Abstract
Epidemiological studies have demonstrated an increased risk of developing B-cell lymphomas in patients with chronic hepatitis C virus (HCV) infection. However, the strength of the association shows great geographic discrepancies, with higher relative risk in countries with high HCV prevalence. It remains unclear whether additional environmental and genetic factors are involved or if the international variability is simply a consequence of the variable infection prevalence. Therefore, a causal relationship remains controversial. Other confounding factors may affect the comparability of the different studies, including the method of HCV assessment, the selection of normal controls, the lymphoma classification used, and the year of publication. The most convincing proof is the observation, mainly limited to some indolent subtypes, of B-cell lymphoma regressions after HCV eradication with IFN and ribavirin. However, the molecular mechanisms of the HCV-induced lymphomagenesis are mainly hypothetical. According to the model considered to be most plausible, lymphoma growth is a consequence of the continuous antigenic stimulation of the B-cell immunologic response induced by the chronic viral infection. This review summarizes the current epidemiological and biological evidence of a role of HCV in lymphomagenesis, describing the putative mechanisms for a causative relationship. The clinical characteristics and management difficulties of the HCV-associated lymphomas are also discussed. HCV treatment with IFN cannot be given safely in concomitance with cytotoxic lymphoma treatment because of hematological and liver toxicity. However, novel and better tolerated antiviral regimens are under development and will hopefully make the treatment of both lymphoma and hepatitis easier in the future.
Learning Objectives
To understand the current lines of evidence linking HCV infection and lymphomagenesis
To review the specific problems of the clinical management of patients with chronic HCV infection
Introduction
The hepatitis C virus (HCV) is a major cause of acute hepatitis and chronic liver disease. Worldwide, ∼150 million people are estimated to be chronically infected and up to 500 000 people die each year from hepatitis C-related liver diseases.1,2 There are important geographical variations in the extent of HCV chronic infection, with the highest prevalence (>10%) in Egypt, Central Africa, Mongolia, and Bolivia.3 It has been estimated that ∼3-4 million people in the United States4 and 15 million people in Europe are chronically infected2 ; Asia has the largest population of infected persons, with the highest number in China (estimated at 13 million) and India (estimated at 9.5 million).5
HCV is not only hepatotropic, but also lymphotropic, because it equally infects both hepatocytes and lymphocytes. A large body of epidemiological, clinical, and biological data have suggested an association between HCV infection and the pathogenesis of at least a portion of B-cell non-Hodgkin lymphomas (NHLs). However, a causative relationship has not been fully confirmed and the etiopathogenetic mechanisms remain largely speculative.
Mixed cryoglobulinemia type II and HCV
The initial finding that generated a large number of epidemiological studies on the association between HCV and lymphoproliferative disorders was the extremely high prevalence of HCV infection in patients with type II mixed cryoglobulinemia (MC).6 Type II MC is a disorder characterized by circulating cryoglobulins consisting of complexes of polyclonal IgG and monoclonal IgM rheumatoid factors that become insoluble at reduced temperature. The main clinical features are palpable purpura, arthralgia, weakness, organ involvement (liver, kidney), peripheral neuropathy, and vasculitis. The histological lesions are secondary to vasculitis, which is caused by immune complexes deposited in small vessels. The antigenic component of these immune complexes has been shown to be highly enriched in HCV-RNA.6 Nearly all MC patients are HCV infected, even in the absence of chronic liver disease. Conversely, the MC prevalence in patients with HCV infection shows a great geographic heterogeneity. Low levels of circulating cryoglobulins are frequently detected in HCV-positive individuals, but only a minority has an overt cryoglobulin vasculitis.7,8 The biological basis of the relation between MC and HCV is not entirely elucidated. Analysis of the immunoglobulin heavy- and light-chain genes highlights the predisposition of HCV to select a restricted B-cell repertoire in response to chronic antigenic stimulation, favoring the preferential use of the IGHV1-69, IGKV3-A27, and IGKV3-20.9,10 Monoclonal B lymphocytes have indeed been detected in the liver, bone marrow, and peripheral blood of patients with MC8 and, in a multicenter Italian study, the risk of developing a B-cell lymphoma in HCV-infected patients with symptomatic cryoglobulinemia was 35 times higher than in the general population.11
