Abstract
Regardless of age, patients with relapsed or refractory acute lymphoblastic leukemia (ALL) have extremely poor outcomes. The success of reinduction chemotherapy remains dismal, because complete remission rates are low and seldom durable. Clearly, new and novel strategies are needed to improve the outcome of patients with relapsed or refractory ALL. Patients with early relapse, especially those still receiving chemotherapy, tend to have a much poorer outcome and are often chemotherapy resistant. Although high-dose approaches may overcome chemotherapy resistance, long-term disease-free and overall survival remains limited. Several approaches have been used to target antigens, including cluster of differentiation (CD) 19, CD20, CD22, and CD52, on the surface of the malignant lymphoblast with striking efficacy. This review will focus on the clinical application of the major classes of antibodies, including naked antibodies, drug–antibody conjugates, immunotoxins, and T cell–engaging bispecific antibodies. Hopefully, these novel monoclonal antibodies will result in a significant improvement in the outcome of patients with relapsed or refractory ALL.
There has been tremendous progress in the treatment of patients with acute lymphoblastic leukemia (ALL). Currently, pediatric patients with ALL have a complete remission (CR) rate of 95% with estimated 5-year event-free survival (EFS) rates of 80%-85%.1 The results of adult patients with ALL have not kept pace with their pediatric counterparts. In comparison, although the current adult regimens have CR rates of ∼85%, the 3-year disease-free survival (DFS) and overall survival (OS) rates remain <45%.2,3 Pediatric inspired regimens are currently being explored for young adult patients, which has led to improvements in the EFS and OS rates compared with historical controls.4-6
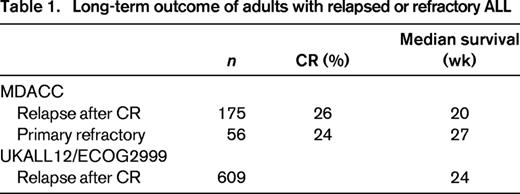
Despite the high CR rates in both pediatric and adult patients with ALL, relapse is an insurmountable problem for many individuals. The higher rate of relapse in adult patients with ALL is likely attributable to higher risk disease. For example, more adult patients have adverse cytogenetics, such as the Philadelphia chromosome or translocations involving the mixed lineage leukemia gene on 11q23, persistent minimal resistant disease (MRD), and poor tolerance of the intense and prolonged chemotherapy protocols. Salvage chemotherapy regimens have shown only modest activity in patients with relapsed or refractory ALL with median remission duration of only 2-7 months (Table 1).7,8 The outcome is particularly poor in patients who relapse while on therapy or with CR duration (CRD) of <24 months. In this group of patients, long-term survival is <5%.
The success of long-term outcomes for patients with relapsed or refractory ALL involves the ability to obtain a CR. However, for the majority of adults, despite the ability to achieve a CR, these are seldom durable in patients with refractory or relapsed disease.7,8 Therefore, the ability to achieve a CR and to have the opportunity to obtain a hematopoietic stem cell transplant (HSCT) remains a reasonable goal for most patients. The DFS and thus OS with second-line therapies are typically short unless HSCT can be performed during the remission period. A variety of treatment regimens have been developed for patients with relapsed or refractory ALL with remission rates slightly <30%.9 Three recent cytotoxic agents have been approved for patients with relapsed or refractory ALL, clofarabine, nelarabine, and liposomal–vincristine, demonstrating respective CR rates in adult patients of 17%, 31%, and 20%.10-12 Although salvage therapy for adult and pediatric ALL patients continues to yield poor results, it remains an area of active research with the hope that the discovery of novel agents will have the ability to improve the outcome of the poor-risk group of patients.
Monoclonal antibodies in ALL
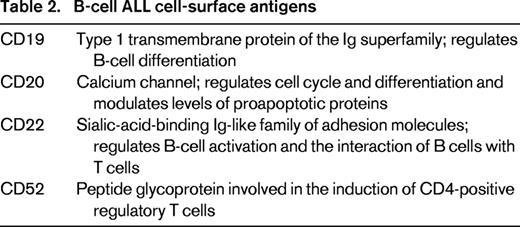
Lymphoblasts express several cell-surface antigens that serve as excellent targets for monoclonal antibodies (Tables 2 and 3). More than 95% of B-cell ALL express cluster of differentiation (CD) 19 and >90% of lymphoblasts express CD22. Thus, targeting these antigens has been the primary focus of monoclonal antibody therapy. In addition, the antigens CD20 and CD52 are expressed in ∼20% and 70% of B-cell ALL, respectively. In addition, lymphoblasts typically express surface antigens at a high antigen density on their cell surface. Although the antigens expressed on the surface of lymphoblasts and normal cells are shared, this has not proven to be a significant impediment in their use in the treatment of patients with ALL.
