Abstract
Silent cerebral infarction (SCI) is a highly prevalent and morbid condition in sickle cell disease (SCD). SCI can occur beginning in the first year of life and becomes more common with increasing age. Potentially modifiable risk factors for SCI include anemia and blood pressure. Headache does not appear to be associated with SCI, so neurologically normal children with headache do not necessarily warrant screening MRI for SCI. SCI does affect cognition, but biological determinants of cognition are not more important than socioeconomic factors. The recent identification of acute silent cerebral ischemic events indicates that the total burden of ischemic injury to the brain in SCD is far greater than previously realized. Acute anemic events appear to increase the risk of acute silent cerebral ischemic events and SCI dramatically. The medical management of SCI is not yet defined, but documentation of the presence of SCI may qualify affected individuals for special resources because comprehensive interventions are needed to optimize patients' academic and vocational outcomes.
Learning Objectives
To summarize the risk factors for SCI in SCD
To rank by relative frequency the overt and covert neurologic events in SCD
To explain the joint role of SCI and socioeconomic factors on cognitive outcomes in SCD
To understand the role of anemia in the genesis of SCI in SCD
To define and recognize ASCIEs in SCD
Background and definition
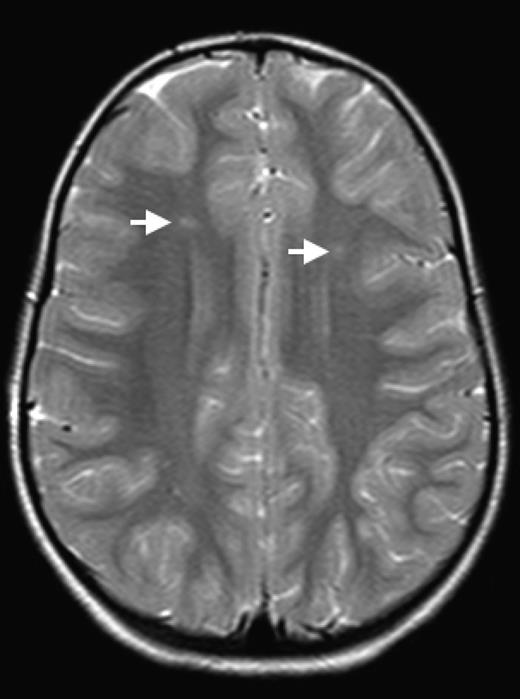
The brain is at constant threat of ischemic injury in sickle cell disease (SCD). The risk of overt stroke for children with SCD is more than 200 times higher than that for the general population, and this burden of overt stroke continues well into adulthood. An even more common form of neurologic injury in SCD is silent cerebral infarction (SCI), which has a prevalence of up to 40%. SCI refers to generally small, permanent brain lesions that are not associated with obvious focal neurologic signs (Figure 1). The term SCI is a misnomer because these strokes, even though they do not produce focal or localizing signs on neurologic examination, are often not “silent.” SCI is a morbid condition associated with neurocognitive impairment, poor academic performance, neurologic soft signs, and increased risk for subsequent overt stroke. Covert cerebral infarction may be a better descriptor, but SCI is the accepted term.
SCI. Two typical SCI lesions (arrows) are shown in the frontal and deep white matter in a T2-weighted magnetic resonance image of the brain in a child with sickle cell anemia.
SCI. Two typical SCI lesions (arrows) are shown in the frontal and deep white matter in a T2-weighted magnetic resonance image of the brain in a child with sickle cell anemia.
The definition of SCI has 2 components: (1) an infarct-like lesion on MRI of the brain and (2) a normal neurologic examination or no abnormality on neurologic examination that can be explained by the location of the infarct-like lesion. Specific definitions of the SCI lesion on MRI vary by study, but the Silent Cerebral Infarct Transfusion (SIT) Trial (www.ClinicalTrials.gov identifier #NCT00072761) rigorously defined it to be an MRI signal abnormality (increased signal) of at least 3 mm in 1 dimension that was visible on at least 2 views of T2-fluid-attenuated inversion recovery images of the brain.1 Because SCI is clinically covert, it must be identified by screening MRI of the brain. SCI is therefore almost always identified incidentally as a remote event well after its onset, which makes the study of its causes and frequency quite challenging. The goal of this chapter is to highlight several of the recent advances in our understanding of the frequency, risk factors, correlates, causes, and management of SCI.