Epidemiological evidence of an association between HCV and NHL
Numerous studies have found a high prevalence of HCV seropositivity in patients with B-cell lymphoproliferative disorders, particularly B-cell NHL, including cases in which MC was not present. The evidence for an association with T-cell lymphoma, Hodgkin lymphoma, and plasma cell disorders is less convincing.12-15 The association with B-cell NHL is particularly evident in countries with a high prevalence of HCV infection. There are important geographic variations of the prevalence of HCV-associated lymphomas, ranging from 0% to 50%. This variability may simply reflect the HCV epidemiology, but other confounding factors may affect the comparability of the different studies, including the timing and rate of HCV assessment in lymphoma patients, the method of HCV assessment, the selection of normal controls, the lymphoma classification used, and the year of publication.12-14 Two systematic reviews of >60 studies indicate that, overall, 13%–18% of B-cell lymphomas are associated with HCV infection (Table 1), but there is an important heterogeneity among studies and in study design.14,16
Table 1 also summarizes the findings of meta-analyses, which take into account the case-control studies and cohort studies published up to 2008.12-14,16,17 Globally, the relative risk of being infected is approximately 2-4 times higher among B-cell lymphoma patients than in the general population. However, because the virus is sporadic in some areas and endemic in others, a clear correlation between HCV and lymphoma has emerged mainly in populations with a high prevalence of the virus. Lower or no evident correlation has been demonstrated in low prevalence countries such as Scandinavia, the United Kingdom, and or Canada. In the United States, 2 large studies involving the National Cancer Institute–Surveillance, Epidemiology, and End Results (NCI-SEER) registry18 and the US Veterans Affairs health system19 have documented a modest, but significant, increase of the risk of B-cell lymphoma in patients with chronic HCV infection. Similarly, the large European multicenter case-control study EPILYMPH reported an estimated 2-fold lymphoma risk.20 Nevertheless, it should be noted that some studies have reported a low prevalence of lymphoma in infected people living in areas with a high prevalence of HCV (Thailand, Macedonia) and others have reported a relatively high prevalence of HCV-associated lymphomas in countries with a relatively low prevalence of the virus (Japan), indicating that additional genetic or environmental factors may be needed for the development of an overt lymphoma. In most meta-analyses, a high geographic heterogeneity is evident; nevertheless, in the countries where HCV prevalence is high, up to 10% of NHL may be attributable to the infection. Compared with the strong correlation between HCV and hepatocarcinoma, there is only a moderate risk for lymphoma development in HCV-positive patients and the presence of a causal link between HCV and lymphomagenesis remains controversial.21 However, interestingly, elimination of the virus may reduce the incidence of NHL in patients with chronic HCV infection.22
HCV is currently classified into at least 6 major genotypes that are variably distributed in different countries (Table 2). The influence of viral genotypes on the pathogenesis and course of hepatitis is controversial, but genotype determination is important for the prediction of response to antiviral treatment. No clear association has emerged in the published literature between genotypes and the risk of lymphoma, and the genotype distribution in HCV-associated lymphoma apparently mirrors that of the countries where the epidemiologic studies have been conducted.15
Principal characteristics of HCV genotypes*

HCV Genotype 7, extremely rare, is a provisional entity discovered in patients who were presumably infected in Central Africa.
†HCV is a bloodborne virus and infection is commonly due to unsafe injection practices: inadequate sterilization of medical equipment and blood transfusion. Transfusion of unscreened blood and blood products was the main route before the 1990s; today, in Western countries, HCV is rarely transmitted by transfusion due to thorough screening of the blood and effective viral inactivation procedures.
RBV indicates ribavirin; HCC, hepatocellular carcinoma; and IVDU, intravenous drug use.
