Abstract
Direct oral anticoagulants (DOACs) have at least noninferior efficacy compared with other oral anticoagulants and have ancillary benefits, including overall better safety profiles, lack of the need for routine monitoring, rapid onset of action, and ease of administration. Reversal of these agents may be indicated in certain situations such as severe bleeding and for perioperative management. DOAC-associated bleeding should be risk stratified: patients with moderate or severe bleeding should have the DOAC discontinued and reversal strategies should be considered. Laboratory testing has limited utility in the acute management of bleeding; thrombin time and activated partial thromboplastin time may be useful for excluding clinically relevant levels of dabigatran. Prothrombin time is potentially useful for rivaroxaban and edoxaban, but calibrated anti-Xa assays are optimal for determining clinically relevant levels of factor Xa inhibitors. Because specific reversal agents are not widely available, supportive care and interventions for local hemostasis remain the cornerstones of therapy in the patient with DOAC-associated bleeding. Nonspecific reversal agents should be considered only in the event of severe bleeding because their efficacy is unknown, and they are associated with risk of thrombosis. Recent results from phase 3/4 studies demonstrate efficacy for an antidote to dabigatran (idarucizumab, a monoclonal antibody fragment with specificity for dabigatran) and an antidote to factor Xa inhibitors (andexanet alfa, a recombinant and inactive form of factor Xa that binds inhibitors). A universal reversal agent (ciraparantag) for many anticoagulants, including the DOACs, shows promise in results from phase 1 and 2 studies.
Learning Objectives
To have a rational approach to direct oral anticoagulant reversal that stratifies by bleeding severity
To describe the range of different strategies, both specific and nonspecific, for reversal of direct anticoagulants
To describe emerging evidence for specific reversal strategies
Introduction
Direct oral anticoagulants (DOACs), also known in the literature as new oral anticoagulants or target-specific anticoagulants, have been approved for the prevention of stroke and systemic embolization in atrial fibrillation, treatment and secondary prevention in venous thromboembolism, and thromboprophylaxis after major orthopedic surgery. Randomized clinical trial data demonstrate noninferior or increased efficacy compared with other anticoagulants such as vitamin K antagonists (VKAs) and low-molecular-weight heparin. Practical advantages with DOACs include fewer drug and food interactions, reduced need for monitoring, and a rapid onset of action. DOACs also have a favorable safety profile compared with warfarin; a meta-analysis of more than 100 000 patients demonstrated a reduction of 28% in major bleeding and a reduction of approximately 50% in intracranial and fatal bleeding.1 The severity of intracranial bleeds is also decreased in DOACs compared with warfarin.2 In patients with renal insufficiency (creatinine clearance <50 mL/min), hemorrhagic stroke events occur significantly less often in patients taking DOACs compared with VKAs.3 All-cause mortality was significantly lower with the use of DOACs compared with warfarin in clinical trial patients.4 Safety data for dabigatran outside clinical trials supports the findings from clinical trial data.5
However, there is still concern regarding bleeding events associated with DOACs. Unlike warfarin and heparins, options for reversing the DOACs are limited. Although evidence is beginning to emerge regarding targeted therapies for reversal, lack of accessibility of these agents will preclude their use in many settings. This review aims to delineate a practical approach to reversal of DOACs in the context of the bleeding patient.
General approach to the bleeding patient
A stepwise algorithm for treating the bleeding patient is provided in Figure 1. In general, increasing severity of the bleed will lead the clinician further down the algorithm. Expert consultation, if available, should be requested early. Each of the steps will be discussed in detail.
General approach to treating the bleeding patient. A, apixaban; E, edoxaban; fVIIa, [recombinant] factor VIIa; R, rivaroxaban.
General approach to treating the bleeding patient. A, apixaban; E, edoxaban; fVIIa, [recombinant] factor VIIa; R, rivaroxaban.
Risk stratification of the patient
We suggest that a targeted history and physical examination in the setting of the bleeding patient on DOACs should include, but not be limited to:
Assessing hemodynamic stability
Identifying the source, severity, risk factors, and history of bleeding
Obtaining a full medication history to identify relevant concomitant medications, assess potential drug interactions, and assess other medications that may potentiate bleeding
Determining the time elapsed since the last dose of DOAC
Determining whether life-threatening anemia and renal function are present
Depending on the severity of bleeding, we advocate the following general approaches.
Minor bleeding.
