Abstract
Thrombocytopenia develops in 5% to 10% of women during pregnancy or in the immediate postpartum period. A low platelet count is often an incidental feature, but it might also provide a biomarker of a coexisting systemic or gestational disorder and a potential reason for a maternal intervention or treatment that might pose harm to the fetus. This chapter reflects our approach to these issues with an emphasis on advances made over the past 5 to 10 years in understanding and managing the more common causes of thrombocytopenia in pregnancy. Recent trends in the management of immune thrombocytopenia translate into more women contemplating pregnancy while on treatment with thrombopoietin receptor agonists, rituximab, or mycophenylate, which pose known or unknown risks to the fetus. New criteria to diagnose preeclampsia, judicious reliance on measurement of ADAMTS13 to make management decisions in suspected thrombotic thrombocytopenic purpura, new evidence supporting the efficacy and safety of anticomplement therapy for atypical hemolytic uremic syndrome during pregnancy, and implications of thrombotic microangiopathies for subsequent pregnancies are evolving rapidly. The goals of the chapter are to help the hematology consultant work through the differential diagnosis of thrombocytopenia in pregnancy based on trimester of presentation, severity of thrombocytopenia, and coincident clinical and laboratory manifestations, and to provide guidance for dealing with some of the more common and difficult diagnostic and management decisions.
Learning Objectives
Gain a working understanding of the differential diagnosis of thrombocytopenia during and soon after pregnancy based on trimester of onset, severity of the thrombocytopenia, and concomitant clinical features at presentation
Learn a management algorithm for ITP to prevent bleeding, optimize delivery, and ensure fetal well-being
Understand guideposts for intervention in women with TMAs based on the need to decide among early delivery, plasma exchange, or anticomplement therapy
Introduction
Thrombocytopenia develops in 5% to 10% of women during pregnancy or in the immediate postpartum period. A low platelet count is often an incidental feature of pregnancy, but it might also provide a biomarker of a coexisting systemic or gestational disorder and a potential reason for a maternal intervention or treatment that might pose harm to the fetus. This chapter reflects our personal approach to the more common causes of thrombocytopenia in pregnancy with an emphasis on recent advances in understanding and management made since the topic was last reviewed at the American Society of Hematology (ASH) educational session in 2010.1 The reader is also referred to a recent comprehensive review of this topic published in Blood,2 a recent ASH guideline,3 and a practice bulletin from the American College of Obstetrics and Gynecology4 for additional details and perspectives. The reader is also referred elsewhere for consideration of pregnancy in patients with hereditary thrombocytopenia (HT),5 type IIb von Willebrand disease6 and immune thrombocytopenia (ITP) secondary to systemic lupus7 and antiphospholipid syndrome.8
Gestational thrombocytopenia
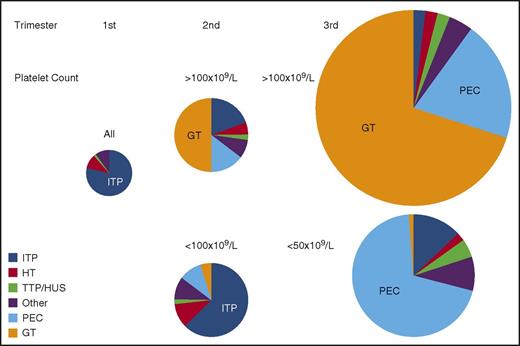
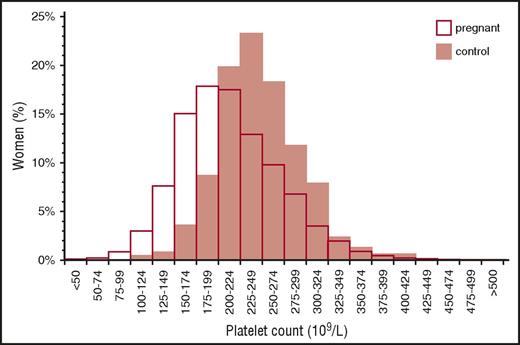
Gestational thrombocytopenia (GT; defined as a platelet count below 150 × 109/L) occurs in 4.4% to 11.6% of pregnancies, accounting for about 75% of all cases of thrombocytopenia in pregnancy2,9 (Figure 1). The distribution of platelet counts at term in uncomplicated pregnancies is shown in Figure 2. These data are representative of several such analyses performed in the United States, Europe, and Japan, but their applicability to other populations is not established. Thrombocytopenia is more prevalent in twin and triplet gestations.11 Platelet counts in many women show a gradual downward trajectory beginning in the second trimester, which is most likely from hemodilution related to an increase in plasma volume during pregnancy and possibly increased platelet clearance as mean platelet volumes, platelet volume distribution width, and platelet-derived cyclooxygenase products rise. A subset of women with GT develop a more significant decline in platelet count and a reduction in antithrombin III, suggesting a discrete pathogenesis that lies on a continuum with the hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome and acute fatty liver of pregnancy (AFLP) and that may be associated with a higher risk of recurrence in subsequent pregnancies.12
Prevalence of causes of thrombocytopenia based on trimester of presentation and platelet count. The size of each circle represents the relative frequency of all causes of thrombocytopenia during each of the 3 trimesters of pregnancy. All etiologies and all platelet counts are considered together in the first trimester when thrombocytopenia is uncommon. Distribution of etiologies during the second and third trimesters is subdivided by platelet count. All results are estimates based on personal experience and review of the literature. “Other” indicates miscellaneous disorders, including infection, DIC, type IIB von Willebrand disease, immune and nonimmune drug-induced thrombocytopenia, paroxysmal nocturnal hemoglobinuria, bone marrow failure syndromes (aplastic anemia, myelodysplasia, myeloproliferative disorders, leukemia/lymphoma, and marrow infiltrative disorders), among others. HUS, hemolytic uremic syndrome; PEC, preeclampsia/HELLP; TTP, thrombotic thrombocytopenic purpura.