HCV prevalence by lymphoma subtype
It is still debated whether some specific lymphoma subtypes are more closely associated with HCV. The B-cell NHL subtypes most frequently described as being associated with HCV are marginal zone lymphomas (MZLs), in particular splenic MZLs (SMZLs), extranodal (mainly nongastric) MZL of mucosa-associated lymphoid tissue (MALT), lymphoplasmacytic lymphoma (LPL), and diffuse large B-cell lymphoma (DLBCL).10,15,23 In a meta-analysis of 15 case-control studies and 3 prospective cohorts published between 1997 and 2005, no clear difference emerged to suggest that NHL develops more frequently in specific histological subtypes.13 However, the EPILYMPH study identified DLBCL, MZL, and LPL as being the most frequently associated with HCV infection.20 Subsequently, the very large International Lymphoma Epidemiology Consortium (InterLymph) case-control study provided a subtype-specific analysis confirming that HCV infection was increasingly associated with DLBCL [odds ratio (OR) = 2.24], MZL (OR = 2.47), and LPL (OR = 2.57).17 DLBCL is also the most common subtype among HCV-negative NHL, whereas MZL and LPL are relatively uncommon. Table 3 reports the lymphoma subtype distribution of 38 HCV-associated lymphoma treated at the Oncology Institute of Southern Switzerland between 2000 and 2013, which shows a more frequent involvement of extranodal marginal zone and DLBCL. This seems mostly in keeping with the aforementioned reports; it should be noted, however, that HCV antibodies were investigated in less than half of the patients seen in the same period at our institution.
Frequency of anti-HCV antibodies detection in different histological subtypes in a cohort of 384 B-cell lymphoma patients with HCV serology tested at diagnosis and treated at the Oncology Institute of Southern Switzerland between 2000 and 2013

CI indicates confidence interval.
*One-sided 97.5% CI.
†This estimate of HCV prevalence among B-cell lymphoma may be biased. Indeed, ∼550 additional lymphoma patients seen in the same period were not tested for HCV at diagnosis. Most likely, patients with an expected increased risk for the infection (eg, those from endemic countries, those with a history of blood transfusions or of intravenous use of drugs, and those with elevate transaminases or a history of liver disease) were tested more frequently than the others, leading to a potential overestimation of the HCV prevalence.
Clinical characteristics of HCV-associated lymphomas
In general, the broad clinicopathological heterogeneity of NHLs is maintained in the subgroup of HCV-associated lymphoma. However, at least in some studies, in addition to the predominance of diffuse large cell and marginal-zone histology, HCV-associated lymphomas appear to carry distinctive clinicopathological features that may partly depend on the presence of HCV infection. At onset, they often present splenic localization or extranodal involvement at target organs of HCV infection such as the liver and the salivary glands.24 Compared with HCV-negative patients, they also show a more frequent presence of increased transaminase levels, monoclonal gammopathies, autoimmune phenomena, rheumatoid factor, and asymptomatic cryoglobulinemia.15
DLBCLs are the most common aggressive lymphomas arising in HCV-infected patients. A few studies25-28 have specifically described the main clinicopathological characteristics of HCV-associated DLBCL (Table 4) that very frequently present at an advanced stage mainly due to extranodal localizations, elevated lactate dehydrogenase (which may, at least in part, result from the concomitant hepatitis), and unfavorable International Prognostic Index score (Table 4). Whether these patients have a significantly worse outcome than HCV-negative lymphoma patients remains unclear. In a large survey of patients included in the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials, HCV-positive DLBCL patients had more frequent spleen involvement and were more frequently transformed from low-grade lymphoma than HCV-negative DLBCL patients, but histological transformation was not associated with a worse outcome.25 The Fondazione Italiana Linfomi (FIL, Italian Lymphoma Foundation) recently developed a specific prognostic score for this subset of patients based on a multicenter retrospective series of 535 patients with HCV-associated DLBCL treated with anthracycline-based regimens (with rituximab in 255 cases). Severe hepatotoxicity was observed in 14% of patients and it was not increased in patients receiving rituximab. The 3-year overall survival and progression-free survival rates were 71% and 55%, respectively. At multivariate analysis, Eastern Cooperative Oncology Group performance status of 2 or over, serum albumin below 3.5 g/dL, and HCV-RNA viral load over 1000 KIU/mL displayed an adverse prognostic significance. The combination of these 3 factors in a new “HCV-Prognostic Score” discriminated 3 risk groups with significantly different outcomes (low = 0; intermediate = 1; high-risk = ≥2 factors). The estimated overall survival at 3 years was 92% for the low-risk group, 77% for the intermediate-risk group, and 39% for the high-risk group. The 3-year progression-free survival rate was 81%, 61%, and 19% in the low-, intermediate- and high-risk groups, respectively. This score retained prognostic value in the subgroups of patients treated with and without rituximab and performed better than the International Prognostic Index.28
Comparison of clinical features at presentation between different studies of DLBCL associated with HCV25-28

NR indicates not reported; OS, overall survival; RFS, relapse-free survival; EFS, event-free survival; and PFS, progression-free survival.