There are four different types of monoclonal antibodies that are currently being developed in ALL. Rituximab, epratuzumab, and alemtuzumab are naked antibodies that destroy the target cell through antibody-dependent cell-mediated cytotoxicity (ADCC). Inotuzomab and SGN19a are antibody–drug conjugates, and SAR3419 and Combotox are antibody immunotoxins. These monoclonal antibodies rely on the efficacy of the chemotherapeutic agent or immunotoxin for its disease activity, which is dependent on the pharmacokinetics of the antibodies. A fourth approach is the bispecific T cell–engaging single-chain (BiTE) antibodies (blinatumomab). BiTE therapy uses a “bridging” approach with variable regions of two antibodies, CD19 on the lymphoblast cell surface and CD3, a membrane protein on the surface of T cells and is a component of the T-cell receptor.13 The binding of the BiTE antibody to the T cell–specific surface protein leads to T-cell activation and killing of the lymphoblasts independent of the major histocompatibility antigen (HLA).
Targeting CD20
Rituximab
In B-cell ALL, the expression of CD20 is associated with a poor prognosis.14 Approximately 40% of pre-B ALL cases are CD20 positive using the tradition cutoff of at least 20% of CD20 expression on lymphoblasts. Importantly, the percentage and the intensity of CD20-positive cases is increased in patients with persistent MRD positivity.15 Rituximab, a chimeric anti-CD20 antibody, is one of the first monoclonal antibodies that was evaluated in combination with chemotherapy for patients with B-cell ALL. At the MD Anderson Cancer Center (MDACC), 97 patients with de novo CD20-positive Philadelphia chromosome–negative B-cell ALL were treated with 8 doses of rituximab at 375 mg/m2/dose added to the first 4 courses of the hyper-CVAD regimen (R-hyper-CVAD; rituximab, cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with cytarabine and methotrexate).16 The results demonstrated an improved 3-year CRD, a lower relapse rate, and an improved OS but only in younger patients under the age of 60 years compared with historical controls (70% versus 38%, p < 0.001; and 75% versus 47%, p = 0.003). However, older patients with CD20-positive ALL did not seem to benefit from the R-hyper-CVAD regimen.
Similarly, the German ALL study group evaluated the addition of rituximab to their backbone chemotherapy regimen.17 In their study, a total of 263 patients with CD20-positive B-cell ALL patients (age range, 15-55 years) received 8 doses of rituximab along with chemotherapy during induction and consolidation. Although there was no improvement in the CR rate, a higher percentage of patients who received rituximab achieved an MRD-negative status after induction and consolidation therapy (60% versus 19% and 90% versus 9%, respectively). This may be the reason that the German group was able to demonstrate an improvement in the 3-year CRD and OS rates (64% versus 58%, p = 0.009; and 75% versus 54%, no p value given). As a result of these studies, the French cooperative group GRAALL (Group for Research on Adult Acute Lymphoblastic Leukemia) is currently evaluating the addition of rituximab in a randomized phase 3 study.
Ofatumumab
Ofatumumab is a humanized, anti-CD20 antibody that binds to a different epitope of CD20 from that of rituximab.18 Ofatumumab binds CD20 with greater avidity compared with rituximab, which leads to higher ADCC activity. Early results of a phase 2 study of ofatumumab combined with hyper-CVAD in adult patients with newly diagnosed as CD20-positive ALL.19 Ofatumumab was administered at a dose of 2 g during the first 4 courses of the hyper-CVAD regimen. Twenty-five patients with de novo ALL were treated with a CR and MRD-negative rate, as measured by flow cytometry, which were both 96%. The toxicities observed were similar to those for hyper-CVAD. The 1-year DFS and OS are 94% and 92%, respectively. These very preliminary results suggest that ofatumumab may be a promising therapeutic approach for use in CD20-positive patients with ALL. Obinutuzumab is another novel glyco-engineered type II CD20 monoclonal antibody, but there is no clinical trial data available for its use in ALL.