Prevalence of causes of thrombocytopenia based on trimester of presentation and platelet count. The size of each circle represents the relative frequency of all causes of thrombocytopenia during each of the 3 trimesters of pregnancy. All etiologies and all platelet counts are considered together in the first trimester when thrombocytopenia is uncommon. Distribution of etiologies during the second and third trimesters is subdivided by platelet count. All results are estimates based on personal experience and review of the literature. “Other” indicates miscellaneous disorders, including infection, DIC, type IIB von Willebrand disease, immune and nonimmune drug-induced thrombocytopenia, paroxysmal nocturnal hemoglobinuria, bone marrow failure syndromes (aplastic anemia, myelodysplasia, myeloproliferative disorders, leukemia/lymphoma, and marrow infiltrative disorders), among others. HUS, hemolytic uremic syndrome; PEC, preeclampsia/HELLP; TTP, thrombotic thrombocytopenic purpura.
Distribution of platelet counts in healthy pregnant women at term. Reprinted from Boehlen et al10 with permission.
Distribution of platelet counts in healthy pregnant women at term. Reprinted from Boehlen et al10 with permission.
From the perspective of the hematology consultant, practical points to be emphasized include: (1) a platelet count of 115 × 109/L is ∼2 standard deviations below the mean at term. (2) GT does not become apparent before the mid-second trimester. (3) Only 1% to 5% of women develop platelet counts below 100 × 109/L and few have counts below 75 × 109/L, but counts below 50 × 109/L have been attributed to GT on rare occasions and only when other possible etiologies have been ruled out. (4) There are no biomarkers to provide an affirmative diagnosis, which might preclude distinction from mild ITP, the onset of preeclampsia/HELLP (see “Preeclampsia and hemolysis, elevated liver enzymes, low platelets” section), or other diagnoses of exclusion. (5) GT does not intrinsically reflect or affect the health of the mother and fetal thrombocytopenia is uncommon (<2%) and mild. (6) GT does not respond to IV immune globulin (IVIG) or corticosteroids, which has been tried when thrombocytopenia is so severe as to compromise epidural anesthesia or delivery.13 (7) If thrombocytopenia does not resolve within 1 to 2 months of delivery, the diagnosis of ITP or HT may become evident only in hindsight.
Immune thrombocytopenia
Incidence and diagnosis
ITP occurs in 1 in 1000 to 10 000 pregnancies. Although ITP accounts for only ∼3% of all cases of thrombocytopenia during pregnancy, it is the most common cause of a platelet count below 50 × 109/L detected in the first and second trimesters (Figure 2). Platelet counts may fall during gestation, and at least 15% to 35% of mothers require treatment even prior to management of labor and delivery.14-16 This figure depends on practice patterns, so that the need for treatment is likely to be more prevalent in tertiary-care referral centers. Maternal and neonatal outcomes are generally favorable. Therefore, ITP is not a contraindication to pregnancy per se. However, in unusually severe or refractory cases or for women reliant on potentially teratogenic medications, deferring pregnancy may be indicated. There is no laboratory test to distinguish ITP from GT or some of the other causes of maternal thrombocytopenia. Therefore, the diagnosis of ITP is based on a personal history of bleeding, a low platelet count prior to pregnancy, and/or a family history that excludes HT; the diagnosis of ITP is made by excluding other disorders when possible or may be made only retroactively based on response to ITP-directed therapy. A few women lack an antecedent (pregestational) history. ITP should be suspected when an otherwise healthy mother (bleeding excepted) who is taking no medications and has no relevant family or gestational history of concern presents with a platelet count below 70 × 109/L to 80 × 109/L in the first or second trimester and has a peripheral blood smear notable only for thrombocytopenia without unusually small or giant platelets.