*The working formulation was used to define histology by Tomita et al,27 reporting 20 large cell lymphomas and other aggressive histologies; this study included also 4 cases of T-cell origin.
Among indolent lymphomas, 2 rare, peculiar clinical presentations were recently described in HCV-infected patients: primary SMZL with MC type II and subcutaneous “lipoma-like” extranodal marginal zone B-cell lymphoma of MALT type. In the former, ∼70% of patients are women and most have symptomatic cryoglobulinemia (vasculitis, arthralgia, peripheral neuropathy) and circulating villous lymphocytes. HCV eradication with IFN and ribavirin has been reported to induce a complete lymphoma remission in most cases and should be the first choice of treatment.29 In subcutaneous “lipoma-like” extranodal marginal zone B-cell lymphoma of MALT type, the large majority of cases were described in elderly women. The disease is characteristically confined to the subcutaneous tissue; the skin and the cutaneous adnexa are not involved. Typical lesions are single or multiple soft and mobile subcutaneous nodules underlying a normal-appearing skin. The clinical course is usually indolent. Analogous to what has been described in splenic lymphomas, objective lymphoma regressions have been reported after HCV eradication.30
Oncogenic role of HCV in lymphoma development
HCV is an enveloped, positive-stranded RNA virus belonging to the Flaviviridae family that infects and replicates directly inside hepatocytes,31 but does not integrate into the host genome and does not contain an obvious oncogene.9 Being a positive-stranded RNA virus, HCV has to convert its positive strand into a negative intermediate RNA strand before replication. Detection of a positive HCV-RNA strand indicates the presence of the virus, but only the detection of a negative-stranded intermediate provides evidence of a true viral replication in a target cells. Viral replication has been demonstrated clearly in hepatocytes, supporting a direct role for HCV in the development of hepatocellular carcinoma.31 The HCV-RNA negative strand and the NS3 viral nonstructural protein, which is a main component of the HCV replication cycle and indicates viral mRNA translation and polyprotein production, have been detected in PBMCs.32,33 However, which is the principal HCV-target population of the immune system is still debated, as is the capacity of the virus to replicate in vivo in the infected lymphocytes.34
Replication in the lymphocytes is not sufficient to fully demonstrate a pathogenetic role in NHL development. The strongest evidence for a causal relationship between HCV and lymphoma comes from the observation of lymphoma regression after antiviral treatment. This was first shown in 9 patients with splenic lymphoma who had a lymphoma remission after IFNα and ribavirin antiviral treatment.35 A direct antitumor effect of IFNα therapy was ruled out (in fact, 6 additional patients with splenic lymphoma, but without HCV infection, had no hematologic response to the antiviral treatment).35 These results were reproduced by other studies showing that viral eradication can be an effective treatment for HCV-associated indolent lymphomas (Table 5). The hematological response rate across different studies is ∼75%.36,37 A large Japanese study comparing 501 consecutive patients with chronic HCV who had never received IFNα therapy with 2708 consecutive HCV-infected patients who received IFNα therapy showed a significantly higher incidence of lymphoma in patients with persistent infection than in patients with sustained virologic response (SVR), demonstrating that HCV eradication protects against the development of malignant lymphoma.22

RBV indicates ribavirin; CR, complete response; ORR, overall response rate (complete + partial); SLVL, splenic marginal zone lymphoma with circulating villous lymphocytes; SMZL, splenic marginal zone lymphoma; EMZL, extranodal marginal zone lymphoma of the mucosa-associated lymphoid tissue; and NMZL, nodal marginal zone lymphoma.
*Five additional patients were treated with PegIFNα alone.
