Abstract
Dabigatran, rivaroxaban, and apixaban are orally active anticoagulants that are approved in many countries. Dabigatran inhibits thrombin, whereas rivaroxaban and apixaban are factor Xa inhibitors. In clinical trials, these novel oral anticoagulants were at least as effective as warfarin for preventing stroke in patients with atrial fibrillation, but with a lower rate of serious bleeding. However, the lack of true antidotes for these agents has caused concern when patients suffer life-threatening bleeding or trauma or require emergent invasive procedures. True antidotes are under development for all of these agents. In the meantime, activated and nonactivated prothrombin complex concentrates have been used as reversal agents. Factor VIIa may also be effective for reversal of the factor Xa inhibitors. Reversal of novel oral anticoagulants by these hemostatic agents has not been studied in bleeding human patients, so their true efficacy and appropriate dosing are not known.
Learning Objective
To understand the currently available options for enhancing hemostasis in a patient on one of the targeted oral anticoagulants
Terminology
First of all, what do we mean by “targeted oral anticoagulants”? We are referring to the orally active agents introduced to the market within the past few years that specifically inhibit either thrombin or coagulation factor Xa (FXa). There is now a bit of dilemma on what to call them. They had been termed “novel oral anticoagulants” or “NOACs.” With the passage of time, that name has become less and less appropriate. More recently, some clever thought leaders in this area have suggested keeping the term “NOAC,” but using it to refer to “non-vitamin K antagonist oral anticoagulants.”1 This is very reasonable because, until recently, warfarin-like agents have been the only oral anticoagulants available. In addition, we will not have to change our terminology or search terms much. In this chapter, I refer to the agents in question as “NOACs” for this reason. Only time will tell whether this terminology will stick.
What is different about NOACs?
The NOACs are small molecules that each inhibit one of the proteases in the coagulation process, either thrombin or FXa. The first of this class of drugs to be approved in the United States was the thrombin inhibitor dabigatran etexilate mesylate (Pradaxa). Two FXa inhibitors have now also been approved in the United States: apixaban (Eliquis) and rivaroxaban (Xarelto).
In the United States, dabigatran etexilate is approved to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation and for the treatment and reduction of recurrence of deep vein thrombosis and pulmonary embolism. In the European Union and Canada, it is additionally approved for prevention of deep vein thrombosis in patients undergoing knee or hip replacement surgery. Rivaroxaban is approved in many countries to reduce the risk of stroke in patients with nonvalvular atrial fibrillation, for prevention of deep vein thrombosis after knee or hip replacement surgery, and to treat and reduce the risk of deep vein thrombosis or pulmonary embolism. In the European Union, it is additionally approved to prevent atherothrombotic events after an acute coronary syndrome. Apixaban is approved both in the United States and European Union to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation; for the prophylaxis of deep vein thrombosis after hip or knee replacement surgery; and to treat and reduce the risk of deep vein thrombosis or pulmonary embolism.
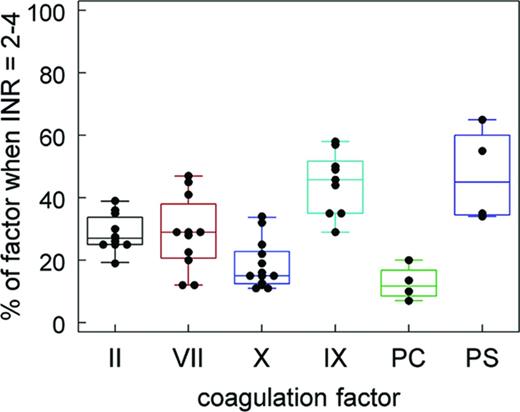
If we look at these anticoagulants in terms of the traditional “coagulation cascade,” we see that each of these molecules inhibits a component of the “common pathway” (Figure 1). In contrast, vitamin K antagonists (VKAs) such as warfarin inhibit the posttranslational modification of all of the vitamin K–dependent factors. This results in production of nonfunctional proteins, effectively inhibiting the coagulation process at multiple points. Not only is the activity of the vitamin K–dependent procoagulants [prothrombin or factor II (FII), factor VII (FVII), factor IX (FIX), and factor X (FX)] reduced, but also the activity of the vitamin K–dependent anticoagulants (proteins C and S). Interestingly, the different vitamin K–dependent factors are not all reduced to the same degree, as shown in Figure 2. That figure was constructed from levels of the factors published in the peer-reviewed literature.2-8
Targets of NOAC (non-VKA oral anticoagulants) and VKAs in a “coagulation cascade” format.