Minor bleeding includes most cases of epistaxis, ecchymosis, and menorrhagia, which can generally be managed with local hemostatic measures. Drug discontinuation may be considered when weighing the balance between the benefit of reducing bleeding and the risk of thromboembolism. For example, withdrawing the DOAC or reducing the dose can be considered with recurrent menorrhagia. In some of these patients, discontinuation of the DOAC and reinitiation of warfarin may be considered because current literature suggests a higher risk (as high as 25%) of heavy menstrual bleeding if the patient is taking rivaroxaban.6-8 Whether this is specific to rivaroxaban or is attributable to factor Xa inhibitors as a class requires further study.8
Moderate bleeding.
Moderate bleeding includes subacute gastrointestinal bleeding or severe forms of the types of bleeding mentioned in minor bleeding. DOACs should be discontinued in these patients, and supportive care and local hemostasis should be emphasized. Adjuncts and reversal agents (such as tranexamic acid for menorrhagia) may be considered. Investigation of the site (in the case of gastrointestinal or genitourinary bleeding) is required because bleeding is likely to recur unless the underlying lesion is identified and corrected.
Severe bleeding.
Severe bleeding encompasses forms of bleeding that are life threatening. We recommend all of the measures mentioned in moderate bleeding along with adjuncts and consideration of targeted reversal agents whenever possible or nonspecific reversal agents if targeted agents are not available.
Determination of clinically significant drug levels
One of the key benefits of DOACs is that routine drug monitoring of patients can be eliminated. However, when a drug level or a surrogate of coagulation function is needed, DOACs are problematic. Liquid chromatography/tandem mass spectrometry is the gold standard, but it is generally unavailable outside of research settings; its utility is limited because of real-world variability and lack of data relating drug levels and clinical outcomes.9,10
For the vast majority of laboratories and clinicians, the only coagulation testing available 24 hours a day is the prothrombin time/international normalized ratio (PT/INR) and the activated partial thromboplastin time (aPTT). However, these tests have inadequacies when used to measure the effect of DOACs, and there is significant variability in methodology and reagents used. These tests should be ordered when the patient presents and can be useful in ruling out therapeutic or supratherapeutic drug levels. However, if the patient is having moderate or severe bleeding, supportive care and other measures should be instituted before coagulation test results become available. Other centers use more specialized testing such as dilute thrombin time (dTT), ecarin clotting time (ECT), the ecarin chromogenic assay (ECA), and anti-Xa levels. The effect of DOACs on coagulation tests and recommended testing for DOACs are provided in Table 1.
Effects of DOACs on coagulation testing and recommended testing

Color key: red, inappropriate testing; yellow, may be useful for excluding clinically relevant drug levels and may approximate drug levels; green, best test available. Adapted from Siegal et al35 with permission.
↑, increase; ↓, decrease; ↔, no change; N/A, not advised.
Direct thrombin inhibitor dabigatran.
Thrombin time.
The test we suggest to exclude clinically relevant drug levels in most settings is the thrombin time (TT). If the TT is normal, this excludes clinically relevant dabigatran levels. However, the test is likely too sensitive for most applications. TT is above the limit of measurement with some reagents at a dabigatran concentration of 25 ng/mL, which is well below the median trough of 90 ng/mL.11 Subtherapeutic levels of dabigatran may therefore prolong the TT above the normal range. Many clinicians do not have immediate access to a TT; however, it is a straightforward test that is easily performed with most coagulation systems found in hospital laboratories. A rapidly available TT is useful in assisting clinicians who are making decisions regarding the use of reversal agents, although immediate management before test results are available should be considered if bleeding is severe or life threatening. Modifying the TT by diluting the patient sample with normal plasma (dTT) decreases the excessive sensitivity of the TT. In-house solutions and commercially available tests have been evaluated and have been demonstrated to reflect dabigatran concentrations across its usual achieved range, but the availability of the dTT is limited.12 The commercial dTT is not approved for clinical use in the United States but is available in other countries.13
aPTT.
aPTT is useful for excluding clinically relevant levels of dabigatran but is inappropriate for quantification at higher concentrations because aPTT reaches a plateau at higher concentrations.11 The aPTT is not as sensitive as TT. Sensitivity varies with different commercial aPTT reagents, and coagulation laboratories should perform validation studies with their chosen method.14 Thus, an elevated aPTT in a patient receiving dabigatran indicates drug presence, and the degree of prolongation of the aPTT generally reflects levels. However, a normal aPTT does not exclude clinically relevant concentrations of drug.
Other tests.
