Abstract
Hematopoietic cell transplantation (HCT) is the only curative therapeutic modality for myelofibrosis (MF) at present. The optimal timing of HCT is not known in the presence of wider availability of less risky nontransplant therapies such as JAK 1/2 inhibitors. Careful review of patient, disease, and transplant-related factors is required in the appropriate selection of HCT vs the best available nontransplant therapies. We highlight some of the relevant issues and positioning of HCT in light of evolving data on JAK 1/2 inhibitors. The goal of this study is to provide the reader with updated evidence of HCT for MF, recognizing that knowledge in this area is limited by the absence of comparative studies between HCT and nontransplant therapies. Prospective studies are needed for better information on: the determination of optimal timing and conditioning regimens, the best way to integrate JAK inhibitors in the HCT protocols, and the impact of JAK inhibitors on graft-versus-host disease.
Learning Objectives
Understand the candidacy for HCT
Recognize and appreciate the necessity of careful review of patient, disease, and transplant-related factors in the appropriate selection of HCT vs best available nontransplant therapies
Introduction
At present, hematopoietic cell transplantation (HCT) is the only curative therapy for primary (PMF) and secondary (post-essential thrombocythemia or post-polycythemia vera) myelofibrosis (collectively termed “MF”). However, HCT is associated with significant risk of treatment-related morbidity and mortality. The optimal timing of HCT for MF has been a matter of debate. The complexity of decision-making for transplantation has increased further following the wider availability of JAK 1/2 inhibitor therapy.
The scarcity of data on various aspects of HCT for MF has led to continued controversy on a number of issues, such as the role of age and disease risk in the selection of patients for HCT. Elsewhere, new controversies have sprung from emerging data and new therapies, such as the role of JAK inhibitors in the HCT setting and optimal donor type. In this study, we will examine some of these controversial issues and discuss the evidence for HCT in MF, highlighting instances where evidence is currently lacking.
Trends in HCT for MF
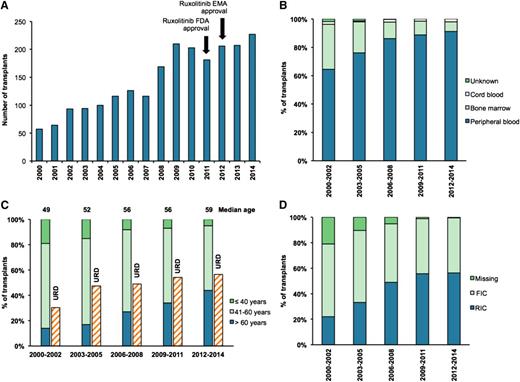
It was initially anticipated that the role of HCT may decline in this disease with the wider availability of JAK inhibitor therapy, similar to that observed in chronic myeloid leukemia following the adoption of tyrosine kinase inhibitor therapy. Registration data from the Center for International Blood and Marrow Transplant Research (CIBMTR) indicate a progressive increase in the number of transplants performed for MF every year over the last decade (Figure 1A). This time-period also saw an increase in the use of peripheral blood grafts for HCT, with a corresponding decrease in bone marrow stem cells (Figure 1B). The median age of transplantation increased by a decade between 2000 and 2014 (49 vs 59 years), with >40% of transplant recipients from 2012 to 2014 being >60 years of age. The number of transplants using unrelated donors (URDs) has also increased (Figure 1C). However, unlike acute leukemia, the use of alternative donors (ADs) such as cord blood or haplo-identical donors, does not appear to have increased in MF. RIC regimens have increased in popularity (Figure 1D).
Data from CIBMTR showing trends in HCT for primary MF between 2000 and 2014. (A) The number of transplants carried out in each year. (B) The percentage of transplants that used cord, bone marrow, or peripheral blood stem cells as the stem cell source. (C) The percentage of transplant recipients in different age groups (≤40 years, 41 to 60 years, and >60 years) and the percentage of transplants using a URD. The median age of MF HCT recipients for each time period is shown above each bar. (D) The percentage of transplants using RIC or FIC regimens. EMA, European Medicines Agency; FDA, Food and Drug Administration; FIC, full-intensity conditioning; RIC, reduced-intensity conditioning. Data used from CIBMTR with permission.