Targets of NOAC (non-VKA oral anticoagulants) and VKAs in a “coagulation cascade” format.
The effect of VKA anticoagulation on a PT-INR of 2-4 on the levels of the vitamin K–dependent factors. These values were compiled from literature references. Each dot represents the value reported in one source.
The effect of VKA anticoagulation on a PT-INR of 2-4 on the levels of the vitamin K–dependent factors. These values were compiled from literature references. Each dot represents the value reported in one source.
The prothrombin time (PT) assay only reflects the levels of 3 of the 4 procoagulants affected by VKAs. However, it has been adapted and standardized via the International Sensitivity Index (ISI)/International Normalized Ratio (INR) system to reflect the relative intensity of VKA anticoagulation. Note that this is not because the PT-INR is fundamentally a measure of hemostatic capability in vivo, but rather because it has been empirically determined that an INR in the 2-3.5 range represents the best compromise between the risk of thrombosis and risk of bleeding for the overall population of patients on VKAs.
Monitoring the intensity of VKA anticoagulation with the PT-INR is necessary because of the variability in response to these agents between individuals and because of the many foods and drugs that can have an influence on the effects of VKAs. In addition, the fact that VKAs reduce the activity of multiple factors means that the level of VKA effect does not have a linear relationship with the intensity of anticoagulation and contributes to the relatively narrow therapeutic window of these agents.
Inhibition of multiple factors has a synergistic effect on the degree of anticoagulation, making it much more difficult to titrate the level of anticoagulation than with an agent that has a single target. This is a good strategy if profound anticoagulation is desired, but makes it more difficult to “fine-tune” the degree of anticoagulation than when only a single step is inhibited.
If we look at the effects of NOACs in terms of a cell-based model of coagulation, we see that each of the NOACs can actually have effects on thrombin generation or activity on 2 different cell surfaces in vivo (Figure 3). Each of them can have effects on initiation of coagulation and on the burst of thrombin produced on platelet surfaces that is responsible for clotting fibrinogen. A thrombin inhibitor, of course, directly inhibits thrombin activity, whereas an FXa inhibitor decreases the production of thrombin.
In a cell-based model of coagulation, the small-molecule thrombin and FXa inhibitors (NOACs) can affect coagulation at both the initiation phase and the propagation phase. During initiation, a small amount of thrombin is produced, which participates in many amplification loops. The propagation phase produces the large burst of thrombin that leads to stable clot formation.
In a cell-based model of coagulation, the small-molecule thrombin and FXa inhibitors (NOACs) can affect coagulation at both the initiation phase and the propagation phase. During initiation, a small amount of thrombin is produced, which participates in many amplification loops. The propagation phase produces the large burst of thrombin that leads to stable clot formation.
Thrombin generation during hemostasis generally follows the stylized curve shown in Figure 4. During the “initiation” and “amplification” phases of hemostasis, little or no thrombin can be measured in the fluid phase. Only small amounts of thrombin are generated, and most of it exerts its activity on or near the surfaces of tissue factor–bearing cells and platelets.9 During the amplification phase of hemostasis, the small amount of thrombin that has been formed is involved in multiple loops that promote platelet and coagulation factor activation. Inhibition of thrombin generation (by small-molecule FXa inhibitors) or activity (by small-molecule thrombin inhibitors) results in prolongation of the lag in the thrombin generation curve (Figure 4). During the “propagation” phase, large-scale thrombin generation takes place on the activated platelet surface. Inhibition of thrombin generation or activity during this phase is responsible for reducing the total amount of active thrombin available to clot fibrinogen. Therefore, the NOACs both prolong the lag and reduce the total amount of thrombin activity produced.