Other tests such as the ecarin-based ECT and ECA assays are promising for quantification of dabigatran levels but are limited in their availability.11 The PT/INR is inappropriate for assessing dabigatran levels. Dabigatran may be normal in patients with therapeutic or supratherapeutic levels.15 The sensitivity of commercial PT reagents is variable.14
Direct factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Anti-Xa activity.
Assays calibrated to the specific drug are effective in measuring drug levels, although precision is decreased outside therapeutic levels.11,16 Most laboratories do not have DOAC-calibrated assays, and most current assays are intended for heparin or low-molecular-weight heparin. Assays calibrated to the heparins are less accurate, but they are the best choice in the absence of optimized tests.17 A normal anti-Xa level using any calibrator excludes clinically relevant levels of any of the Xa inhibitors.
PT.
Rivaroxaban and edoxaban mildly increase the PT/INR, but a normal PT/INR does occur at typical trough levels.18 A normal PT does not exclude clinically relevant concentrations of rivaroxaban or edoxaban. Differences in sensitivity are observed with various PT reagents. The conversion of PT to an INR generated by a calibrated DOAC-specific international sensitivity index has been demonstrated to improve assay precision.19 Laboratories should assess the sensitivity of their PT method to rivaroxaban and edoxaban. A normal PT level is helpful in excluding clinically relevant drug levels of rivaroxaban and edoxaban in the absence of anti-Xa levels. PT/INR should be avoided when assessing levels of apixaban because in most assay systems, little PT prolongation is seen, even at high apixaban concentrations.
Other tests.
The aPTT is inappropriate for assessing drug levels in patients taking direct factor Xa inhibitors. A normal aPTT does not exclude therapeutic or supratherapeutic levels.20
Withdrawal of the medication and supportive care
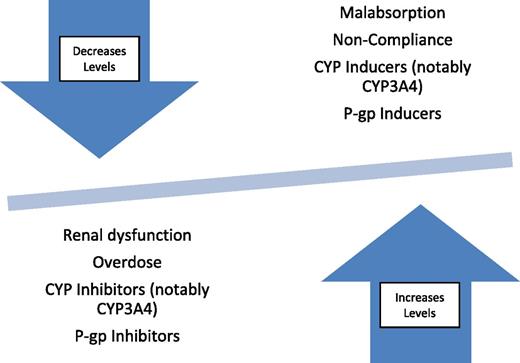
Patients who have moderate or more severe bleeding should have their DOACs and any other medications contributing to bleeding discontinued if possible. Factors that contribute to changes in drug levels (such as interacting drugs) should be considered and should be managed to optimize DOAC metabolism (Figure 2). Because of the shorter half-lives of DOACs compared with warfarin, moderately severe bleeding may be treated with supportive care until the drug is eliminated, which avoids the risks (eg, thrombosis) associated with some reversal strategies. Pharmacokinetic properties of the DOACs are provided in Table 2. The following are other aspects of supportive care that should be managed.
Pharmacokinetic properties of DOACs
| . | Direct thrombin inhibitor dabigatran . | Factor Xa inhibitor . | ||
|---|---|---|---|---|
| Rivaroxaban . | Apixaban . | Edoxaban . | ||
| Time to peak onset | 22 min-4.5 h | 1-3 h | 1-2 h | Unknown |
| Half-life | 12-14 h | 5-9 h | 8-15 h | 10-14 h |
| >24 h if CrCl is <30 mL/min | 9-13 h if patient is elderly | |||
| Drug interactions | P-gP | CYP3A4, CYP3A5, CYP2J2, P-gP | CYP3A4,P-gP | P-gP |
| Renal excretion (%) | 80 | 33 | 25 | 35 |
| . | Direct thrombin inhibitor dabigatran . | Factor Xa inhibitor . | ||
|---|---|---|---|---|
| Rivaroxaban . | Apixaban . | Edoxaban . | ||
| Time to peak onset | 22 min-4.5 h | 1-3 h | 1-2 h | Unknown |
| Half-life | 12-14 h | 5-9 h | 8-15 h | 10-14 h |
| >24 h if CrCl is <30 mL/min | 9-13 h if patient is elderly | |||
| Drug interactions | P-gP | CYP3A4, CYP3A5, CYP2J2, P-gP | CYP3A4,P-gP | P-gP |
| Renal excretion (%) | 80 | 33 | 25 | 35 |
CrCL, creatinine clearance.
Hemodynamic stability and metabolic disturbances.
