prevention of stroke in atrial fibrillation and for the prevention and treatment of venous thrombosis without the need for laboratory monitoring. However, there are clinical settings in which laboratory measurement of anticoagulant effect is needed. These may include patients with life-threatening bleeding or those requiring emergency surgery, in the setting of renal or hepatic failure, or patients with thrombosis on therapy. This chapter reviews the use of laboratory testing to assess the anticoagulant effect of these drugs. In addition, because these drugs can interfere with other laboratory testing, available data on these interactions are presented.
Learning Objectives
To understand the effect of TSOACs on laboratory screening tests and specific tests developed to measure their activity
To understand the effects of TSOACs on other coagulation testing
Introduction
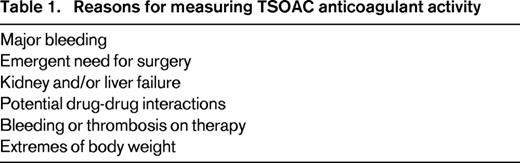
Target-specific oral anticoagulants (TSOAC)s, also termed direct oral anticoagulants, have received regulatory approval for the prevention and treatment of venous thromboembolism and atrial fibrillation without a need for dose adjustment based on laboratory testing. When used in this manner in clinical trials, these agents were associated with less or similar bleeding and thrombotic complications.1-6 However, in certain clinical settings, measurement of anticoagulant activity is needed to inform patient care. These situations include life-threatening bleeding, emergency surgery, renal or liver failure, patients taking medications that affect TSOAC plasma concentrations, and recurrent thrombosis or bleeding on recommended doses (Table 1).
True therapeutic ranges based on clinical outcomes have not been established for TSOACs. Levels that correlate with efficacy and/or adverse outcomes are just now being studied. Instead, we have levels that were measured in study populations on standard dosing, most commonly at peak and trough concentrations using liquid chromatography/tandem mass spectrometry (LC-MS/MS) methodology. Therefore, instead of therapeutic levels, the terms “on-therapy drug concentrations” or “on-therapy levels” are more accurate.
Drug characteristics
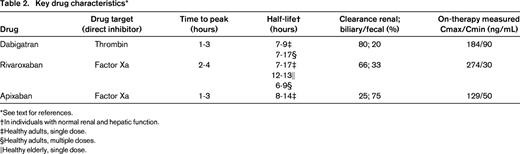
Three TSOACs, dabigatran, rivaroxaban, and apixaban, are licensed for treatment of patients with venous thromboembolism and atrial fibrillation by regulatory agencies in the United States and several other countries. Key characteristics of these agents are shown in Table 2. Several additional agents are under study, including edoxaban, but are not included in this review. Knowledge of drug characteristics is key in informing when and if to measure TSOAC-induced anticoagulant activity in an individual patient.
Key drug characteristics*
See text for references.
†In individuals with normal renal and hepatic function.
‡Healthy adults, single dose.
§Healthy adults, multiple doses.
‖Healthy elderly, single dose.