Data from CIBMTR showing trends in HCT for primary MF between 2000 and 2014. (A) The number of transplants carried out in each year. (B) The percentage of transplants that used cord, bone marrow, or peripheral blood stem cells as the stem cell source. (C) The percentage of transplant recipients in different age groups (≤40 years, 41 to 60 years, and >60 years) and the percentage of transplants using a URD. The median age of MF HCT recipients for each time period is shown above each bar. (D) The percentage of transplants using RIC or FIC regimens. EMA, European Medicines Agency; FDA, Food and Drug Administration; FIC, full-intensity conditioning; RIC, reduced-intensity conditioning. Data used from CIBMTR with permission.
Nontransplant therapies for MF
Ruxolitinib is the only approved JAK 1/2 inhibitor therapy for MF, and can result in significant improvement of splenomegaly, constitutional symptoms, performance status, and quality of life (QOL).1-3 Anemia and thrombocytopenia are two major toxicities. Long-term follow up data are available on COMFORT-I and COMFORT-II trials, and no additional safety concerns have arisen on the use of ruxolitinib in MF patients.4,5 Although there is no debate on the salutary effects of ruxolitinib in decreasing the disease symptom burden, the issue of improvement on survival is contentious. Moreover, ruxolitinib has limited activity against JAK2-mutant clones and there are no convincing data on the resolution of fibrosis. Long-term data show that ∼50% of patients will discontinue ruxolitinib by 3 years due either to side effects or loss of response.4
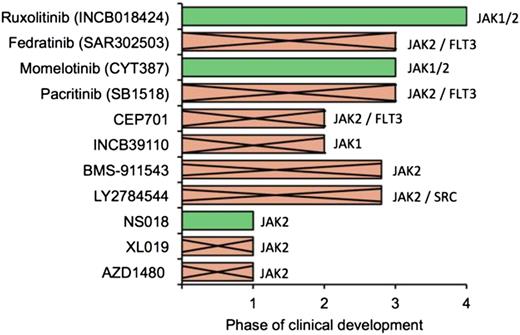
Some patient subgroups are difficult to treat with ruxolitinib, such as those with severe thrombocytopenia and heavily transfusion-dependent patients, whereas others such as those with ≥3 mutations may have an inferior response and shorter time to treatment failure (Table 1).1,2,6-19 Other investigational JAK 1/2 inhibitors or combination therapies may be an appropriate alternative in some of these patients. Among various JAK 1/2 inhibitors, only momelotinib is in advanced development, because drug development has been halted on several JAK 1/2 inhibitors due to unanticipated toxicities or lack of perceived benefit over ruxolitinib (Figure 2). Several other agents such as PRM151, imetelstat, and SL-401 are in early stages of clinical development for patient subgroups, who are either not suitable candidates for JAK inhibitors or are refractory to JAK inhibitors.
Factors influencing the choice between HCT vs nontransplant therapies
| Factors . | . |
|---|---|
| Characteristics Severe thrombocytopenia (<50 × 109/L) | Reason for poorer outcomes with nontransplant therapy No data on the use of ruxolitinib in this subgroup |
| Challenging to safely deliver adequate doses of ruxolitinib in severely thrombocytopenic patients | |
| Heavily transfusion-dependent anemia | Anemia is a major toxicity of JAK inhibitor therapy, and may worsen with treatment1,2 |
| ≥3 mutations | Shorter time to treatment failure with ruxolitinib6 |
| Increased risk of LT9,10 | |
| High-risk cytogenetics | Increased risk of LT7,8 |
| Impact of high-risk cytogenetics on ruxolitinib-treated patients not well studied | |
| Increasing blasts in peripheral blood | Increasing blasts is a risk factor for LT7 |
| Characteristics | Reason for poorer outcomes with HCT |
| Poor performance status | Increased NRM and decreased survival14 |
| Comorbidities | Severe comorbidities result in higher NRM15,18 |
| Advanced age | Very advanced age adversely impacts HCT outcomes11,19 |
| Response to JAK inhibitor therapy is not impacted by advanced age13 | |
| Mismatched donor | Mortality almost double compared with MSD/well-matched URD12,17 |
| Severe portal hypertension | Possible increase in regimen-related hepatotoxicity16 |
| Factors . | . |
|---|---|
| Characteristics Severe thrombocytopenia (<50 × 109/L) | Reason for poorer outcomes with nontransplant therapy No data on the use of ruxolitinib in this subgroup |
| Challenging to safely deliver adequate doses of ruxolitinib in severely thrombocytopenic patients | |
| Heavily transfusion-dependent anemia | Anemia is a major toxicity of JAK inhibitor therapy, and may worsen with treatment1,2 |
| ≥3 mutations | Shorter time to treatment failure with ruxolitinib6 |
| Increased risk of LT9,10 | |
| High-risk cytogenetics | Increased risk of LT7,8 |
| Impact of high-risk cytogenetics on ruxolitinib-treated patients not well studied | |
| Increasing blasts in peripheral blood | Increasing blasts is a risk factor for LT7 |
| Characteristics | Reason for poorer outcomes with HCT |
| Poor performance status | Increased NRM and decreased survival14 |
| Comorbidities | Severe comorbidities result in higher NRM15,18 |
| Advanced age | Very advanced age adversely impacts HCT outcomes11,19 |
| Response to JAK inhibitor therapy is not impacted by advanced age13 | |
| Mismatched donor | Mortality almost double compared with MSD/well-matched URD12,17 |
| Severe portal hypertension | Possible increase in regimen-related hepatotoxicity16 |
MSD, HLA-matched sibling donor; NRM, nonrelapse mortality.