Targeting CD52
Alemtuzumab
Alemtuzumab is a humanized monoclonal anti-CD52 antibody that induces ADCC. Alemtuzumab has limited single-agent activity in the treatment of ALL20 ; however, when combined with chemotherapy, it appears to have more clinical activity. A phase 1 trial led by the Cancer and Leukemia Group B (CALGB 10102) treated 24 newly diagnosed patients with ALL with alemtuzumab during their fourth treatment post-remission module.21 All patients were CD52 positive defined by >10% expression on the lymphoblast. On this trial, 80% of the cases were B-cell ALL, and the median age was 37 years. The median OS was 55 months, and the median DFS was 53 months. In the 11 cases with serial MRD measurements by polymerase chain reaction (PCR), 8 patients had at least a 1-log reduction in MRD. A 70-patient phase 2 trial has completed accrual, which will hopefully further confirm the above findings. Of course, there are important safety criteria to consider when adding alemtuzumab to chemotherapy, such as cytomegalovirus, herpes simplex, and herpes zoster infections.
Targeting CD22
Epratuzumab
Epratuzumab is a humanized immunoglobulin G1 (IgG1) antibody that targets CD22. Recently, the Children's Oncology Group (COG) combined epratuzumab with their relapsed chemotherapy backbone regimen in children with relapsed CD22-positive B-cell ALL.22 This small pilot study of 15 patients tested the addition of four doses of epratuzumab (360 mg/m2 intravenously twice a week) during induction, followed by 3 modules of chemotherapy, with an additional 3 doses of epratuzumab administered only during the first module. In this study, the CR rate was 60%, with 40% of patients achieving an MRD-negative status. A larger study of 112 patients, aged 2-30 years, with relapsed CD22-positive ALL added epratuzumab, either weekly or twice weekly, to the standard COG chemotherapy backbone.23 The CR rate was not only identical in both arms (65% versus 66%) but was also no better when compared with historical controls treated with the same chemotherapy backbone. Furthermore, although the MRD-negative rate was higher with the addition of epratuzumab, it did not reach statistical significance.
Epratuzumab was added to chemotherapy in adult patients with relapsed CD22-positive ALL in the Southwestern Oncology Group Trial S0910.24 In this small multicenter study of 32 patients with a median age of 41 years, epratuzumab was administered in 4 weekly doses combined with clofarabine and cytarabine. The overall response rate (ORR) was 52% [CRs plus CR without peripheral count recovery (CRi)], and this was significantly higher compared with a historical cohort using the same chemotherapy backbone (52% versus 17%). IntReALL (International Study for Treatment of Childhood Relapsed ALL) is an ongoing randomized phase 3 trial that will test the effect of epratuzumab in relapsed pediatric patients and will hopefully better elucidate the potential role of this antibody in the relapsed setting.
BL22 and HA22
The immunoconjugate BL22 is a monoclonal anti-CD22 antibody fused to a fragment of the Pseudomonas aeruginosa exotoxin A.25 A phase 1 study was conducted in 23 pediatric patients with relapsed ALL. The dose of BL22 ranged from 10 to 40 μg/kg every other day for 3 or 6 doses repeated every 3-4 weeks.26 Although 16 patients demonstrated a reduction in blast counts and 4 patients had clearance of peripheral blasts, no CRs were observed. Toxicities included hypoalbuminemia, transaminase elevation, fatigue, and peripheral edema.
HA22 is a second-generation immunoconjugate similar to BL22 with a 15-fold increase in binding affinity CD22-positive cell lines.27 HA22 was administered in a phase 1 study in children with relapsed refractory hematological malignancies at a dose between 5 and 40 μg/kg IV every other day for 12 days.28 Of the 17 evaluable patients, 30% achieved a CR (24%), with an ORR of 68%. The dose limiting toxicity (DLT) was a capillary leak syndrome seen in 2 of 7 patients in the 30 μg/kg dose level. Unfortunately, high-titer anti-HA22 neutralizing antibodies developed in 14% patients.