Prevalence and incidence of SCI
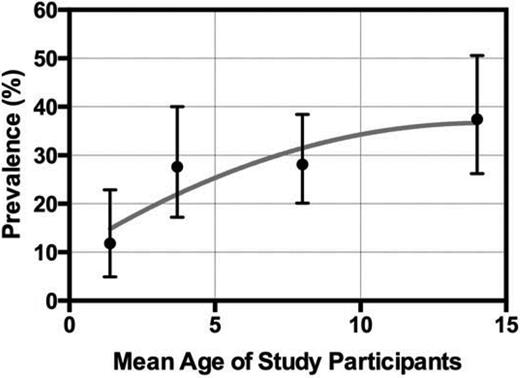
SCI is highly prevalent in children and adults with SCD. Like overt stroke, SCI occurs much more frequently (at least twice as frequently) in individuals with sickle cell anemia (HbSS) or sickle-β0-thalassemia (HbSβ0) than in those with sickle-hemoglobin C disease or sickle-β+-thalassemia. SCI begins to occur in the first year of life. In the Hydroxyurea to Prevent Organ Damage in Children With Sickle Cell Anemia (Baby HUG study; www.ClinicalTrials.gov identifier #NCT00006400), 13% [95% confidence interval (CI), 3-34] of neurologically asymptomatic children with HbSS at a mean age of 13.7 months already had SCI detected on screening MRI of the brain.2 Several additional studies including children of different ages show that the prevalence of SCI increases with age throughout childhood and approaches 40% in adolescents (Figure 2).2-5 Studies in adults also show a high prevalence of unsuspected brain lesions. Silva et al6 found a prevalence of 60% of several MRI abnormalities classified as leukoencephalopathy, encephalomalacia, atrophy, and lacunar infarction. Vichinsky et al7 found that a highly selected population of adults with HbSS (baseline Hb concentration <10 g/dL and no history of stroke, focal neurologic deficits, known brain imaging abnormalities, serious cognitive impairment, or depression) had a 13% prevalence of SCI (lacunar infarction) on screening MRIs. Comparisons of prevalence across age groups, especially between pediatric and adult groups, are difficult because the studies to date provide cross-sectional estimates of prevalence at different ages, which can be biased by several factors (selection bias, survivorship bias, etc) and there are no comprehensive longitudinal data about the prevalence of SCI across the lifespan. The definitions of SCI and the terminology of covert brain lesions also vary across studies.