Status of JAK 1/2 inhibitor clinical trials. The status of clinical development of various JAK 1/2 inhibitors. Drugs that are no longer in development are indicated by a cross.
Status of JAK 1/2 inhibitor clinical trials. The status of clinical development of various JAK 1/2 inhibitors. Drugs that are no longer in development are indicated by a cross.
Understanding the natural history of MF for HCT decision making
It is important to understand the natural history of the disease for transplant decision making, including anticipated survival and risk of leukemic transformation (LT). Current guidelines recommend that HCT be offered to patients predicted to have poor survival based on prognostic risk scores (International Prognostic Scoring System [IPSS], Dynamic IPSS [DIPSS], and DIPSS Plus). This encompasses patients with intermediate-2 and high-risk disease.20,21 These recommendations are based upon the poor survival in this patient group (median overall survival [OS] ≤5 years). In contrast, low- and intermediate-1–risk patients are not routinely offered HCT. A retrospective comparison between HCT and non-HCT therapies in PMF patients <65 years old indicated that intermediate-2/high-risk patients benefited more from HCT in comparison with non-HCT therapies, low-risk patients had inferior outcomes with HCT, and outcomes for intermediate-1 risk patients were equivalent.22
Is there a role for HCT in patients with intermediate-1–risk disease?
Severe thrombocytopenia, increased peripheral blood blasts, and high-risk cytogenetics are associated with a higher risk of LT.7 A single-center study by Caramazza et al23 found that an unfavorable karyotype (complex karyotype or sole or two abnormalities that include +8, −7/7q-, i(17q), inv(3), −5/5q-, 12p-, or 11q23 rearrangement) was predictive of a poorer prognosis. Consequently, unfavorable karyotype has been included in the DIPSS Plus risk stratification system.8
Recent years have seen a rapid expansion in mutations identified in MF patients, and to some extent, in our understanding of their prognostic value. Following the recent identification of the calreticulin (CALR) driver mutation,24,25 CALR has been found to be associated with a milder disease phenotype and superior OS relative to JAK2/MPL-positive patients.26 However, when CALR patients are divided into type I and type II, only CALR type I is associated with superior survival compared with JAK2V617F.27 Patients who do not harbor a detectable JAK2, MPL, or CALR driver mutation (the so called “triple-negative” patients), are an additional group identified as high-risk associated with decreased survival and increased LT.26,28
Several other subclonal mutations such as ASLX1/SRSF2/IDH1/2/EZH2 have prognostic value in MF.29-31 The number of detrimental mutations also has a prognostic role in PMF. Guglielmelli et al9 analyzed survival and leukemia-free survival based on the presence and number of “prognostically detrimental” (nondriver) mutations (ASXL1, EZH2, SRSF2, and IDH1/2). Presence of any of these mutations shortened survival (7 years for one mutation vs 12.3 years for no mutations), and the presence of two or more of these mutations predicted the worst survival (median, 2.6 years) and shortened leukemia-free survival. Similarly, in an analysis of 197 myeloproliferative neoplasm (MPN) patients,10 survival was poorer and risk of LT higher in patients with a greater number of mutations (excluding JAK2V617F or CALR). These studies highlight the current inconsistency in inclusion of driver mutations when reporting the effect of mutation numbers on outcomes for MPN/MF patients.