Wide-bore intravenous access should be instituted to correct hypovolemia and hypotension, and the patients may need to be placed in a monitored or intensive-care setting.
Drug excretion is achieved by optimizing renal blood flow. Antihypertensives should be held. Metabolic disturbances such as acidosis and hypocalcemia that occur with large volumes of transfused red blood cells (RBCs) as well as hypothermia should be corrected to optimize hemostasis and patient stability.
Blood component transfusion.
Although no studies have been performed on DOAC-associated bleeding, transfusion of RBCs should be kept to a restrictive transfusion trigger when possible. This recommendation is consistent with studies that demonstrate worse patient outcomes with increased RBC transfusion.21,22
Plasma transfusion is inappropriate for DOAC-associated bleeding because it lacks efficacy and confers unnecessary risk to patients.23 Plasma, platelet, and cryoprecipitate transfusions should be based on conventional indications such as dilutional coagulopathy from massive transfusion or concomitant disseminated intravascular coagulation. Massive transfusion protocols should be implemented promptly (on the basis of institutional protocol) in patients with massive hemorrhage.24 These recommendations apply even in the absence of reversal agents.
Local hemostasis
Whenever possible, mechanical compression and local hemostatic measures should be applied. Early referral for definitive procedural or surgical interventions remains the cornerstone of management, even with use of reversal agents and adjuncts. For patients with moderate or severe bleeding, early consultation with experts in gastroenterology, interventional radiology, or surgery should be considered. Imaging may help delineate the location and extent of bleeding, for example, in retroperitoneal bleeding.
Adjunct treatments
Antifibrinolytics.
Although not studied in DOAC-associated bleeding, tranexamic acid and ε-aminocaproic acid promote clot stability by reducing fibrinolysis. They are effective in other patient groups with pathological bleeding and could be considered in patients with moderate or severe bleeding. Although they are not generally associated with an increased risk of thrombosis, they have not been evaluated in patients at increased risk of thrombosis which, by definition, includes all patients who are taking anticoagulants.25,26
Desmopressin.
Desmopressin has not been studied in DOAC-associated bleeding but may promote hemostasis because it increases levels of von Willebrand factor through release from endothelial cells. A meta-analysis demonstrated no increased risk of thrombosis perioperatively.27 However, caution is needed because of the risk of hyponatremia and potential prothrombotic risk. Repeated doses over short time periods lead to tachyphylaxis.
Targeted reversal agents
Three DOAC-specific antidotes are currently being investigated. Their properties along with summaries of clinical studies are provided in Tables 3 and 4.
Characteristics of DOAC-specific reversal agents
| . | Ciraparantag . | Idarucizumab . | Andexanet alfa . |
|---|---|---|---|
| Anticoagulants indicated for reversal | Direct thrombin inhibitors, factor Xa inhibitors, heparins | Dabigatran | Factor Xa inhibitors (rivaroxaban, apixaban, edoxaban, enoxaparin) |
| Mechanism of action | Reported to bind anticoagulants via noncovalent hydrogen bonds and charge-charge interactions | Monoclonal antibody fragment to bind dabigatran | Inactive form of factor Xa to bind inhibitors |
| Suggested administration | Phase 2 study used 100-300 mg single intravenous dose | Total of 5 g given as two 2.5-g 50 mL boluses within 15 minutes of each other | 800 mg bolus and 960 mg infusion over 2 h; patients who take apixaban or rivaroxaban more than 7 hours before andexanet administration: 400 mg bolus and 480 mg infusion over 2 hours |
| Time to onset | Within 10-30 minutes | Within minutes (between vials in REVERSE-AD31 ) | Within 2-5 minutes |
| . | Ciraparantag . | Idarucizumab . | Andexanet alfa . |
|---|---|---|---|
| Anticoagulants indicated for reversal | Direct thrombin inhibitors, factor Xa inhibitors, heparins | Dabigatran | Factor Xa inhibitors (rivaroxaban, apixaban, edoxaban, enoxaparin) |