Prevalence of SCI by age. The prevalence (mean ± 95% CI) of SCI across 4 different studies of children with SCD (HbSS and HbSβ0) is shown by the mean age of the participants in the separate studies. (Data for figure obtained from Debaun et al.13 )
Prevalence of SCI by age. The prevalence (mean ± 95% CI) of SCI across 4 different studies of children with SCD (HbSS and HbSβ0) is shown by the mean age of the participants in the separate studies. (Data for figure obtained from Debaun et al.13 )
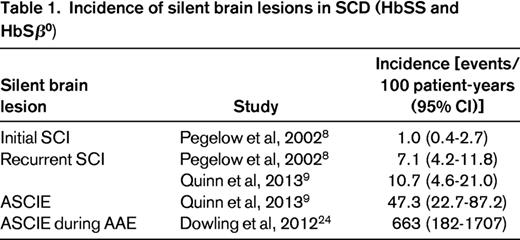
Little has been published about the incidence of SCI, properly defined as new SCI events that occur in a specified time interval (one must be cautious when reading the literature because the term “incidence” is often used incorrectly). In a seminal study, Pegelow et al8 estimated that the incidence of first SCI was 1.01 per 100 patient-years and the incidence of recurrent or progressive SCI was 7.06 per 100 patient-years (age range of the study population, 6-19 years). No other studies report the incidence of first SCI for comparison. Recently, Quinn et al9 estimated the incidence of recurrent or progressive SCI to be 10.7 events per 100 patient-years (age range of the study population, 5-15 years).9 The incidence rates for recurrent or progressive SCI found by Pegelow et al8 and Quinn et al9 are quite similar and have largely overlapping 95% CIs (Table 1), so this is evidence that the true rate in the SCD population is near these estimates. For comparison, the historical overall incidence of stroke in HbSS is lower at 0.61 events per 100 patient-years.10 The incidence of first SCI (6-19 years of age) is approximately equivalent to the incidence of first overt stroke in the highest-risk age groups for overt stroke (2-5 years of age and ≥50 years of age).10 The incidence of recurrent or progressive SCI is 7- to 10-fold higher than this. Despite limited data, the obvious conclusion from these studies of prevalence and incidence is that SCI begins in early life, increases in frequency with increasing age, and is a common finding across all age groups.
Risk factors for SCI
The identification of risk factors for SCI can provide insight into the pathogenesis of the lesion and inform strategies for prevention. Prior studies have implicated several risk factors, some of which differ from the risk factors for overt stroke. A multivariate model from the Cooperative Study for Sickle Cell Disease identified a lower pain event rate, history of seizures, increased total leukocyte count, and the Senegal β-globin haplotype as risk factors for SCI. Notably, the degree of anemia was not retained in this final model.11 Several recent studies have provided new observations, especially about the importance of anemia in the genesis of SCI. Kwiatkowski et al5 found that SCI was associated with cerebral arterial stenosis by magnetic resonance angiography, lower rates of vasoocclusive pain and acute chest syndrome, and a lower hemoglobin concentration in univariate analyses. Bernaudin et al3 also found that a lower hemoglobin concentration increased the risk of SCI (hazard ratio = 1.7 for every 1 g/dL decrease in hemoglobin concentration) in multivariate analysis.
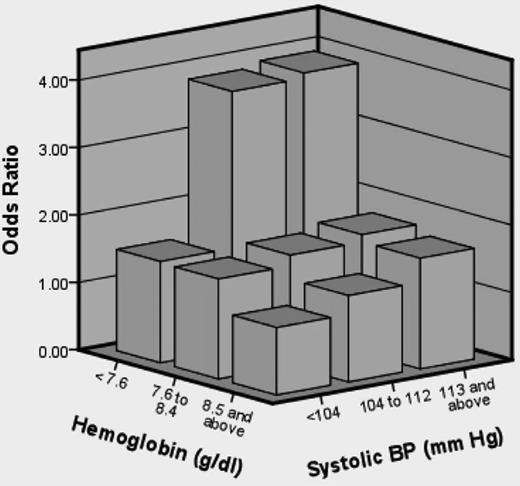
Recently, the SIT trial study group reported on risk factors for SCI in 814 patients.12 The study population was children 5-15 years of age who had screening MRIs of the brain to determine eligibility for the main SIT trial. None had a history of overt stroke or seizures. Several clinical and laboratory variables were studied as predictors of SCI. In univariate analyses, male sex (P = .005), baseline hemoglobin concentration (P < .001), baseline systolic blood pressure (SBP; P = .016), and baseline oxygen saturation (P = .04) were all associated with SCI. For multivariate analysis, hemoglobin concentration and SBP were considered in tertiles. In logistic regression analysis, lower baseline hemoglobin concentration (P < .001), higher baseline SBP (P = .018), and male sex (P = .030) were statistically significantly associated with an increased risk of an SCI. Participants who had a hemoglobin concentration in the lowest tertile (<7.6 g/dL) had a higher risk of SCI compared with the reference tertile (≥8.6 g/dL). Participants in the 2 highest tertiles for SBP (≥104 and ≤112 and ≥113 mmHg) had a higher risk of SCI compared with those in the reference tertile (<105 mmHg). It is important to note that the study identified a higher relative SBP as a risk factor for SCI and not hypertension per se. A joint effect of hemoglobin concentration and SBP was also seen, where the highest risk for SCI was found in patients who were in both the lowest tertile for hemoglobin concentration (<7.6 g/dL) and the highest tertile for SBP (≥113 mmHg) (Figure 3). This is an important study because it identified 2 potentially modifiable risk factors for SCI.