Stylized curve of thrombin generation during hemostatic coagulation. The lag before the onset of measurable thrombin generation corresponds to the initiation and amplification phases. During this period, a small amount of thrombin is produced, which participates in feedback amplification of the procoagulant signal. The large burst of thrombin generation that takes place on platelet surfaces during the propagation phase is responsible for the peak of measurable thrombin activity. Both the rate of thrombin generation (#2) and the total amount of thrombin activity (#3) play important roles in determining clot structure and stability.
Stylized curve of thrombin generation during hemostatic coagulation. The lag before the onset of measurable thrombin generation corresponds to the initiation and amplification phases. During this period, a small amount of thrombin is produced, which participates in feedback amplification of the procoagulant signal. The large burst of thrombin generation that takes place on platelet surfaces during the propagation phase is responsible for the peak of measurable thrombin activity. Both the rate of thrombin generation (#2) and the total amount of thrombin activity (#3) play important roles in determining clot structure and stability.
Assaying and monitoring NOACs
One of the advantages of NOACs is that they do not require routine monitoring. However, there are circumstances under which one would like a convenient assay. The “gold standard” assay methodology is liquid chromatography-tandem mass spectrometry. The coagulation “cascade” model (Figure 1) is a good model for the common PT and activated partial PT (aPTT) assays. Based on the effects of NOACs on the terminal steps of clot formation, it appears that they would prolong both the PT and aPTT and that these assays could perhaps be used to assay NOAC levels. In practice, the PT is more sensitive to the FXa inhibitors and the aPTT to thrombin inhibitors. The responsiveness of these assays to NOACs depends on the specific reagents used. Therefore, the PT and aPTT may be normal despite therapeutic NOAC levels.10,11 Individual laboratories can determine the responsiveness of their own aPTT and PT reagents, which may allow estimation of thrombin or FXa inhibitor levels. Anti-FXa activity assays are available at many hospitals and have reasonably good correlation with the reference method for estimating levels of FXa inhibitors. Specific tests to measure thrombin inhibitor levels include ecarin chromogenic assay (ECA; Diagnostica Stago), dilute thrombin time, and Prothrombinase Induced Clotting Time (PiCT; Pefakit). These, however, are not routinely available at most hospitals.
Do we really need antidotes for NOACs?
Physicians hold a very wide range of views on NOACs. Many focus on the fact that a large number of patients with atrial fibrillation who, by current guidelines, should be on anticoagulation12 are not. Patients with atrial fibrillation who are not on anticoagulants have a risk of stroke ranging from ∼2% to 19% per year depending on age and comorbidities.13 It has been estimated that there are >50 000 preventable strokes per year in the United States. Key clinical trials have shown that, at least in the population under study, the NOACs are as effective as warfarin in preventing strokes in patients with atrial fibrillation and are associated with a lower risk of life-threatening bleeding than warfarin. A meta-analysis including the 71 683 participants in the RE-LY, ROCKET AF, ARISTOTLE, and ENGAGE trials concluded that, overall, patients on one of the NOACs had a 19% lower risk of stroke or embolic events compared with those on warfarin.14 This benefit was mainly driven by a reduction in hemorrhagic stroke. Patients on NOACs also had a lower all-cause mortality but a higher risk of GI bleeding.14 Bleeding in these trials was managed primarily by stopping the NOAC and providing supportive care. In addition to better outcomes, patients on dabigatran (RE-LY trial) were more likely to stay on their anticoagulant regimen than patients taking warfarin.15 Of course, these benefits of NOACs were based on results of major clinical trials in which subjects were more highly selected than in the real world [after US Food and Drug Administration (FDA) approval]. However, at least in this patient population, outcomes appear to be better with NOACs than with warfarin, even without antidotes for the NOACs. Therefore, many physicians (and presumably regulatory agencies) conclude that these agents should be used even more widely. In a public health context, the main reason antidotes are needed is to make more doctors and patients comfortable using the NOACs and reaping their benefits.