The clinical responses observed after IFNα treatment in splenic HCV-positive lymphomas are similar to those observed in gastric MALT lymphoma after antibiotic treatment38 and clearly indicate a pathogenic role for HCV and lymphomagenesis, but do not explain the mechanism(s) by which HCV may promote lymphoma growth. A causal link may be based on different theoretical mechanisms (summarized by Marcucci and Mele9 ): a viral immunosuppressive effect on the tumor cells, but a significant immunodeficiency is not usually detectable; the coinfection by another unknown oncogenic virus, but no evidence has emerged supporting this hypothesis; a direct oncogenic role of HCV; and an indirect, antigen-driven stimulation of the lymphoma growth. These different putative oncogenic mechanisms of HCV-induced lymphoma-genesis do not necessarily have to be mutually exclusive.
Chronic stimulation of lymphocyte receptors by viral antigens
Apparently, the lymphoma subtypes that are most frequently HCV associated originate from germinal center or postgerminal center lymphocytes, suggesting a possible antigen-driven proliferation. Indeed, a monoclonal/oligoclonal B-cell expansion has been shown in circulating B cells, as well as in the bone marrow or intrahepatic B cells of HCV-infected patients.31
The HCV-E2 envelope protein binds the CD81 expressed on B cells. At the same time, HCV may bind a specific BCR on the B-cell surface. It has been hypothesized that dual binding of both the CD19/CD21/CD81 complex and the BCR can result in a decreased threshold for B-cell activation and proliferation.31 This mechanism, however, has been challenged by Ng et al, who did not confirm the ability of lymphoma BCRs from patients with B-NHL and chronic HCV infection to bind HCV antigens.39 Nevertheless, chronic antigenic stimulation may play a significant role in the development of an initially polyclonal B-cell expansion and predispose to genetic aberrations.
The monoclonal rheumatoid factor-like IgM component of the type II MC and monoclonal antibodies derived from HCV-infected patients were found to have restricted combinations of the IGHV gene repertoire.9,10 HCV-NHLs have been found to express analog regions of the IgV genes and to undergo somatic hypermutation, indicating affinity maturation driven by a selective antigen pressure. Analogous to gastric MALT lymphoma associated with Helicobacter pylori chronic infection, it can be hypothesized that, in the context of a persistent antigenic stimulation, successive genetic abnormalities can progressively affect a B-cell clone among the reactive B cells of the chronic inflammatory tissue and give rise to the HCV-associated B-cell lymphoma. In this model, type II MC can be considered a premalignant condition associated with chronic antigenic stimulation of B-cell clones, which, after “a second hit” represented by additional genomic alterations, can progress to overt lymphoma. It should be noted that HCV eradication is an effective treatment for HCV-associated MC. However, cryoglobulinemia may sometimes persist after antiviral treatment as an expression of acquired independence from the antigenic stimulus.
Signaling pathways potentially involved in HCV-induced lymphomagenesis
As summarized in a recent review,21 it remains unclear which signaling pathways mediate HCV-induced lymphomagenesis. The proinflammatory IL-6 has been suggested to contribute to the development of cryoglobulinemia and B-cell NHL in HCV-infected patients via stimulation of TLR2.21 In transgenic mice, the expression of B-lymphocyte stimulator/B-activating factor (BLyS/BAFF, a TNF-family cytokine) has appeared associated with the clinical progression of HCV infection and may activate NF-κB, JNK, and ERK pathways, stimulating B-cell proliferation.21 Gene expression profiling of lymphoma B cells from HCV-transgenic mice has revealed a modified expression of various genes, suggesting that the activation of both canonical and alternative NF-κB pathways and down-regulation of miR26b may contribute to lymphomagenesis, and a reduced expression of miR-26b has been found in HCV-positive SMZL patients.21
Cytokines, chemokine, and chromosomal aberration and instability in HCV-associated B-lymphoproliferations
Several studies have addressed the role of chromosomal aberration in HCV-induced lymphomagenesis (reviewed in Zignego et al).40 The presence of t(14;18) and the rearrangement of bcl-2 were reported to be present with increased frequency in patients with chronic HCV-related MC and to disappear after successful antiviral therapy, suggesting that the antiapoptotic function of Bcl-2 can be relevant in HCV-infected B cells. Different studies have identified genetic instability, increased aneuploidy, and reduced Rb protein expression in HCV-infected cells, potentially favoring a neoplastic transformation. In addition to the BLyS/BAFF contribution to an increased B-cell survival, other studies have suggested a role of cytokines and chemokines, particularly in MC, in which Th1 cytokines (IFNγ and TNFα) and some chemokines (MIP-1α, MIP-1β, CXCL10 and CXCR-3, CXCL13) levels have been found elevated in the vasculitic lesions, suggesting a potential pathogenic role.