Joint effect of hemoglobin concentration and SBP on the odds of SCI. The highest risk for SCI was found in patients who were in both the lowest tertile for hemoglobin concentration (<7.6 g/dL) and highest tertile for SBP (≥113 mmHg). (Figure reprinted with permission from DeBaun et al.12 )
Joint effect of hemoglobin concentration and SBP on the odds of SCI. The highest risk for SCI was found in patients who were in both the lowest tertile for hemoglobin concentration (<7.6 g/dL) and highest tertile for SBP (≥113 mmHg). (Figure reprinted with permission from DeBaun et al.12 )
SCI and associated morbidity
Prior studies have documented that SCI is a morbid condition associated with neurocognitive impairment, poor academic performance, neurologic soft signs, and increased risk for subsequent overt stroke.13 Two recent studies add to our understanding of the factors associated with SCI, whether causal or not. An important clinical question is whether there is an association between headaches or migraines and SCI, both of which are common in SCD. Prior studies have shown an association between headaches and white matter lesions in adults without SCD14 and that children are more likely to present with acute headaches at the onset of first cardioembolic stroke than adults.15 Although acute headache can be a presenting feature of CNS events in children with SCD,16 no association between headaches and SCI was identified in 3 single-institution studies of children with SCD.17-19 Recently, Dowling et al20 studied the SIT trial cohort to determine the risk factors for recurrent headaches and recurrent migraines. In multivariable logistic regression analysis, both headaches and migraines were associated with lower steady-state hemoglobin and higher pain rate, but headaches or migraines were not associated with SCI. A practical interpretation of this study is that children with SCD who present with acute headaches or migraines but no other neurologic abnormalities might not need additional evaluation with MRI of the brain.
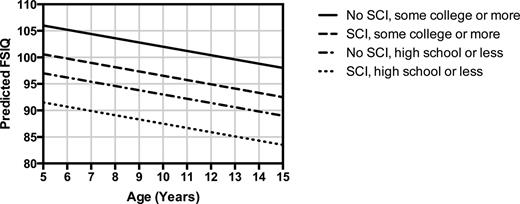
The educational attainment of parents is a strong predictor of the cognitive development of children in the general population. However, individuals with SCD have physiological and neuroanatomical pathology (chronic anemia, cerebral desaturation,21 and SCI) that may also influence cognition, so what is the role of socioeconomic factors on cognition in this setting? To answer this question, King et al22 studied the role of parents' education and family income on cognition in children with HbSS. These investigators studied 150 children (107 with and 43 without SCI) screened for the SIT trial who were administered the Wechsler Abbreviated Scale of Intelligence. In a multivariate model for Full-Scale Intelligence Quotient (FSIQ), the absence of college education for the head of household was associated with a decrease of 6.2 points (P = .005), presence of SCI with a 5.2 point decrease (P = .017), each $1000 of family income per capita with a 0.33 point increase (P = .023), each increase of 1 year in age with a 0.96 point decrease (P = .023), and each 1% (absolute) decrease in hemoglobin oxygen saturation with a 0.75 point decrease (P = .030) in FSIQ. A practical interpretation of this study is that FSIQ in children with HbSS is best accounted for by a model that includes both biologic and socioeconomic factors (Figure 4). A very interesting finding is that parents' educational attainment is a stronger correlate of a child's FSIQ than biological factors, so it will be important to consider the home environment when testing preventive strategies for SCI.