The first attempt to integrate mutation information into a weighted prognostic scoring system was presented by Vannucchi et al.32 Their model, the Molecular IPSS, refines stratification within IPSS categories using triple negativity, JAK2/MPL, ASXL1, and SRSF2 mutation status along with other clinical factors. Although further validation is required, these studies highlight the potential future increased use of mutational profiling to refine patient risk, particularly in those traditionally categorized as low- or intermediate-1 risk.
At present, the authors suggest that decisions regarding HCT in intermediate-1–risk patients are individualized after careful consideration of refractory cytopenias, increasing blasts in the peripheral blood, high-risk cytogenetics, and potentially mutational profile.
Q1. How should candidates be selected for HCT vs nontransplant therapies?
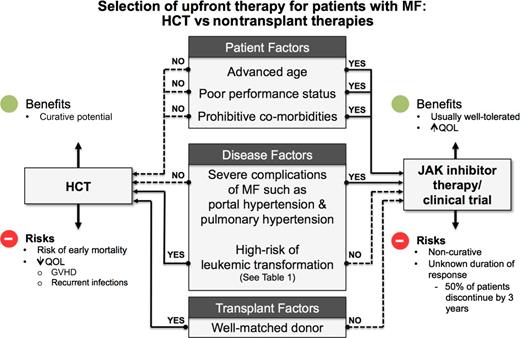
In contrast to acute leukemia patients, the decision regarding HCT in MF is a complex issue due to the chronic nature of MF. The decision whether to proceed with HCT is a delicate one involving multiple factors such as disease risk status, donor availability, performance status, comorbidities of the recipient, and available nontransplant therapies (Figure 320 ; Table 1). Lack of comparative studies on HCT vs nontransplant treatment options have led to several biases, such as provider’s knowledge and beliefs, acceptance of transplant as a treatment modality, and patient’s perceptions and understandings.
Choosing between nontransplant therapies vs HCT.20 GVHD, graft-versus-host disease.
Choosing between nontransplant therapies vs HCT.20 GVHD, graft-versus-host disease.
Age
The upper age of transplant for MF varies considerably depending on the health care setting and availability of resources. As indicated from the eligibility criteria for various transplant studies, transplants up to the age of 70 years are commonly performed (www.clinicaltrials.gov: #NCT01790295 and #NCT01814475). Some studies have no specific upper age limit (#NCT02251821). Variation in age limit is most stark for older patients who rely on Medicare. MF is not an indication for HCT under National Coverage Determination 110.8.1, and is therefore not covered under Medicare insurance, presenting a huge barrier to access to HCT for older patients.33
Although some retrospective studies have reported an association between older age at HCT and inferior outcomes,11 other studies using cohorts transplanted more recently and/or undergoing RIC show no association between age and poor HCT outcomes after controlling for other factors.12,34 However, a potential selection bias in favor of very fit older patients for HCT may contribute to the results of such studies. There is a correlation between increasing age and comorbidities,35 likely contributing to the adverse impact of age observed in older patients with MF undergoing HCT.
Performance status and comorbidities
Performance status is highly predictive of outcomes after transplant. Poor performance status, which may be disease-related, predicts higher NRM and poor survival.14 Some investigators have found that treatment with JAK inhibitor therapy may improve the performance status in some patients, and may make some patients eligible for transplant who were initially considered ineligible.36
A higher comorbidity score is associated with poorer HCT outcomes and reduced survival.15 Because comorbidity scores are generally higher in older patients,34 careful consideration is required when assessing older patients for HCT. Particular attention should be paid to MPN-associated comorbidities such as severe portal or pulmonary hypertension,20,16 because these patients may be less suitable for HCT.
The authors suggest careful attention to performance status and comorbidities in potential HCT candidates. Patients with poor performance status may benefit from a trial of JAK inhibitor therapy, and re-assessment for HCT candidacy after 3 to 6 months of therapy.