| Mechanism of action | Reported to bind anticoagulants via noncovalent hydrogen bonds and charge-charge interactions | Monoclonal antibody fragment to bind dabigatran | Inactive form of factor Xa to bind inhibitors |
| Suggested administration | Phase 2 study used 100-300 mg single intravenous dose | Total of 5 g given as two 2.5-g 50 mL boluses within 15 minutes of each other | 800 mg bolus and 960 mg infusion over 2 h; patients who take apixaban or rivaroxaban more than 7 hours before andexanet administration: 400 mg bolus and 480 mg infusion over 2 hours |
| Time to onset | Within 10-30 minutes | Within minutes (between vials in REVERSE-AD31 ) | Within 2-5 minutes |
Summary of selected studies of DOAC-specific reversal agents
| Drug name . | Clinical trial identifier . | Study name . | Study phase . | Study description . | No. of patients . | Results summary . |
|---|---|---|---|---|---|---|
| Ciraparantag | NCT02205905 | 1 | Open-label, single-dose, nonrandomized pharmacokinetic study in healthy male patients | 6 | Completed; results pending publication | |
| NCT01826266 | 1 | Double-blind RCT of efficacy/safety of escalating doses after single dose of edoxaban 60 mg | 80 | Administration of 100-300 mg reversed anticoagulation within 10-30 min and was sustained for 24 h | ||
| NCT02207257 | 2 | Single-blind RCT of safety/efficacy of escalating doses after steady-state edoxaban and effects after re-anticoagulation and second reversal | 69 | Recruiting as of second quarter of 2016 | ||
| Idarucizumab | NCT01688830 | 1 | Double-blind RCT in healthy patients for (A) an escalating dose assessment | 110 | (A) Rapid peak plasma exposure and elimination; no adverse effects. | |
| (B) For efficacy/safety | 47 | (B) Efficacy in reversal of coagulation test abnormalities (TT, dTT, aPTT, ECT) | ||||
| NCT01955720 | 1 | Double-blind RCT to study pharmacokinetics/pharmacodynamics | 12 | Reinitiation of dabigatran at 24 h led to similar aPTT levels whether previous treatment was idarucizumab or placebo | ||
| NCT02104947 | REVERSE-AD | 3 | Cohort study of efficacy/safety in patients with (A) serious bleeding on dabigatran who (B) required urgent procedure on dabigatran | 300 (planned) | Interim analysis (n = 90): dTT normalized in ≥93% and ECT normalized in ≥88% of patients. (A) median time for cessation of bleeding was 11.4 h; (B) normal intraoperative hemostasis was achieved in 92% of patients | |
| Andexanet alfa | NCT01758432 | 2 | Double-blind RCT to study pharmacokinetics/ pharmacodynamics | 144 | Dose-dependent reduction in factor Xa activity lasting until 2 h | |
| NCT02207725 | ANNEXA-A | 3 | Double-blind RCT of efficacy/safety in reversing apixaban | 48 | Anti-Xa activity reduced by 94%; thrombin generation restored in 100% | |
| NCT02220725 | ANNEXA-R | 3 | Double-blind RCT of efficacy/safety in reversing rivaroxaban | 53 | Anti-Xa activity reduced by 92%; thrombin generation restored in 96% | |
| NCT02329327 | ANNEXA-4 | 3B to 4 | Cohort study of efficacy/safety in achieving hemostasis in those with major bleeding on factor Xa inhibitors | 270 (planned) | Interim analysis (n = 67): anti-Xa activity reduced by 89% with rivaroxaban and 93% with apixaban; 79% had good/excellent hemostasis 12 h after infusion; 18% had thrombotic events |
| Drug name . | Clinical trial identifier . | Study name . | Study phase . | Study description . | No. of patients . | Results summary . |
|---|---|---|---|---|---|---|
| Ciraparantag | NCT02205905 | 1 | Open-label, single-dose, nonrandomized pharmacokinetic study in healthy male patients | 6 | Completed; results pending publication | |
| NCT01826266 | 1 | Double-blind RCT of efficacy/safety of escalating doses after single dose of edoxaban 60 mg | 80 | Administration of 100-300 mg reversed anticoagulation within 10-30 min and was sustained for 24 h | ||
| NCT02207257 | 2 | Single-blind RCT of safety/efficacy of escalating doses after steady-state edoxaban and effects after re-anticoagulation and second reversal | 69 | Recruiting as of second quarter of 2016 | ||
| Idarucizumab | NCT01688830 | 1 | Double-blind RCT in healthy patients for (A) an escalating dose assessment | 110 | (A) Rapid peak plasma exposure and elimination; no adverse effects. | |
| (B) For efficacy/safety | 47 | (B) Efficacy in reversal of coagulation test abnormalities (TT, dTT, aPTT, ECT) | ||||