Inotuzumab ozogamicin
Inotuzumab ozogamicin (INO) is a humanized anti-CD22 antibody conjugated to calicheamicin, a potent chemotherapy agent that leads to double-strand DNA cleavage. INO has been studied in clinical trials in two different dosing schedules. A phase 1/2 study treated 49 patients with relapsed or refractory CD22-positive B-cell ALL with INO, administered at a dose of 1.8 mg/m2 intravenously every 3-4 weeks. An ORR of 57% was achieved, including 18% CR and 39% CRi. In addition, 17 patients (61%) achieved an MRD-negative status.29
Two studies, a single center at MDACC and a multicenter phase 1/2, have explored weekly dosing schedules with INO doses of 0.8 mg/m2 on day 1 and 0.5 mg/m2 on days 8 and 15, repeated every 3-4 weeks. In the MDACC trial, 41 patients were treated, with 28 (68%) in salvage 2 or beyond. The CR and CRi rates were 20% and 32%, respectively, with 78% of responding patients achieving an MRD-negative status.30 These results are similar to the multicenter trial that treated a total of 72 patients with relapsed or refractory CD22-positive B-cell ALL demonstrating an ORR (CR and CRi) of 68%, with 86% of patients an MRD-negative status.31,32
A recent phase 3 trial evaluated INO compared with standard chemotherapy in patients with relapsed ALL in first or second salvage. This trial accrued a total of 326 patients, with the first 218 patients showing an improved ORR of 80.7% versus 33.3% (p < 0.0001) in the INO versus the standard of care (SOC) arms and an MRD negative status of 78% versus 28% (p < 0.0001).33
Based on these studies, INO is being combined with chemotherapy in the frontline setting in older patients using a lower-intensity regimen, mini-hyper-CVD (rituximab, dexamethasone, cyclophosphamide, vincristine, and intrathecal chemotherapy). Of the 28 patients treated with this combination regimen, 27 patients (96%) achieved a CR/CRi (21 CR, 5 CRi). All patients achieving CR/CRi have also achieved flow-cytometric MRD-negative status. The 1-year PFS and OS were 88% and 81%, respectively.34
The most common side effects of INO include liver function test (LFT) abnormalities, thrombocytopenia, and veno-occlusive disease (VOD), especially in patients who proceed to HSCT. Although the majority of abnormal LFTs are reversible, VOD after HSCT has been one of the major concerns, especially given previous experiences with gemtuzumab ozogamicin, but in the case of INO, this may be partly attributable to the preparative regimens, specifically those containing double alkylating agents.
Targeting CD19
SGN19a
SGN-CD19A is a humanized anti-CD19 monoclonal antibody conjugated to the microtubule-disrupting agent monomethyl auristatin F. A phase 1 study of SGN-CD19A in adult and pediatric patients with relapsed or refractory B-cell ALL or highly aggressive B-cell lymphoma including Burkitts is ongoing.35 So far, 51 adult patients, with a median age of 43 years (range, 18-77 years), and 12 pediatric patients, with a median age of 9.5 years (range, 1-18 years), have been treated on Schedule A (days 1 and 8 of a 21-day cycle; dose range, 0.3-2.3 mg/kg) or Schedule B (day 1 of a 3-week cycle; dose range, 4-6 mg/kg). Objective responses have been observed in 11 of 43 adult patients with ALL/lymphoma, 6 of 29 (21%) on the weekly Schedule A and 5 of 14 on the 3-week Schedule B (36%), with 6 of 8 CR/CRp patients achieving an MRD-negative status. Impressively, 4 of 7 adult patients with Philadelphia-positive ALL showed an objective response, including 3 that were MRD-negative. SGN-CD19A was generally well tolerated with superficial microcystic keratopathy as the most common toxicity requiring prophylactic eye drops to reduce the severity of the corneal adverse events.
Combotox
The immunoconjugate known as Combotox is actually a 1:1 mixture of RFB4-deglycosylated ricin A (dgA) and HD37-dgA, which are immunotoxins that target both the CD22 and CD19 antigens. A phase 1 dose-escalating study of Combotox in pediatric patients with refractory or relapsed B-cell ALL treated 17 children, aged 1-16 years, at 4 dose levels (2, 4, 5, and 6 mg/m2).36 Three patients achieved a CR, and 6 additional patients showed a marked decrease in the peripheral blood blast count. The maximum tolerated dose was 5 mg/m2, and the DLT was CLS similar to that seen in the HA22 and other immunotoxins. Unfortunately, in adult ALL patients, the peripheral blast counts rebounded rapidly after the last dose of Combotox, suggesting that continued Combotox administration at lower doses may lead to more durable remissions.37 Combination studies with Combotox and cytarabine are ongoing in adult patients with relapsed B-cell ALL.