Dabigatran etexilate is a prodrug that is hydrolyzed by plasma esterases to the direct thrombin inhibitor dabigatran, an inhibitor of both free and clot-bound thrombin.7 The prodrug has relatively poor bioavailability. Dabigatran has predominantly renal clearance (∼80%) and renal insufficiency results in bioaccumulation due to prolonged clearance. A wide variation in drug levels has been observed in patients with normal renal function receiving standard dosing (Table 2).8,9
Rivaroxaban and apixaban are both small, orally available, competitive reversible antagonists of activated factor X (FXa), preventing subsequent conversion of prothrombin to thrombin.10-13 They inhibit both free and prothrombinase complex-bound forms of FXa. Rivaroxaban bioavailability is dose dependent, being higher with lower doses (80%–100% after a 10 mg dose; 66% after a 20 mg dose). Drug plasma concentrations peak 2-4 hours after oral administration; absorption of higher doses is enhanced when taken with food.10 Rivaroxaban has renal (∼66% with ∼33% as active drug) and biliary/fecal (∼33%) clearance and a half-life of 6-13 hours, which has been shown to be dependent on both dosing regimen and age (Table 2). As for dabigatran, there is wide interpatient variability in trough and peak drug concentrations measured on standard dosing.10
Apixaban reaches peak plasma concentration ∼3 hours after ingestion and, in persons with normal renal function, has a half-life of ∼12 hours.13 It has approximately 50% bioavailability and is highly protein bound in plasma.11 Apixaban has slightly less renal clearance of active drug than rivaroxaban, with ∼25% renal and 75% biliary and fecal clearance. As for dabigatran and rivaroxaban, there is wide interindividual variability in on-therapy-measured drug concentrations (Table 2).11
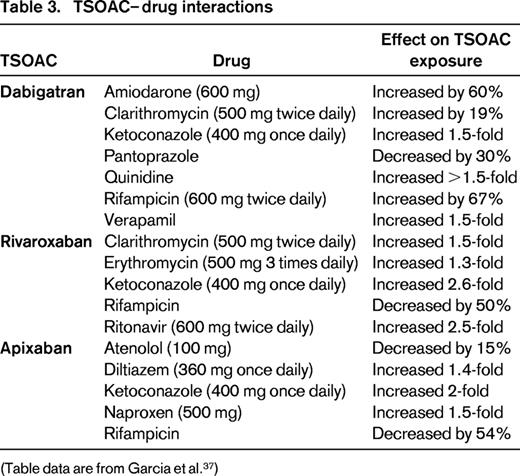
Although drug–drug interactions with TSOACs are less common than with warfarin, certain drugs, particularly strong modulators of P-glycoprotein and CYP 3A4, will increase or decrease drug exposure and maximum observed plasma concentrations. Drugs that affect TSOAC levels are shown in Table 3.
Laboratory assays and TSOACs
TSOACs differ in their effect on the commonly used screening coagulation tests, the prothrombin time (PT) and activated partial thromboplastin time (aPTT), and neither of these assays can be used to exclude significant drug concentrations of dabigatran, rivaroxaban, or apixaban (Table 4). Cuker et al recently performed a systematic review of data on laboratory measurements of anticoagulant activity of these agents published through December 1, 2013.14 In their review, they included only studies in which the measurement was correlated with LC-MS/MS measured or LC-MS/MS-validated calibration standards. This review uses those same standards.
Laboratory tests to assess and/or monitor TSOAC anticoagulant activity
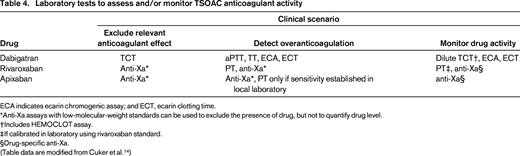
ECA indicates ecarin chromogenic assay; and ECT, ecarin clotting time.
*Anti-Xa assays with low-molecular-weight standards can be used to exclude the presence of drug, but not to quantify drug level.
†Includes HEMOCLOT assay.
‡If calibrated in laboratory using rivaroxaban standard.
§Drug-specific anti-Xa.
(Table data are modified from Cuker et al.14 )
Clinicians may consider measurement of TSOAC-induced anticoagulant activity in several settings, including in patients with renal or hepatic failure, thrombosis, or bleeding symptoms on therapy and in the setting of potential drug–drug interactions. One should be cautioned before testing clinically stable patients given the wide range of on-therapy drug concentrations that have been measured in subjects receiving standard dosing. In addition, given the relatively short half-life of these drugs, the levels will not be a measure of drug adherence as the international normalized ratio can be for warfarin therapy. If activity is measured, it must be timed to assess trough or peak activity so that results can be interpreted in light of published data. Because there is more variability in peak concentrations among the TSOACs, trough levels may be easiest to interpret. This requires knowledge of time since the last dose.
To date, no laboratory assays developed to measure TSOAC activity have been approved by the Food and Drug Administration for the United States. And importantly, there is no evidence to support dose adjustment based on test results.