| NCT01955720 | 1 | Double-blind RCT to study pharmacokinetics/pharmacodynamics | 12 | Reinitiation of dabigatran at 24 h led to similar aPTT levels whether previous treatment was idarucizumab or placebo | ||
| NCT02104947 | REVERSE-AD | 3 | Cohort study of efficacy/safety in patients with (A) serious bleeding on dabigatran who (B) required urgent procedure on dabigatran | 300 (planned) | Interim analysis (n = 90): dTT normalized in ≥93% and ECT normalized in ≥88% of patients. (A) median time for cessation of bleeding was 11.4 h; (B) normal intraoperative hemostasis was achieved in 92% of patients | |
| Andexanet alfa | NCT01758432 | 2 | Double-blind RCT to study pharmacokinetics/ pharmacodynamics | 144 | Dose-dependent reduction in factor Xa activity lasting until 2 h | |
| NCT02207725 | ANNEXA-A | 3 | Double-blind RCT of efficacy/safety in reversing apixaban | 48 | Anti-Xa activity reduced by 94%; thrombin generation restored in 100% | |
| NCT02220725 | ANNEXA-R | 3 | Double-blind RCT of efficacy/safety in reversing rivaroxaban | 53 | Anti-Xa activity reduced by 92%; thrombin generation restored in 96% | |
| NCT02329327 | ANNEXA-4 | 3B to 4 | Cohort study of efficacy/safety in achieving hemostasis in those with major bleeding on factor Xa inhibitors | 270 (planned) | Interim analysis (n = 67): anti-Xa activity reduced by 89% with rivaroxaban and 93% with apixaban; 79% had good/excellent hemostasis 12 h after infusion; 18% had thrombotic events |
RCT, randomized controlled trial.
Ciraparantag as a reversal agent for all DOACs and heparins.
Ciraparantag (PER977; Perosphere, Danbury, CT) is a small synthetic molecule that binds to direct Xa inhibitors, direct thrombin inhibitors, and heparins. In animal studies, it demonstrated normalization of thromboelastography in a rabbit model and reduced bleeding in both rat and rabbit models.28,29 In a preliminary study of 80 healthy patients receiving a single dose of edoxaban, ciraparantag normalized hemostasis based on whole-blood clotting time.30 Ciraparantag is currently being studied in healthy volunteers (NCT02207257).
Idarucizumab as an antidote to dabigatran.
Idarucizumab (anti-Dabi-Fab; Boehringer Ingelheim, Biberach, Germany) is a humanized mouse monoclonal antibody fragment that binds free dabigatran and thrombin-bound dabigatran and is supplied as a refrigerated solution in single-use 2.5-g vials. It is administered as two 2.5-g intravenous injections (5 g total) usually given over 15 minutes or less. Normalization of the dTT and ECT occurs minutes after infusion.31 Patients with high levels of drug after an initial reversal and who have a persistent clinical need for reversal may require a second exposure, which seems to be effective on the basis of a phase 1 study in volunteers.32 An interim analysis of the prospective cohort REVERSE-AD study reported results in 51 patients with major bleeding (group A) and 39 patients needing urgent surgery (group B) who required reversal with idarucizumab after taking dabigatran. In group A, the median time for cessation of bleeding was 11.4 hours. In group B, normal intraoperative hemostasis was achieved in 92% of patients.31 Rapid correction of the functional effect of dabigatran was demonstrated in all patients. Thrombotic events were observed in 5 patients after reversal and before reinstitution of anticoagulant therapy. Procoagulant effects were not observed in phase 1 studies.33,34 Patients are started on anticoagulant therapy because of their increased thrombotic risk, and these events should not be attributed solely to the use of a reversal agent. In healthy volunteers, similar aPTTs were seen when they restarted dabigatran 24 hours after reversal compared with starting dabigatran after placebo.32 Idarucizumab is currently approved by the US Food and Drug Administration, Health Canada, and the European Medicines Agency and is indicated for patients treated with dabigatran when reversal of its anticoagulant effects are needed for emergency surgery, urgent procedures, or life-threatening or uncontrolled bleeding. It should be considered the agent of choice for reversal of major or life-threatening bleeding attributable to dabigatran in centers where it is available.
Andexanet alfa as an antidote to direct factor Xa inhibtors.