SAR3419
The monoclonal antibody SAR3419 is a humanized anti-CD19 antibody conjugated to maytansin, a potent tubulin inhibitor. After the binding of SAR3419 to the surface of CD19-positive lymphoblast, SAR3419 is internalized and processed to release the active maytansin metabolites that induce both cell-cycle arrest and apoptosis.38 In phase 1 studies conducted in patients with non-Hodgkin lymphoma, the DLT is reversible corneal toxicity.38,39 In studies using CD19-positive pre-B-cell ALL xenografts, the administration of SAR3419 delayed disease progression, even in chemotherapy-resistant xenograft models.40 SAR3419 is being evaluated in phase 2 clinical trials in adult patients with relapsed ALL.
Blinatumomab
Blinatumomab is a BiTE antibody with variable regions recognizing both CD3 and CD19. Adequate T-cell activation requires binding to both the CD3 and CD19 and works in a HLA-independent pathway. Single-agent blinatumomab was administered to patients with B-cell ALL in morphologic remission but detectable MRD.41 In this trial, 21 patients received blinatumomab by continuous intravenous infusion at a dose of 15 μg/m2/d for 4 weeks on a 6-week cycle. Of the 20 evaluable patients with MRD data, 80% (16 patients) achieved a negative MRD status, including 3 of 5 patients who were Philadelphia-chromosome positive. The DFS was 78%, with a median follow-up of 405 days.
A subsequent trial evaluated blinatumomab in relapsed or refractory ALL, using the same administration schedule but at a reduced dose during the first week (5 μg/m2/d) to reduce the frequency of infusion reactions.42 Of the 36 evaluable patients, 25 achieved CR/CRi (69%), and 22 patients (88%) achieved a MRD-negative status. The median OS and DFS were 9.8 and 7.1 months, respectively. This study led to a larger multicenter phase 2 trial of 189 patients using a fixed-dose format of 9 μg/kg during week 1 and 28 μg/kg during weeks 2-4 via a 4-week continuous infusion schedule with a 2-week break before proceeding to the next cycle.43 Subsequent cycles were administered at a fixed dose of 28 μg/kg for 4 weeks on a 6-week cycle. After 2 cycles, the ORR was 43%, including 33% CR and 10% CR with incomplete hematologic recovery (CRh). In addition, 60 of 73 (82%) patients achieved an MRD-negative status. Given the encouraging single-agent activity of blinatumomab in relapsed and refractory ALL, a phase 3 randomized trial [Eastern Cooperative Oncology Group (ECOG) 1910] is actively enrolling patients to evaluate the role of blinatumomab during consolidation in adults with newly diagnosed Philadelphia chromosome–negative B-cell ALL.
Blinatumomab is associated with several important side effects, including cytokine release syndrome, characterized by fever, hypotension, and respiratory symptoms, in up to 70% of patients.41-43 In addition, reversible CNS events, including encephalopathy, seizures, and cerebellar symptoms, occur in up to 20% of cases. The severity of these symptoms has led to the requirement that patients remain hospitalized during the first 10 days of the initial cycle and for the first 2 days during each subsequent cycle. In an effort to reduce the incidence and severity of the CRS and neurologic side effects, steroids are used as a premedication and blinatumomab is administered at a lower dose during the first week of cycle 1.
Conclusion
The use of monoclonal antibody therapy is now entering common practice in the treatment of adults with ALL. These novel monoclonal antibody therapies are generally well-tolerated and have demonstrated high single-agent activity in patients with relapsed and refractory disease and, more importantly, with high rates of MRD negativity (Table 4). However, longer follow-up is still needed to confirm these outcomes and the toxicity profiles. The Achilles' heel of monoclonal antibody therapy is the emergence of cell-surface antigen-negative clones, suggesting that combination chemotherapy approaches may improve outcomes especially in patients with newly diagnosed ALL. There are several clinical trials that are actively ongoing, including two large phase 3 studies comparing either blinatumomab or INO with SOC chemotherapy. These results are eagerly awaited and will expand our knowledge for the treatment of patients with relapsed ALL.
Summary of clinical evidence of antibody therapy in ALL and selected ongoing/pending clinical trials

Adapted with permission from Ai and Advani.44 CMR indicates complete modular remission; RFS, relapse-free survival; hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone.
Correspondence
Dr Daniel J. DeAngelo, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; Phone: 617-632-2645; Fax: 617-632-6771; e-mail: daniel_deangelo@dfci.harvard.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has consulted for Amgen, Novartis, BMS, Ariad, Incyte, and Pfizer.
Author notes
Off-label drug use: None disclosed.