The opposing view does not focus on the majority of patients with atrial fibrillation who do well on NOACs. Rather, it focuses on the relatively small number of patients who experience serious bleeding, suffer trauma, or need an urgent/emergent invasive procedure. These patients can be extremely challenging and can leave a lasting impression on those who are called upon to manage them. Some physicians who have been in this situation have the opinion that it was irresponsible of the FDA to approve new anticoagulants without requiring that there also be antidotes available. For example, in a letter to the New England Journal of Medicine, Cotton et al16 presented their experience with several patients taking dabigatran who had poor outcomes after traumatic injuries. They opine that “when prescribing a drug with side effects that include life-threatening hemorrhage, reversal is not “desirable,” it is essential.”17
We are not aware of any clinical trials directly evaluating the outcome of injury-associated hemorrhage in patients on NOACs compared with warfarin. In the RE-LY trial of the thrombin inhibitor dabigatran (total of 18 113 patients), 46 intracranial (intracerebral, subarachnoid, and subdural) hemorrhages occurred in association with trauma.18 Overall mortality was 24% in these patients. The mortality rate for patients on dabigatran (6 of 22) who suffered traumatic intracranial hemorrhage was not different from patients on warfarin (5 of 24).
One small, retrospective study suggests that outcomes after injury may be worse for patients on dabigatran than for those on warfarin.19 A group from a trauma center reviewed the outcome of closed head injuries due to ground level falls at their institution. Although the overall mortality rate was 14%, the mortality rate of patients on dabigatran was 40% (2 of 5); 0% (0 of 15) for patients on warfarin and 0% (0 of 25) for patients not on an anticoagulant. The 2 deaths in the dabigatran group were related to the progression of intracranial hemorrhage. Despite the extremely small numbers of subjects, the risks of progression of intracerebral bleeding and death were significantly greater in the patients on dabigatran.
Better data, although still retrospective, are available for bleeding in patients on rivaroxaban. Data from the Dresden NOAC registry was used to assess rates, management, and outcome of rivaroxaban-related bleeding in patients who were not part of a clinical trial.20 Between 2011 and 2013, 1776 patients taking rivaroxaban were enrolled. Of these, 762 patients experienced 1082 bleeding events, including 6.1% with major bleeding. The majority (71.2%) of major bleeding events occurred spontaneously, whereas 10.6% occurred after trauma and 6.9% after surgical or interventional procedures. Most major bleeding events were managed with supportive care. Surgical or interventional treatment was needed in 37.8% and a prohemostatic agent [prothrombin complex concentrate (PCC)] was given to 9.1%. The 6 patients given PCC received 18-47 IU/kg. Five of them showed stabilization of hemorrhage; 4 of these survived without sequelae at day 90. The fifth patient died of septic pneumonia on day 16. The only PCC-treated patient in whom hemorrhage did not stabilize died of intracranial bleeding on day 7. This patient had received the lowest dose of those treated with PCC, 18 IU/kg.
Cardiovascular events occurred in 6 of the Dresden registry patients within 90 days of the bleeding event. None of them had received procoagulant treatment (PCC) during the bleeding situation. This is an important observation given the prothrombotic risk of PCC.21
Fatality rates were 5.1% and 6.3% at 30 and 90 days after bleeding in the Dresden NOAC registry, respectively. Recent data from large cohorts of patients on VKA estimate the fatality rate of VKA-related major bleeding at ∼15%-20%, up to 50% for intracranial bleeding.22,23 Although there was no direct comparison with patients treated with VKA, it appears that rates of rivaroxaban-related major bleeding may be lower and outcomes are not worse than in VKA-treated patients. In summary, only small numbers of patients with rivaroxaban-associated bleeding required a reversal strategy, with prohemostatic therapy being given only in life-threatening situations. The investigators did note that, if indicated, PCC should be given as early as possible.
It seems likely that many thousands of patients could benefit from the NOACs and only a small proportion of those will suffer major bleeding and require a reversal agent However, if you are caring for even one individual who is injured or suffering serious bleeding while anticoagulated, you would surely like to have an antidote—or at least a reversal strategy—available. Therefore, all would probably agree that we should have antidotes, either to make us feel better about using NOACs or to allow us to better manage those patients who develop life-threatening bleeding.
What do we mean when we talk about “reversing” an anticoagulant?
Reversal of NOAC effects can mean 2 different things. First, it could refer to a direct “antidote” that inactivates the drug/protease inhibitor. In the case of warfarin and related drugs, we do have true antidotes. Reduced forms of vitamin K directly antagonize the effect of warfarin on coagulation factor synthesis, thus allowing synthesis of the normal active forms of the proteins. In addition, one can directly replace the deficient factors with either plasma or PCCs.