Andexanet alfa (Portola Pharmaceuticals, San Francisco, CA) is a recombinant, inactive form of factor Xa that binds all factor Xa inhibitors (including enoxaparin). Two randomized, placebo controlled trials (ANNEXA-A and ANNEXA-R) evaluated the efficacy of reversal and safety in healthy older (age 50 to 75 years) volunteers who received anticoagulation therapy with apixaban and rivaroxaban.35 In both studies, after administration of andexanet alfa with a bolus, the time to effect was within minutes and the effect wore off after approximately 2 hours. Anti-Xa levels were reduced by more than 90% in both studies by bolus treatment, and thrombin generation was restored in nearly all patients. Administration with a bolus followed by a continuous infusion over 2 hours sustained the near complete reversal of anti-Xa activity for the duration of the infusion. ANNEXA-4 is a prospective open-label cohort study of patients with acute major bleeding associated with factor Xa inhibitors (including edoxaban and enoxaparin), and it is currently enrolling patients. Most patients were given a bolus dose of 800 mg and an infusion dose of 960 mg over 2 hours, unless more than 7 hours had passed since factor Xa inhibitor was ingested, in which case doses were halved. An interim analysis of 67 patients demonstrated reduction in anti-Xa levels similar to that in the ANNEXA-A and ANNEXA-R studies that persisted after a 2-hour infusion. Effective clinical hemostasis 12 hours after infusion was observed in 79% of evaluable patients. Andexanet alfa forms a complex with tissue factor pathway inhibitor and causes transient increases in D-dimer and prothrombin fragments 1 and 2 in volunteers as well as increased thrombin generation when used to reverse rivaroxaban.36 Thrombotic events were not observed in ANNEXA-A and ANNEXA-R but did occur in 18% of patients at 30 days in the interim analysis of the ANNEXA-4 study; of those patients, only 2 had restarted anticoagulation.
Nonspecific reversal strategies
Hemodialysis and activated charcoal: potential options for dabigatran.
Dabigatran is amenable to removal via hemodialysis because of its low plasma protein binding and small molecular size. A systematic review with evidence derived from case studies found that hemostasis can be achieved, but rebound is common after renal replacement therapy is discontinued.37 However, if idarucizumab is available, it should be administered instead of removing dabigatran via hemodialysis.
Activated charcoal can reduce absorption of dabigatran and apixaban. Given that they are rapidly absorbed, this is an option only within the first 2 to 3 hours of administration for dabigatran and within 6 hours for apixaban.38,39 It is unclear whether charcoal is effective with other DOACs, but dialysis is unlikely to be effective because of high protein binding.
Prothrombin complex concentrates.
Prothrombin complex concentrates (PCCs) are plasma-derived products containing vitamin K–dependent coagulation factors, and they are used for the reversal of VKAs. All PCCs contain factors II, IX, and X. PCCs with normal amounts of factor VII are known as 4-factor PCCs and those lacking factor VII are known as 3-factor PCCs. The efficacy of PCCs in DOAC reversal is difficult to interpret given different durations for DOAC administration, different PCC products, and various results in different studies. Four-factor PCC is an option for reversal when given at a dose of 50 U/kg, although evidence for its use in dabigatran is lacking.40-42 When used for VKA reversal, the reported risk of thromboembolism is 1.4%.43
In a randomized, placebo-controlled, crossover study, a 4-factor PCC (Cofact) corrected PT prolongation and increased endogenous thrombin potential in healthy subjects (n = 12) receiving rivaroxaban 20 mg twice per day for 2.5 days. In the same study, PCCs did not normalize PT, ECT, or TT in dabigatran-treated subjects.40 Another study in healthy volunteers treated with rivaroxaban suggests that 4-factor PCC (Beriplex/Kcentra) may normalize PT more effectively than 3-factor PCC (Profilnine), but it had less of an effect on normalizing thrombin generation. Neither PCC product corrected anti-Xa activity.41 A randomized placebo-controlled study reported reversal to baseline for bleeding duration and a trend toward reduced bleeding volume after 4-factor PCC (Beriplex), although confidence intervals for the effect on bleeding duration overlap with those for placebo.42 In ex vivo studies, PCCs corrected PT, aPTT, and TT in dabigatran and variably correct thrombin generation abnormalities in dabigatran and rivaroxaban.44,45 It is not known whether reversal in healthy subjects or ex vivo studies accurately reflects patients with bleeding. The effect of PCCs on DOACs requires further study to prove their efficacy.
Despite the lack of supportive data in actively bleeding patients, PCCs have been used in patients with major or life-threatening bleeding who are receiving DOACs; the use of PCCs should be considered experimental and not the standard of care. If idarucizumab is available, it should be used in preference to PCCs for patients with dabigatran-associated bleeding.