Impact of donor type
Donor type affects HCT outcomes for MF patients. Use of mismatched donors has been found to result in inferior HCT outcomes consistently in several studies; mismatched donors were found by the European Blood and Marrow Transplant (EBMT) Group to have higher NRM compared with well-matched donors (38% vs 12% at 1 year).17 A high mortality rate was also found in mismatched URD transplants by the CIBMTR.12 A prospective study from the Myeloproliferative Disorders Research Consortium also found inferior survival (32% vs 75% at 25 months) and NRM (59% vs 22% at 25 months) following transplants using URDs compared with MSDs.37 However, importantly several studies have found no significant difference in outcomes between MSDs and well-matched URDs.17,34,38
The availability of an MSD or well-matched URD may prompt earlier consideration for HCT in a patient. Conversely, HCT may be delayed for patients without an MSD or well-matched URD, and may be more appropriate for patients who have failed nontransplant therapies, are unlikely to do well with JAK 1/2 inhibitor therapy (Table 1), or are at higher risk of LT.
Q2. What is the optimal timing of HCT in the era of JAK inhibitor therapy?
The optimal timing of HCT in MF has yet to be determined. The aim is to proceed with transplant before LT, given the poor outcomes associated with transformed acute myeloid leukemia.39 A major dilemma is what to do in a patient who has been started on JAK inhibitor therapy and responds well to treatment. Early HCT may lead to significant treatment-related morbidity and loss of QOL in some patients who could have years of reasonable QOL with other treatment options. Conversely, delaying HCT may result in a worse outcome due to a multitude of factors, such as increasing age, worsening splenomegaly, transfusion-associated iron overload, and LT. A recent case series highlighted the issue of LT in patients showing symptom benefit on ruxolitinib.40 There are currently no prospective studies addressing this question, which is a major unmet need in this area.
In the largest retrospective study so far on the use of JAK 1/2 inhibitors in HCT for MF,38 higher survival (91% vs 56%) and lower NRM were observed in patients who had clinical improvement on JAK inhibitor therapy at the time of HCT (n = 23) compared with those who had either stable disease or developed clinical issues such as significant cytopenenias, increasing blasts, or loss of response to JAK inhibitor therapy at HCT. It is not possible to discern whether JAK inhibitor therapy influences HCT outcomes directly through clinical improvement, or indirectly by identifying patients with clinical improvement as those with favorable disease biology. This study demonstrates that early HCT might be a valid option for JAK inhibitor-responsive patients, although the question of early vs delayed HCT must be examined prospectively before drawing any strong conclusions. Due to logistical challenges in conducting such studies, investigators at the CIBMTR are planning to use Markov modeling, similar to that previously used for myelodysplastic syndrome.
The authors believe that for patients responding to JAK inhibitor therapy, a balanced discussion about early vs delayed HCT is required and individualized decisions should be made based on patient goals and wishes (Figure 3).
Q3. JAK inhibitors in transplant protocols: beneficial or harmful?
Use of JAK inhibitor therapy prior to HCT is a topic of active interest. Salutary effects of JAK inhibitor therapy in decreasing the symptom burden of the disease are particularly attractive in the pre-HCT setting.1,2 Potential beneficial and detrimental effects of JAK inhibitor therapy in the HCT setting are summarized in Table 2.36,38,41-46 The key questions have been how to best integrate JAK inhibitor therapy into HCT protocols prior to transplant and whether there is any role in the posttransplant setting.