In contrast, there are no direct antidotes yet available for the NOACs. These would be agents that directly bind and inactivate the anticoagulant molecules. At the present time, the only reversal strategies for NOACs are more akin to the use of “bypassing” agents in hemophiliacs with inhibitors. In other words, instead of replacing something that is missing, we try to overcome the effects of the inhibitor on thrombin generation or activity.
Although no direct antidotes are yet available for the NOACs, several strategies are in development. One is a humanized Fab antibody fragment against dabigatran (idarucizumab). This agent binds to dabigatran with high affinity and inhibits its activity.24 Findings were presented at the American Heart Association (AHA) 2013 Scientific Sessions, showing that this approach rapidly and completely reverses the effects of dabigatran in normal volunteers. Idarucizumab is being evaluated in a phase 3 study, RE-VERSE AD, of patients on dabigatran who have uncontrolled bleeding or require emergency surgery or procedures. The FDA has granted breakthrough therapy designation to idarucizumab. This designation is intended to expedite the review of drugs for serious or life-threatening conditions if preliminary clinical evidence indicates the therapy may demonstrate a substantial improvement over existing therapies on one or more clinically significant end points.
Two potential antidotes for FXa inhibitors are also in development. One is a recombinant modified FXa protein (andexanet alpha, PRT064445; Portola Pharmaceuticals). It is catalytically inactive and lacks the membrane-binding domain of wild-type FXa, but can still bind to FXa inhibitors and antithrombin.25 Therefore, it has no procoagulant activity, but it inactivates FXa inhibitors. Data on andexanet were presented at the ASH Annual Meeting in 2013. It immediately and completely reversed the anticoagulant effect of factor Xa inhibitors with no indication of prothrombotic effects. It is currently being tested in clinical trials (www.ClinicalTrials.gov identifiers #NCT02220725 and #NCT02207725).
A second potential antidote in early stage clinical trials is a small molecule that binds to FXa inhibitors, as well as to heparin (Aripazine, PER977; Perosphere). Therefore, it appears that true antidotes are on the horizon for both dabigatran and FXa inhibitors.
In the meantime, there are 3 agents available that been proposed for reversing NOAC anticoagulation: activated coagulation factor VII (FVIIa), PCCs, and activated PCCs (aPCCs). PCCs are products containing vitamin K–dependent factors in the unactivated (zymogen) form. They are purified from plasma and treated to inactivate or remove pathogens. They were originally used as replacement therapy for FIX (hemophilia B), so their activity is expressed in FIX units. Some of the PCCs contain relatively low levels of FVII and are referred to as “3-factor” PCCs because they primarily contain FII, FIX, and FX. “Four-factor” PCCs also contain FVII at a level comparable to their content of FII, FIX, and FX. aPCCs, of which there is only one currently on the market, FEIBA (Baxter), also contain zymogen vitamin K–dependent factors, but some of the factors have been partially activated during the purification process.
Because dabigatran was the first of the NOACs to reach the market, there are more data on its reversal than on the FXa inhibitors. There are no clinical trials testing the effectiveness of reversal agents for any of the NOACs in bleeding patients. All of the available data were obtained by testing the ability of reversal agents to improve various coagulation assays (as a surrogate for hemostatic function) and to reduce bleeding in a variety of animal models.
Quite a few reviews have been published detailing the data from existing studies on reversal agents. Therefore, we will summarize and draw some conclusions from the available data rather than recounting all of the results.
Most of the studies on reversing FXa inhibitors in animal bleeding models showed that PCCs, aPCCs, and FVIIa were all partially or fully effective, even at extremely high levels of the FXa inhibitor.26-30 Findings have been more mixed in reversing dabigatran. PCCs and aPCCs were partially or fully effective in reversing dabigatran in most animal studies.31,32 In general, fewer studies found FVIIa to be effective than PCC or aPCC, and some studies found both PCC and FVIIa to be without benefit.33 The reason for the inconsistent findings with FVIIa might be explained by the fact that in vitro studies suggest that FVIIa can reverse the effect of dabigatran at therapeutic levels, but the effectiveness of FVIIa declines as the dabigatran levels increase.34 The animal models for studying reversal used a range of NOAC doses, with those using extremely high levels of dabigatran being less likely to show effective reversal.