Activated prothrombin complex concentrates.
Activated prothrombin complex concentrates (aPCCs) are most often used in the setting of patients with hemophilia who have a factor inhibitor. aPCCs are available commercially as factor eight inhibitor bypassing activity (FEIBA) (Baxter Bioscience, Vienna, Austria), and they contain the factors found in PCCs and activated factor VII. Thrombosis has been reported at a rate of 4 to 8 events per 105 infusions.46 It is an option for reversal given at a dose of 50 to 100 U/kg, but it has not been studied in clinically bleeding patients. In ex vivo studies, aPCCs correct all abnormal thrombin generation indices in rivaroxaban and some in dabigatran.45 A porcine polytrauma model showed improved survival and decreased total blood loss when reversal of dabigatran occurred with aPCCs compared with placebo.47 In vitro studies demonstrate aPTT correction in dabigatran and PT correction in rivaroxaban.44,45
Despite a complete lack of supportive data in actively bleeding patients, aPCCs have been used in patients with major or life-threatening bleeding who were receiving DOACs; their use should be considered experimental and not the standard of care. If idarucizumab is available, it should be used in preference to aPCCs for patients with dabigatran-associated bleeding.
Recombinant factor VIIa.
Recombinant factor VIIa correction of DOAC-induced abnormalities in thrombin generation parameters is variable. In vitro, it does not correct aPTT in dabigatran or anti-Xa activity in rivaroxaban, but it does correct prolonged PT in rivaroxaban.44,45 We recommend against the use of recombinant factor VIIa for treatment of DOAC-associated bleeding.
Conclusion
DOACs have favorable safety profiles and have similar or less major bleeding than VKAs. DOAC-associated bleeding should be risk stratified to determine management and should be managed with expert consultation whenever possible. Moderate or life-threatening bleeding requires temporary discontinuation of the drug and supportive care. Supportive care includes maintaining hemodynamic stability, transfusion, and local hemostasis. Although specific antidotes are promising, availability is limited, but idarucizumab is becoming more widely accessible. When available, specific reversal agents should be used in patients with moderate to severe bleeding because they have greater efficacy. Adjuncts and nonspecific reversal agents are best considered in severe bleeding, but there is a lack evidence for both efficacy and safety.
Correspondence
Andrew W. Shih, McMaster University, HSC 3H50, 1280 Main St West, Hamilton, ON L8S 4K1, Canada; e-mail: andrew.shih@medportal.ca.
References
Competing Interests
Conflict-of-interest disclosures: A.W.S. declares no competing financial interests. M.A.C. is on the Board of Directors or on an advisory committee for CSL Behring, Asahi Kasei Pharma America, Bayer AG, Boehringer-Ingelheim, LEO Pharma, Octapharma, Pfizer, and Portola Pharmaceuticals; has consulted for CSL Behring, Alexion Pharmaceuticals, Bayer AG, LEO Pharma, Octapharma, Pfizer, and Portola Pharmaceuticals; has received honoraria from Ortho Clinical Diagnostics, Bayer AG, Boehringer-Ingelheim, Bristol-Myers Squibb, Pfizer, Celgene, Daiichi Sankyo, LEO Pharma, and Pfizer; and has been affiliated with the Speaker’s Bureau for CSL Behring, Alexion, Bayer AG, and Leo Pharma.
Author notes
Off-label drug use: None disclosed.

![Figure 1. General approach to treating the bleeding patient. A, apixaban; E, edoxaban; fVIIa, [recombinant] factor VIIa; R, rivaroxaban.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2016/1/10.1182_asheducation-2016.1.612/6/m_hem088393f1.jpeg?Expires=1767824773&Signature=FNm~hU7KOj4BLGya8wuEpF7Pj7EjWGnGJc7XYz~Y7qY7nucky0rYbQbg4oV9I94X2N-f7hRTYDRTP-1SrQsFLp5v9vdW6FzjjdcbYNrNsFbg5OfWd1Z~6ULKKbrg16wgaHEVzeaFP6~svLpIPJUKj4LJ875PtNmZtDf96dxXphK-nzWrOQftZWloosB~kmuMQgpdT1jU9U~7TWItzhcnO7QFAdjtvVJhrqgaRis~T6yZd6V6EbZHuWnQWQCOpTr2QIJLBSnzks4AYQAJK6mQQpnGBLnoEz1swqi0oyGWNoXDa9fRSfovjuoxPWinAd8aiD5XG9JnQMohFRpkSI0Gyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)