Potential beneficial and detrimental effects of JAK 1/2 inhibitors in the transplant setting
| Potentially beneficial effects . | Potentially detrimental effects . |
|---|---|
| Improvement in performance status | Withdrawal effect |
| Potential to improve the pre-HCT performance status by effective control of MF-related symptom burden36 | If stopped suddenly prior to HCT, JAK 1/2 inhibitors can cause rapid return of MF-related symptoms38,41 |
| Several transplant studies have shown a relationship between better performance status with improved survival after transplant36 | Gentle tapering over 4 to 5 days prior to HCT is recommended |
| Reduced splenomegaly | Hematopoietic recovery |
| Reduced spleen size may aid faster hematologic recovery42 | Myelosuppressive properties of JAK 1/2 inhibitors may impact hematologic recovery after HCT, limiting post-HCT use |
| Decrease in GVHD | Increased risk of infections |
| JAK 1/2 inhibitor therapy has recently been shown to be effective in reducing the risk of GVHD in a mouse model by inhibiting donor T-cell expansion and inflammatory cytokine production43,44 Beneficial effect also demonstrated on 6 patients with steroid refractory GVHD43 | Murine models show silencing of T-helper cytokine secretion and profound reduction in T-regulatory cells45 Proven increased risk of urinary tract infections and zoster infection in comparison with other available treatments46 Anecdotal case reports of opportunistic infections (reactivation of hepatitis, mycobacterial infections, and invasive fungal infections) |
| Decreased GVL effect | |
| Through impaired function of NK cells and dendritic cells | |
| Drug-drug interactions | |
| Potential for significant drug interactions with routine transplant medications such as calcineurin inhibitors and anti-fungal medications etc via CYP3A4 inhibition | |
| Tumor lysis syndrome | |
| Cases of tumor lysis syndrome reported in a recent prospective study41 | |
| It is not clear if ruxolitinib causes chemo-sensitization to drugs used in conditioning therapy |
| Potentially beneficial effects . | Potentially detrimental effects . |
|---|---|
| Improvement in performance status | Withdrawal effect |
| Potential to improve the pre-HCT performance status by effective control of MF-related symptom burden36 | If stopped suddenly prior to HCT, JAK 1/2 inhibitors can cause rapid return of MF-related symptoms38,41 |
| Several transplant studies have shown a relationship between better performance status with improved survival after transplant36 | Gentle tapering over 4 to 5 days prior to HCT is recommended |
| Reduced splenomegaly | Hematopoietic recovery |
| Reduced spleen size may aid faster hematologic recovery42 | Myelosuppressive properties of JAK 1/2 inhibitors may impact hematologic recovery after HCT, limiting post-HCT use |
| Decrease in GVHD | Increased risk of infections |
| JAK 1/2 inhibitor therapy has recently been shown to be effective in reducing the risk of GVHD in a mouse model by inhibiting donor T-cell expansion and inflammatory cytokine production43,44 Beneficial effect also demonstrated on 6 patients with steroid refractory GVHD43 | Murine models show silencing of T-helper cytokine secretion and profound reduction in T-regulatory cells45 Proven increased risk of urinary tract infections and zoster infection in comparison with other available treatments46 Anecdotal case reports of opportunistic infections (reactivation of hepatitis, mycobacterial infections, and invasive fungal infections) |
| Decreased GVL effect | |
| Through impaired function of NK cells and dendritic cells | |
| Drug-drug interactions | |
| Potential for significant drug interactions with routine transplant medications such as calcineurin inhibitors and anti-fungal medications etc via CYP3A4 inhibition | |
| Tumor lysis syndrome | |
| Cases of tumor lysis syndrome reported in a recent prospective study41 | |
| It is not clear if ruxolitinib causes chemo-sensitization to drugs used in conditioning therapy |
GVL, graft-versus-leukemia; NK, natural killer.
Integrating JAK 1/2 inhibitors in the pretransplant protocol
Although integrating JAK inhibitor therapy in HCT protocols is theoretically attractive, controversial data have emerged in the last few years. Preliminary results from a prospective study (JAK-ALLO) to examine JAK inhibitors in the HCT setting showed serious adverse events (SAEs). These included cardiogenic shock and tumor lysis syndrome, resulting in a temporary hold on participant recruitment.41 The exact causes of these SAEs are not well understood. It is speculated that the abrupt discontinuation of JAK inhibitor prior to HCT and splenectomy procedure may have contributed to some of these SAEs. Several retrospective studies did not observe such SAEs, and no harmful effect on early posttransplant outcomes was observed in these studies (Table 3).36,38,47,48 In the largest retrospective study to date,38 SAEs occurred in 2 patients out of a total of 66 patients who continued JAK inhibitor therapy close to HCT (Table 3). Both patients experiencing SAEs discontinued JAK inhibitor therapy >6 days before conditioning therapy, whereas very few patients experienced adverse symptoms when JAK inhibitor therapy was continued close to conditioning therapy.