Direct oncogenic effect on B-lymphocytes
HCV replication in B-lymphocytes is controversial and may be restricted to a subset of the B-cell population, those that are CD5-positive, which also expresses high CD81 levels. Additional events (eg, EBV coinfection) may be needed for the B cell to become permissive for viral replication.21 There is limited evidence of a direct role of intracellular viral proteins. HCV may be able to induce cellular transformation without intracellular viral replication with different putative, still poorly known oncogenes and tumor suppressor genes.34
Multifactorial models
Marcucci and Mele have recently reviewed the potential lymphomagenic mechanisms of HCV and proposed a multifactorial model for HCV lymphomagenesis.9 According to this model, all the previously described oncogenic events may individually have a relatively low lymphomagenic potential, and the accumulation of at least 2 oncogenic signals is required for lymphoma occurrence. These required signals may derive from HCV itself or may result from the integration of an HCV signal with HCV-independent events generated by either genetic damage or coinfection with other viruses such as EBV.9
Management of B-cell NHL in HCV-positive individuals
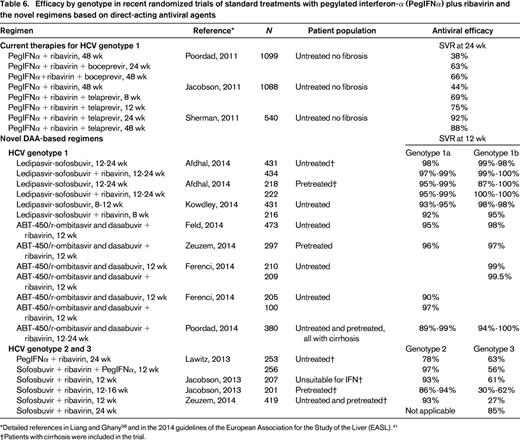
The purpose of antiviral therapy in HCV-related chronic hepatitis is to prevent hepatic and extrahepatic complications. The recent approval of new direct-acting antiviral agents (boceprevir, telaprevir) that specifically inhibit viral replication has provided a tool to improve the virologic response rate in the resistant genotypes as well.41,42 The current first-line treatment of chronic HCV infection is the combination of pegylated IFNα (PegIFNα) and ribavirin for genotypes 2–6. The addiction of boceprevir or telaprevir is indicated for the treatment of chronic hepatitis C genotype 1. Several other novel compounds, orally available and clinically well tolerated, are being tested in clinical trials in various combinations with or without ribavirin or PegIFNα (Tables 6, 7); some of these will be registered soon.41,42 The indication to treat, as well as the timing and type of treatment, have thus far been based on viral genotype, patient conditions and comorbidities, and degree of liver fibrosis. The novel anti-HCV agents may, however, dramatically change the treatment scenario in the very next future.42
Efficacy by genotype in recent randomized trials of standard treatments with pegylated interferon-α (PegIFNα) plus ribavirin and the novel regimens based on direct-acting antiviral agents
Anti-HCV oral compounds in clinical development at the beginning of 201459

In principle, HCV eradication is indicated in patients with lymphoma because it may induce, in certain subtypes, lymphoma regression and may remove the antigenic stimulation that may promote a relapse. However, there are specific management issues associated with presenting clinical features and histological type.
Indolent NHLs
As already mentioned, the use of antiviral therapy to treat HCV-associated lymphomas was established after the demonstration by Hermine et al of SMZL regression after HCV eradication.35 Later, other reports demonstrated that antiviral therapy can be considered as front-line therapy for other HCV-associated indolent B-NHLs.30,43-45 This treatment approach has been more frequently explored in MZLs, in which the achievement of a virologic response can be followed by a lymphoma response in up to 75% of the cases. With respect to the other indolent histologies, hematologic responses to antiviral therapy have been shown in follicular lymphoma and lymphoplasmacytic lymphoma. Although most studies included very small numbers of patients (Table 5), a very recent large retrospective study of 134 patients has confirmed that antiviral therapy is effective in either MZL or non-MZL indolent entities and that HCV-RNA clearance is needed to attain lymphoma response.45 At present, in HCV-positive patients with MZL who do not need immediate conventional treatment for lymphoma, antiviral treatment with PegIFNα and ribavirin should be considered as first-line treatment.45,46 A similar approach may also be considered for the asymptomatic HCV-infected patients with lymphoplasmacytic and follicular lymphomas, but the evidence from published studies is less strong and further studies are needed to proper evaluate the role of front-line antiviral treatment in non-MZL indolent subtypes.