Many of the studies only examined the effects of reversal agents on laboratory parameters. When both in vivo assessment and laboratory assays were performed (summarized in Lee et al35 ), the effects of reversal agents on the common clinical tests (PT, PTT, thrombin time, activated clotting time) did not correlate well with bleeding time or amount of blood loss. The laboratory tests having the best correlation with hemostasis were thrombin generation assays. Although these can be difficult to standardize and are too cumbersome to perform as routine clinical assays, they may be useful in predicting which treatments deserve further study.
In examining the effects of hemostatic agents in thrombin generation assays in the presence of the FXa or thrombin inhibitors,34,36,37 PCCs could correct all parameters of thrombin generation except the lag. aPCCs could also correct the lag, but were more likely to “overshoot” and increase parameters of thrombin generation to supranormal levels. Although this tendency may enhance efficacy, it could also possibly increase the risk of thrombotic complications. FVIIa could shorten the lag and increase the rate of thrombin generation, but did not increase the total amount of thrombin generated. FVIIa also had a greater effect when the dabigatran level was in the therapeutic range than when it was supratherapeutic. These results have been interpreted to mean that aPCCs or PCCs are likely the most effective agents for reversal of NOACs given that there are no trials of any agent to reverse the effects of NOACs in bleeding patients.
Mechanisms of NOAC reversal
How could “bypassing” agents such as FVIIa, PCC, and aPCC enhance hemostasis in this setting? Studies of thrombin generation in vitro may shed some light on mechanism.
The addition of FVIIa can shorten the lag before onset of thrombin generation (Figure 4) by more rapidly producing FXa, which then generates thrombin during initiation and amplification.38,39 This same mechanism can also increase the rate of thrombin generation during the propagation phase (#2 in Figure 4). However, it does not result in more prothrombin being converted to thrombin (#3 in Figure 4), because that parameter is limited by the amount of prothrombin present.
In contrast, PCCs do not shorten the lag phase.36 However, primarily by providing prothrombin (with minor contributions from FIX, FX, and FVII), PCC increases the rate of thrombin generation and also the total amount of prothrombin converted to thrombin.34 aPCC can both shorten the lag due to the presence of activated factors and also increase the rate and total amount of thrombin activity generated.
The prothrombin content of PCCs and aPCCs appears to be important for reversal of anticoagulation. The prothrombinase (FXa/FVa) complex is not saturated at normal plasma levels of prothrombin. Therefore, increasing the level of prothrombin leads to more rapid production of thrombin. Even if the number of active FXa/FVa complexes is reduced due to FXa inhibition, those that remain will produce more thrombin if the prothrombin level is increased.
Summary
In summary, true antidotes to NOACs are in development and will be useful in cases that cannot be managed by waiting for clearance of the drug and/or providing supportive care. Animal and in vitro studies suggest that nonspecific prohemostatic agents can likely overcome NOAC effects by increasing FXa and/or thrombin generation. In vivo and in vitro studies suggest that FXa inhibitors may be more readily reversible than thrombin inhibitors. PCCs and aPCCs are the hemostatic agents most commonly used in patients on NOACs with life-threatening bleeding. The true efficacy and appropriate dosing of these agents have not been studied in bleeding patients and may depend on the level of anticoagulant present.
Disclosures
Conflict-of-interest disclosures: M.H. has received research funding from CSL-Behring, Boehringer Ingelheim, and Novo Nordisk; has consulted for CSL-Behring; and has received honoraria from Novo Nordisk. D.M.M. declares no competing financial interests. Off-label drug use: Any drug used for reversal of oral anticoagulants (other than warfarin) constitutes an off-label use. PCCs, activated PCC, and FVIIa for this use are discussed.
Correspondence
Maureane Hoffman, Pathology & Lab Medicine Service, Durham VA Medical Center, 508 Fulton St., Durham, NC 27705; Phone: (919)286-0411 (x6494); Fax: (919)286-6818; e-mail: maureane@med.unc.edu.