Summary of retrospective studies describing combining JAK inhibitors in HCT protocols
| Reference . | # Patients . | Study Design . | Results . | Conclusions . |
|---|---|---|---|---|
| 38 | 100 | Retrospective | No adverse impact on early outcomes of HCT | Continuing JAK inhibitor therapy near to start of conditioning therapy is associated with very low risk of withdrawal symptoms |
| HCT was delayed in 2 patients due to significant clinical events following JAK 1/2 inhibitor discontinuation: 1 patient developed pulmonary infiltrates and rebound splenomegaly, a second patient experienced fever and hypoxic respiratory failure | ||||
| 47 | 22 | Retrospective | 1-y OS of 100% in patients with a good response to ruxolitinib vs 60% in others | Continuing ruxolitinib until conditioning without tapering resulted in no unexpected SAEs |
| 36 | 14 | Retrospective | Engraftment in 13 patients (93%); graft fibrosis (n = 1) and treatment-related sepsis (n = 1) | Tapering ruxolitinib until conditioning did not result in unexpected SAEs |
| 48 | 11 | Retrospective | Good engraftment rates | Differing schedules of ruxolitinib tapering associated with high engraftment rates |
| Reference . | # Patients . | Study Design . | Results . | Conclusions . |
|---|---|---|---|---|
| 38 | 100 | Retrospective | No adverse impact on early outcomes of HCT | Continuing JAK inhibitor therapy near to start of conditioning therapy is associated with very low risk of withdrawal symptoms |
| HCT was delayed in 2 patients due to significant clinical events following JAK 1/2 inhibitor discontinuation: 1 patient developed pulmonary infiltrates and rebound splenomegaly, a second patient experienced fever and hypoxic respiratory failure | ||||
| 47 | 22 | Retrospective | 1-y OS of 100% in patients with a good response to ruxolitinib vs 60% in others | Continuing ruxolitinib until conditioning without tapering resulted in no unexpected SAEs |
| 36 | 14 | Retrospective | Engraftment in 13 patients (93%); graft fibrosis (n = 1) and treatment-related sepsis (n = 1) | Tapering ruxolitinib until conditioning did not result in unexpected SAEs |
| 48 | 11 | Retrospective | Good engraftment rates | Differing schedules of ruxolitinib tapering associated with high engraftment rates |
Given the observation that “withdrawal”-like effects are more common in patients who undergo early discontinuation of JAK inhibitor therapy prior to conditioning therapy, we recommend that JAK inhibitor therapy be continued and tapered until the start of conditioning therapy. Prospective trials are in progress to evaluate this strategy (#NCT01790295).
Do JAK 1/2 inhibitors have a role in the posttransplant setting?
In our opinion, JAK 1/2 inhibitor therapy has a limited role in the posttransplant setting because there is a limited anticlonal activity.4,49,50 Moreover, resultant cytopenias and drug interactions in the post-HCT setting may pose difficulties in use after HCT.
The available data suggest that JAK inhibitor therapy is ideally suited for the pre-HCT setting and should be continued to the start of conditioning therapy. It is important to gradually taper rather than suddenly discontinue therapy prior to the start of conditioning.
Q4. What is the optimal conditioning intensity and regimen?
The success of HCT in MF patients can be hampered by disease relapse, transplant-related toxicities, and graft failure, with results relatively poor compared with other myeloid malignancies.20 RIC is increasingly used in MF (Figure 1D), and age is often the major factor in decision-making regarding conditioning intensity. No prospective data are available comparing FIC and RIC regimens, although multiple retrospective studies have compared RIC with FIC in MF.51-54 Overall in these studies, patients transplanted using RIC have similar outcomes to those transplanted using FIC.51,52,54 Despite the higher age of patients in the RIC cohorts of these studies compared with the FIC cohorts, FIC was not superior to RIC. Indeed, in 1 study, RIC resulted in better survival compared with FIC even after adjusting for age.52
There are several limitations to these studies, such as their retrospective nature and lack of statistical power. Another potential bias could be selection for candidacy for transplantation for very fit older patients. Although a comparative prospective study in younger patients is desirable to answer this question, rarity of disease poses logistical challenges, and ongoing observational studies in the transplant registries may provide an answer in the near future. In the meantime, it is reasonable to restrict RIC to patients ≥50 years unless in the setting of clinical trials.
The heterogeneity of various RIC regimens makes comparison between them challenging. However, in a retrospective study of minimal intensity (nonmyeloablative) and RIC regimens, lower intensity was found to be associated with a high graft failure rate of 28% at 60 days post-HCT.55 Registry trends show that fludarabine (Flu) in combination with busulphan (Bu) or melphalan (Mel) are the two most commonly used regimens for HCT in MF.12 A RIC regimen consisting of Flu/Bu was investigated prospectively in 103 patients in a study from the EBMT.17 The probability of OS and progression-free survival at 5 years was 67% and 51%, respectively, and no differences were observed between MSD and well-matched URD. The Myeloproliferative Disorders Research Consortium prospectively investigated Flu/Mel RIC.37 Although very encouraging results similar to Flu/Bu were reported in the matched-sibling cohort, the results of the URD cohort were of concern; a high risk of graft failure was observed in the URD arm in this study.