Aggressive HCV-associated NHL
The management of HCV-associated DLBCL is still based on immunochemotherapy with anthracycline-containing regimens in combination with rituximab and there are no standard recommendations.47,48 In these patients, unlike patients with hepatitis B, the immunosuppression induced by rituximab does not usually cause HCV reactivation. Indeed, hepatic flare after rituximab seems to be rare49,50 and associated with a good outcome and low mortality.28,48,51 Nevertheless, it may lead to discontinuation of potentially life-saving immunochemotherapy.51 Although prophylactic antihepatitis B therapy during chemotherapy is the standard of care, concurrent administration of immunochemotherapy with IFN-based HCV treatments is usually not feasible because of the increased hematological and liver toxicity.52 In most cases, the treatment of HCV can be safely given only after the completion of the immunochemotherapy program and may result in prolonged disease-free survival.53 However, the utility of antiviral treatment in DLBCL in remission after cytotoxic chemotherapy has never been studied properly.
HCV in the hematopoietic stem cell transplantation setting
Liver disease is a well-known complication of hematopoietic stem cell transplantation (HSCT). HCV infection is not an absolute contraindication for HSCT, but is a cause of morbidity and late mortality. A prospective study of the European Group of Blood and Marrow Transplantation (EBMT) followed over time a cohort of 195 HCV-infected patients (134 had allogeneic HSCT and 61 autologous HSCT). Survival probability at 20 years from HSCT was 82%, with a cumulative incidence of severe liver complications (death from liver failure, cirrhosis, and liver transplantation) of 12%. Antiviral therapy with IFN was given to 85 patients (42 in combination with ribavirin), with a SVR rate of 40%. Toxicity was comparable to other patient populations; no patient developed significant exacerbations of GVHD. Patients receiving antiviral therapy had a trend toward a decreased risk of severe liver complications.54
Conclusion
The current challenge is to identify the right combination of antiviral drugs with the highest potency and the best toxicity profile. The role of IFN-based treatments may change rapidly and IFN-free regimens may soon become a suitable option for patients with contraindications to this drug.42 This may be particularly advantageous for the treatment of chronic hepatitis C in lymphoma patients. However, whether the safety profile of novel regimens will allow the administration of antiviral treatment concomitantly with chemotherapy is still unknown. In the light of the changing therapeutic paradigm, and with very active oral regimens (eg, the combination of ledipasvir and sofosbuvir) that will become available in many countries, we strongly recommend that treatment modalities are chosen after consultation with an expert gastroenterologist. Ideally, HCV eradication is indicated in all HCV-infected lymphoma patients and the shortest and least toxic regimen should be used to achieve the highest possible SVR rate. Regrettably, the cost of novel HCV treatments will be extremely high and most HCV-infected patients live in areas where these drugs will not be available for many years.
Acknowledgments
The authors thank Luca Arcaini, Florian Bihl, and Davide Rossi for helpful comments and Sarah Jane Ortelli Giannakis for excellent editorial assistance.
Disclosures
Conflict of interest disclosures: E.Z. is on the board of directors or an advisory committee for Roche, Celgene, and Janssen; has received research funding from Roche and Mundipharma; has consulted for Roche, Mundipharma, Celgene, and Janssen; and has received advisory honoraria from Celgene, Janssen, Gilead, Mundipharma, Roche and Takeda. B.V. declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Emanuele Zucca, MD, Oncology Institute of Southern Switzerland (IOSI), Ospedale San Giovanni, CH-6500 Bellinzona, Switzerland. Phone: +41-91-811-90-40; Fax: +41-91-811-91-82; e-mail: ielsg@ticino.com.