Dabigatran
The aPTT and the PT have variable sensitivity to dabigatran.15 The aPTT is more sensitive than the PT to the presence of dabigatran; however, there is a curvilinear dose response with a varying relationship between drug level and anticoagulant effect.9,16 Results within the normal range of neither the aPTT nor the PT can be used to absolutely exclude an anticoagulant effect of the drug The aPTT is prolonged at dabigatran concentrations >100 ng/mL, the approximate expected trough level in patients with normal renal function on 150 mg twice daily.18 The PT is typically within normal limits at a dabigatran concentration of 100 ng/mL, but elevated at concentrations >400 ng/mL.16-18 As only 10% of subjects in the RE-LY trial had peak levels >383 ng/mL,18 most patients on dabigatran will have a normal PT.
The thrombin clotting time (TCT) measures the conversion of fibrinogen to fibrin by thrombin and is exquisitely sensitive to the presence of dabigatran.16 A normal TCT can be used to exclude the presence of drug and anticoagulant effect. In patients with therapeutic and higher range plasma concentrations, the TCT will often be prolonged above the upper limit of the laboratory's testing range. A modification of the TCT, the dilute thrombin time, can be used to assess the anticoagulant effect of direct thrombin inhibitors,19 and a commercial assay, the HEMOCLOT (Hyphen BioMed), is approved for use in Europe and Canada and can be used for measurement of dabigatran levels.20 In addition, an ecarin chromogenic assay or ecarin clotting time assay can be used for this purpose, although they are not widely available. These assays use the venom of the saw-scaled viper Echis carinatus to convert prothrombin to thrombin via the intermediary meizothrombin, which is sensitive to inhibition by direct thrombin inhibitors. Ecarin clotting activity correlates directly with the dabigatran concentration.15,21 Direct Xa inhibitors have no effect on TCT, HEMOCLOT, or ecarin-based assays.
Because dabigatran etexilate is a prodrug, commercially available dabigatran-spiked plasmas are available for use in establishing standard curves of drug concentrations. When dabigatran calibrators are used, results are expressed in nanograms per milliliter of dabigatran.
Rivaroxaban
The sensitivity of the PT to rivaroxaban varies widely by laboratory reagent used22,23 and, for that reason, a normal PT cannot be used to exclude the presence of clinically relevant anticoagulant activity unless the laboratory sensitivity of the PT reagent is known. As examples, the reagents REcombiPlasTin (Instrumentation Laboratory) and Neoplastin (Diagnostica Stago) are more sensitive to rivaroxaban than Thromborel S or Innovin (both Siemens Healthcare Diagnostics).24 The PT will be prolonged with elevated drug concentrations, with the degree of elevation dependent on the sensitivity of the reagent used.
The level of rivaroxaban activity can be assessed by a PT if the test has been validated with rivaroxaban standards. Rivaroxaban plasma calibrators can be used to determine the sensitivity of the thromboplastin reagent and to standardize the assay in the individual laboratory. The calculation and use of the international normalized ratio using the international sensitivity index established for monitoring of warfarin anticoagulation is not valid for rivaroxaban, or other Xa inhibitors, because this has been shown to increase the drug-induced between-thromboplastin variability.26 Rivaroxaban-specific international sensitivity index methodology is under study and appears feasible for future application.25 Modifications of the PT to improve performance characteristics in assays of rivaroxaban-induced anticoagulation have been developed, but are not widely used.
The aPTT is less sensitive to rivaroxaban than the PT and cannot be used to exclude clinically significant drug concentrations. The aPTT will generally be prolonged in the setting of elevated rivaroxaban levels, but there will be considerable variability depending on the aPTT reagent used by the laboratory.
A drug-specific anti-Xa activity measurement using a rivaroxaban standard is the assay of choice to assess rivaroxaban levels.27-29 In an anti-Xa assay, a FXa chromogenic substrate is used and the color released is proportional to the amount of FXa present. When a known amount of FXa is added to plasma containing the drug, the amount of inhibition (anti-FXa) activity in the plasma can be determined from a standard curve. Unlike heparins and fondaparinux, the direct Xa inhibitors do not require antithrombin for activity or measurement. Some hospital laboratories have anti-Xa assays available on a 24/7 basis, whereas in other settings, it will not be possible to obtain the test in an urgent manner. If the goal is to rule out the presence of rivaroxaban (or apixaban), an anti-Xa assay using a low-molecular-weight standard can be used if available. However, it will not quantify the amount of rivaroxaban present and, in assays with added antithrombin, will overestimate the anti-Xa activity. Dabigatran will have no effect on anti-Xa activity assays.