Effect of age, presence of SCI, and head of household education on predicted FSIQ. The model predictions have household per capita income and hemoglobin oxygen saturation fixed at mean values. (Data for figure obtained from King et al.22 )
Effect of age, presence of SCI, and head of household education on predicted FSIQ. The model predictions have household per capita income and hemoglobin oxygen saturation fixed at mean values. (Data for figure obtained from King et al.22 )
Acute silent cerebral ischemic events
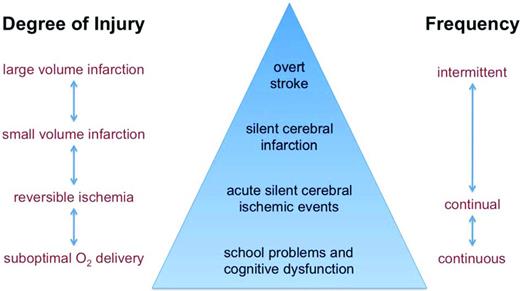
The overall burden of ischemic insults to the brain in individuals with SCD is not actually known. Overt stroke and transient ischemic attacks are clinically apparent events, usually with MRI correlates, so their incidence is relatively easy to calculate. SCI is also quantifiable because it is defined by a discrete MRI lesion of the brain, but the challenge is that it can only be detected by screening MRI because it produces no focal neurologic signs. The combined frequency of these events (overt stroke, transient ischemic attacks, and SCI), however, likely underestimates the overall burden of ischemic insults to the brain in SCD. Recurrent, subclinical, and potentially reversible ischemia occurs in other organ systems in SCD, so the brain is not likely to be an exception. It is possible that some silent cerebral ischemic events could be transient and reversible, leaving no permanent tissue injury. In addition, cerebral ischemia could leave permanent lesions that are smaller than the limits of detection by clinical MRI. Therefore, considering only the frequency of clinically overt events and macroscopic, permanent MRI lesions fails to account for a potentially larger burden of ongoing (chronic, intermittent) subclinical cerebral ischemia. However, it is challenging to detect acutely ischemic and potentially reversible lesions, especially in asymptomatic individuals without focal neurologic signs, because of the expense and inconvenience of frequent MRIs.
A recent report provided evidence that silent cerebral ischemia can be identified during the acutely ischemic phase.23 Patients with SCD who had acute exacerbation of chronic anemia were found to have silent cerebral ischemia (“acute silent stroke”) by diffusion-weighted MRI. None had focal neurologic signs (eg, hemiparesis) by definition, but several patients had transient or subtle neurologic symptoms. The subset of patients who had follow-up imaging in convalescence had what appeared to be typical SCI lesions in the location of the previously detected acute ischemia. These observations indicated not only that SCI could be detected in the acute phase, but that exacerbation of anemia might predispose to SCI and subtle neurologic signs and symptoms might be clues to the onset of SCI.