In another retrospective study from the CIBMTR evaluating the outcomes of RIC HCT for MF, a trend toward better outcomes was observed in the Flu/Mel cohort.12 In a retrospective study of 160 patients, and currently the only direct comparison of Flu/Mel and Flu/Bu regimens in MF,56 OS was comparable. Although it is difficult to make comparisons between these retrospective studies, the differences in outcomes may be related to patient selection, warranting the need for prospective trials.
In summary, to date, there are no conclusive data on the optimal conditioning regimen for HCT in MF patients. Various FIC and RIC regimens have been demonstrated to result in similar outcomes and further prospective studies are required to confirm the best conditioning regimen for MF.
Q5. Is there a role for splenectomy in the JAK inhibitor era?
Spleen size is a risk factor for poorer HCT outcomes, including potential effects on engraftment and mortality.57,58 No consistent data on the role of pretransplant splenectomy have emerged. Although an increased rate of relapse was observed in nonsplenectomized male patients,59 an EBMT study showed increased relapse in splenectomized patients.17 And although splenectomy prior to HCT has been associated with faster engraftment in some retrospective studies60,61 and decreased mortality,34 a large international study by CIBMTR failed to show an effect for splenectomy on OS or incidence of GVHD.62
The above data indicate that use of splenectomy prior to HCT in MF is controversial. Complications of splenectomy include hemorrhage, infection, and thrombosis, and in an analysis of MF patients splenectomy carried a perioperative morbidity of 31% and a mortality rate of 9%.63 Given the effect of JAK inhibitors in decreasing spleen size in MF patients, they are a potential alternative to splenectomy to decrease spleen size prior to HCT.
Consensus guidelines published by EBMT/European Leukemia Net recommend against routine splenectomy of MF patients prior to HCT and use only in carefully selected cases of massive splenomegaly refractory to JAK inhibitor therapy.21
Q6. What is the role of AD transplants in MF?
There are scant data on the use of AD transplants in MF. A recent single center analysis64 of outcomes of AD transplants (haplo-identical and family mismatched) showed somewhat encouraging outcomes in recent years. In the 2011 to 2014 interval, the 3-year survival of matched-sibling and AD-transplant recipients overlapped (mainly haplo-identical).
Although there is little data regarding umbilical cord (UCB) transplants for MF, these appear also to be inferior to those of sibling- or well-matched URD donor transplants. A retrospective analysis that included 11 unrelated UCB transplants revealed that these patients had a lower probability of hematopoietic recovery compared with related-donor transplant recipients, although the OS was not significant.65 In another larger study (n = 35),66 2-year OS and progression-free survival in UCB recipients was 44% and 30%, respectively, although of note, 7 of these patients had undergone LT.
Given the limited experience on the use of AD transplants in MF, we suggest that AD transplants are most appropriate for patients who have a high risk of LT, or are poor candidates for or have failed JAK inhibitor therapy.
Conclusion
HCT for MF is a complex area that requires individualized decision-making at all stages of the process. Careful selection of patients, timing of HCT, and management of the disease are essential. Nontransplant options such as JAK 1/2 inhibitors may be more appropriate in some patients such as lower-risk patients, those without a well-matched donor, and those unsuitable for HCT. Due to the chronic nature of MF, consideration of HCT should be repeatedly revisited during the course of the patient’s disease. Additionally, all patients in the transplant age group should be referred to a specialist center for consideration of whether HCT is appropriate and assessment of HCT timing. Continued enrollment in prospective clinical trials is necessary to improve outcomes of HCT for MF patients, including determination of optimal timing, integration of JAK 1/2 inhibitors in the transplant protocol, and comparison with nontransplant therapies.
Correspondence
Vikas Gupta, The Elizabeth and Tony Comper Myeloproliferative Neoplasm Program, Princess Margaret Cancer Centre, 610 University Ave, Toronto, ON M5G 2M9, Canada; e-mail: vikas.gupta@uhn.ca.
References
Competing Interests
Conflict-of-interest disclosure: V.G. received research funding and honoraria from and has consulted for Novartis and Incyte. R.D. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.