Apixaban
The PT is less sensitive to apixaban than rivaroxaban and may be within normal limits in many patients with clinically relevant anticoagulant activity.14,26,30 The PT would generally be prolonged in a patient with elevated apixaban drug concentrations, but given the low sensitivity of some PT reagents to apixaban, one cannot assume that to be true unless the sensitivity of the PT reagent to apixaban is known.26,30 The aPTT is very insensitive to apixaban and may not be elevated, even in the setting of significantly elevated apixaban levels.14
As for rivaroxaban, drug levels need to be assessed by an anti-Xa assay using an apixaban standard as calibrator. Given the insensitivity of both the PT and aPTT to apixaban,31 hospital laboratories should be encouraged to establish this assay with 24/7 availability. As for rivaroxaban, if the goal is to rule out the presence of apixaban-induced anticoagulant activity, an anti-Xa assay using a low-molecular-weight standard can be used if available. However, it will not quantify the amount of apixaban present.
Influence of TSOACs on other coagulation assays
Because TSOACs inhibit clotting, they will affect many clot-based assays, including those used to assess hypercoagulable states (Table 5). Therefore, these assays, for example, measures of antithrombin, protein C, or protein S activity, APC resistance, and lupus anticoagulant testing, should not be performed in patients on these drugs.23,32-34 False-positive testing for lupus anticoagulants, even with a positive phospholipid correction step, have been reported in patients on each of these drugs.34 Measures of antigen levels or DNA-based assays will not be affected by these drugs.
Interference by TSOACs in selected coagulation assays*
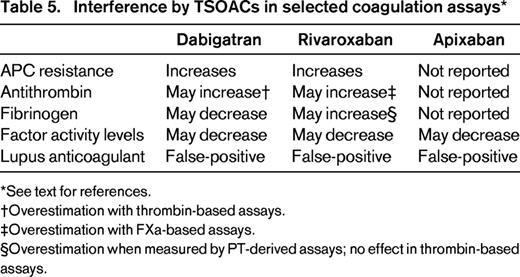
See text for references.
†Overestimation with thrombin-based assays.
‡Overestimation with FXa-based assays.
§Overestimation when measured by PT-derived assays; no effect in thrombin-based assays.
Fibrinogen measurements determined by Claus methodology, the most common approach, may be artifactually lowered in the presence of dabigatran.16,35 Fibrinogen measured using a prothrombin-based method may be artifactually elevated by all 3 drugs, the extent depending on the reagent used.33 Individual clotting factor assays may also be affected. This will depend in part on the reagent and methodology used. Rivaroxaban has been reported to falsely lower one-stage and chromogenic FVIII assays.36 Clinicians should discuss individual assays with their laboratory to understand the potential impact of the drug on the test result.
Summary
Clinicians may need to assess anticoagulant effect of the TSOACs. The clinical circumstance will dictate the urgency for laboratory assessment. The availability of laboratory testing will differ depending on the test. The commonly available TCT assay can be used to exclude clinically relevant dabigatran concentrations and, if the reagent sensitivity is known, the PT can be used to exclude clinically relevant rivaroxaban concentrations. However, an anti-Xa assay is needed to exclude clinically relevant apixaban concentrations. Before assessing on-drug concentrations, it is important to recall that true therapeutic ranges have not been established and on-therapy drug concentrations vary widely between patients on standard therapy. Furthermore, at this time, there is no evidence to support dose adjustment based on test results.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Barbara A. Konkle, MD, Director, Clinical and Translational Research, Medical Director, Hemostasis Reference Laboratory, Puget Sound Blood Center, 921 Terry Ave., Seattle, WA 98104; Phone: (206)689-6191; Fax: (206)292-8030; e-mail: barbarak@psbc.org.