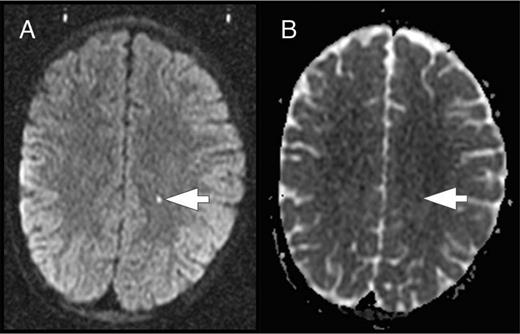
In the course of the SIT trial, some study participants were found to have acute ischemic lesions on screening brain MRIs to determine eligibility for study. These incidentally detected acute ischemic lesions occurred in children who were asymptomatic and clinically well at the time of the MRI. To explore the hypothesis that these acute silent cerebral ischemic events (ASCIEs) were frequent and potentially transient, Quinn et al9 studied the participants of the SIT trial. ASCIE was defined as an area of restricted diffusion on diffusion-weighted imaging (DWI) sequences with a corresponding decrease in signal intensity on the apparent diffusivity coefficient map in the absence of focal neurologic findings that could be explained by the location of the DWI-positive lesion (Figure 5). The temporal information contained in the DWI sequences was used to calculate the incidence of ASCIEs. DWI signal abnormalities occur within 24 hours of the onset of cerebral ischemia and persist for about 10 days, so DWI-positive lesions were considered to be new (incident) and provide 10 patient-days of observation. ASCIEs were detected on 1.3% of MRIs (10 of 771) in 652 children (mean age, 10.0 years), with an incidence of 47.3 events per 100 patient-years (95% CI, 22.7-87.2). In comparison, the incidence of ASCIE was about 40 times higher than the incidence of initial SCI and 4 times higher than recurrent SCI (Table 1). Nine of 10 cases of ASCIEs had no acute medical illnesses in the 2 weeks before MRI. One had just recovered from a recent episode of acute chest syndrome complicated by severe anemia and hypertension and treated with a simple transfusion. Standard neurologic examination was normal for all 10, indicating that the acute cerebral ischemia was clinically covert or silent. Two of 10 children with ASCIEs in this study had follow-up MRIs of the brain, and only 1 had SCI in the same location as the previously detected ASCIE. The main conclusion is that cerebral ischemia occurs far more frequently than previously recognized in SCD. It is important to be aware that ASCIEs often occur during baseline or “steady-state.” Some ASCIEs may be reversible and leave no detectable lesion on MRI, whereas others evolve into typical SCI.
ASCIE. ASCIE is defined as an area of restricted diffusion on DWI sequences (A) with a corresponding decrease in signal intensity on the apparent diffusivity coefficient (ADC) map (B) in the absence of focal neurologic findings that could be explained by the location of the DWI-positive lesion. The lesion has increased signal on T2 and T2-fluid-attenuated inversion recovery images (data not shown).
ASCIE. ASCIE is defined as an area of restricted diffusion on DWI sequences (A) with a corresponding decrease in signal intensity on the apparent diffusivity coefficient (ADC) map (B) in the absence of focal neurologic findings that could be explained by the location of the DWI-positive lesion. The lesion has increased signal on T2 and T2-fluid-attenuated inversion recovery images (data not shown).
Dowling et al24 built upon their earlier observations about the potential role of anemia in the genesis of ASCIEs. These investigators conducted a prospective observational study of children with SCD who were hospitalized for an illness complicated by an acute exacerbation of chronic anemia, such as acute chest syndrome or aplastic crisis. These acute anemic events (AAEs) were defined as a hemoglobin concentration that was ≤5.5 g/dL and decreased at least 30% from baseline (“steady-state”). Using DWI sequences, ASCIEs were detected in 4 (18.2%) of 22 patients with SCD and AAE. All lacked focal neurologic signs, but some had mild abnormalities in the cognitive-behavioral domain with structured testing. Using the temporal information contained in DWI images, the incidence of ASCIEs during AAE was estimated to be 663 events per 100 patient-years, far greater than the incidence of ASCIEs in asymptomatic children with SCD (Table 1). One of 4 patients with ASCIE had no lesion on follow-up MRI corresponding to the focus of prior acute ischemia, consistent with the study by Quinn et al9 showing that some ASCIEs may be reversible and leave no detectable lesion on MRI, whereas others evolve into typical SCI.
In summary, these observations indicate that individuals with SCD experience cerebral ischemia far more frequently than previously recognized (Figure 6). ASCIEs can occur in asymptomatic individuals without antecedent or concurrent acute medical illnesses, which would go unrecognized unless the patient happened to have a screening MRI that identified the unsuspected acute lesion. However, AAEs appear to increase the risk of ASCIEs dramatically. A fraction of ASCIEs appear to be transient and leave no detectable MRI lesion on follow-up scans, whereas others evolve into typical, permanent SCI. Although, by definition, focal neurologic signs (eg, hemiparesis) do not occur with ASCIEs, subtle neurologic signs and symptoms appear to be clues to the occurrence of ASCIEs. Finally, given the association of AAEs with cerebral ischemia, transfusion to correct at least the acute anemia might be beneficial for individuals with AAEs, even in those who do not have “symptomatic” anemia.
Model depiction of different type of brain injury or dysfunction in SCD. Types of brain injury are shown in the triangle and categorized by degree of injury and frequency. Overt stroke and SCI likely represent only a fraction of the burden of ischemic insults to the brain.
Model depiction of different type of brain injury or dysfunction in SCD. Types of brain injury are shown in the triangle and categorized by degree of injury and frequency. Overt stroke and SCI likely represent only a fraction of the burden of ischemic insults to the brain.
Management of patients with SCI
The medical management of SCI has not yet been resolved. Two studies provide evidence that chronic transfusions may decrease the frequency of new SCI in patients with prior stroke or abnormal transcranial Doppler (TCD) velocities. However, there is also evidence that chronic transfusions for first overt stroke may not prevent SCI,25 hydroxyurea may not prevent SCI,26 and that SCI still occurs frequently despite intensification of medical therapy for SCD.3 The results of the recently completed SIT trial were not published at the time this chapter was written, so no comments about the findings can be made here. The SIT trial randomized children (5-14 years of age) with sickle cell anemia and SCI to chronic transfusion therapy or standard care (observation).1 The main exclusion criteria were overt stroke, abnormal TCD velocities, epilepsy, and treatment with hydroxyurea or chronic transfusions. The primary aim was to determine whether 36 months of chronic transfusion therapy decreased the incidence of new or progressive SCI or overt stroke. The secondary aims were to determine whether chronic transfusions limited intellectual decline in children with SCI and to assess the risk-benefit ratio of chronic transfusions for SCI. Ancillary studies from the SIT trial suggest that the overall results, when published, may be broadly clinically applicable. For example, the imaging protocol had a high degree of reproducibility27 and acceptance of random allocation by study participants (and families) was not associated with socioeconomic or demographic factors.28
The role of hydroxyurea for the primary or secondary prevention of SCI has not been established. A currently recruiting study, Hydroxyurea to Prevent Brain Injury in Sickle Cell Disease (www.ClinicalTrials.gov identifier #NCT01389024), randomizes children with sickle cell anemia (12-48 months of age) with no evidence of overt or covert cerebrovascular disease to hydroxyurea or placebo. The primary outcome is a composite of TCD abnormalities, SCI, or overt stroke. The results of this trial will provide guidance about the role of hydroxyurea for the primary prevention of SCI (and CNS injury in general). No randomized trials have addressed the role of hydroxyurea for secondary prevention of SCI as the primary outcome.
Even though we do not yet have high-quality evidence from randomized clinical trials to support a particular medical intervention for SCI, such as chronic transfusion or hydroxyurea therapy, we are not powerless to help. Neuropsychological testing, psychological counseling and support, and school intervention programs are all key components of high-quality, comprehensive SCD care. We know that children with SCI have an average loss of at least 5 points on FSIQ testing (Figure 4) and are at risk for academic failure and future silent and overt cerebral infarction. Fortunately, students with SCD who are documented to have SCI and decreased cognitive function are eligible for specific educational resources for which they would not otherwise be eligible. Other resources are available for young adults who need vocational support or assistance in college. Therefore, even though we are awaiting high-quality clinical evidence for the medical management of SCI from randomized trials, simply the knowledge of the presence of SCI can allow one to obtain special resources to optimize educational and employment opportunities and improve outcomes. The benefits of an MRI of the brain performed without anesthesia or sedation to identify SCI very likely outweigh the few risks of the procedure, so it would be prudent to perform at least one screening MRI for SCI, especially in young school-aged children, and consider it for older individuals with SCD as well.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Charles T. Quinn, MD, MS, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave., Cincinnati OH, 45229; Phone: (513)803-3086; Fax: 513-636-3549; e-mail: charles.quinn@cchmc.org.