Management
Treatment is initiated for bleeding when the platelet count falls below 20 × 109/L to 30 × 109/L, and for procedures and delivery. Data indicate an increased risk of bleeding if platelet count is below 20 × 109/L to 30 × 109/L for a vaginal delivery14 or below 50 × 109/L for a cesarean section.15 Hematomas following neuraxial anesthesia are exceedingly rare in patients with stable ITP14 and a platelet count above 50 × 109/L with no concomitant coagulopathy or exposure to an antithrombotic agent, for example, low-molecular-weight heparin. However, most guidelines suggest a minimum platelet count of 80 × 109/L is advisable for neuraxial anesthesia.17 Platelet counts should be measured more frequently starting at 32-34 weeks and repeated weekly in unstable patients. This generally allows enough time for a change in therapy to improve platelet counts and reduce the risk of bleeding in advance of a planned or unplanned cesarean section or neuraxial anesthesia without the urgent need for platelet transfusions. ε-Aminocaproic acid is a safe and effective adjunct that should be considered before and after delivery in women with severe ITP and other causes of thrombocytopenia that place a woman at high risk for bleeding.
Care should be coordinated with an experienced obstetrician and neonatologist to the extent possible. For initial short-term treatment, for example, in anticipation of delivery, daily oral prednisone may be favored over pulse dexamethasone because there is less concern about placental transfer. IVIG is used if corticosteroid therapy fails or if its use is limited by maternal intolerance. There is limited published experience with IV immunoglobulin G (IgG) anti-RhD, which crosses the placenta and coats fetal erythrocytes.18 Splenectomy has been performed safely in the second trimester, but is rarely required. Persistent exposure to high-dose corticosteroids in the first trimester is associated with a small increased risk of cleft palate and exposure throughout gestation may increase the risk of preterm birth and gestational diabetes. Therefore, greater reliance is often placed on periodic administration of IVIG than would be typical in the management of nonpregnant patients.19
There is little evidence on which to base treatment of women refractory to a combination of corticosteroids and IVIG. Human recombinant thrombopoietin (not currently available in the United States) has been used with success in 1 pilot study,20 and there are anecdotal reports involving the use of thrombopoietin receptor agonists such as romiplostim in the weeks prior to delivery.21,22 It is difficult to define the risk to the fetus of individual immunosuppressive agents due to the lack of disease-specific and disease-severity controls. The effects on pregnancy outcome and on the fetus of other medications used to treat ITP are either: unknown (thrombopoietin receptor agonists), tolerable/associated with some risks but used for other indications in the setting of pregnancy (azathioprine, cyclosporine, and cyclophosphamide), or known to be teratogenic and not used in pregnancy (mycophenylate; danazol). Dapsone has been associated with a risk of hemolytic anemia and hyperbilirubinemia in the newborn. Rituximab is not known to be teratogenic but has been associated with prolonged B-cell lymphocytopenia and the need to delay vaccination in neonates exposed in utero; therefore, when clinically appropriate, rituximab should not generally be used within at least 6 months of planned conception. ITP does not prevent thrombosis; therefore, thromboprophylaxis and anticoagulation should be managed according to standard protocols. Until evidence-based guidelines are available, our preference is to maintain the platelet count above 20 × 109/L to 30 × 109/L for prophylactic intensity anticoagulation and above 40 × 109/L to 50 × 109/L for therapeutic intensity anticoagulation in the absence of complicating features.
Neonatal outcome
Mode of delivery should be based on obstetrical considerations. Although the risk of severe hemorrhagic complications is low, operative vaginal deliveries, such as with forceps or vacuum-assisted vaginal delivery, are avoided whenever possible. One percent to 5% of neonates are born with platelet counts below 20 × 109/L and up to 5% to 15% require therapy,16 but the incidence of intracerebral hemorrhage (ICH) is below 1%.12,14 There is little correlation between maternal and neonatal platelet counts and maternal therapy has not been demonstrated to lessen this risk. The incidence of neonatal thrombocytopenia may be higher in women who are postsplenectomy16 or when there is a sibling who was born with thrombocytopenia.14 Thus, no clinical features or biomarkers in the mother have been found to reliably identify neonatal risk. Management of the newborn should be coordinated with an experienced neonatologist or pediatric hematologist, when available. The child should be assessed carefully for bleeding. A cord platelet count is indicated in all neonates. Neurologic impairment potentially indicative of an ICH should be assessed with an ultrasound and should be considered in neonates with a platelet count below 50 × 109/L at birth. A comprehensive assessment for other causes of neonatal thrombocytopenia is required. IVIG and platelet transfusions for emergent conditions may be needed to manage bleeding or if the platelet count is below 30 × 109/L. Platelet counts may fall during the first 1 to 3 days after delivery, which has been attributed to maturation of the spleen and other clearance mechanisms. Concurrent neonatal/fetal alloimmune thrombocytopenia should be suspected when a neonate is born with a platelet count below 10 × 109/L or there is suspicion of ICH. The diagnosis and management of neonatal/fetal thrombocytopenia are considered in detail elsewhere.23
Thrombotic microangiopathies
The differential diagnosis and management of thrombotic microangiopathies (TMAs) in pregnancy have been the subject of a recent review.24 Operationally, the critical issue is whether a pregnant woman presenting with evidence of TMA can be observed, or requires emergent delivery, or management with plasma infusion or exchange or with an inhibitor of complement. Features that help to differentiate among the various causes of TMA are shown in Table 1 and are discussed in detail in the following sections.
Clinical and laboratory features of pregnancy-associated microangiopathies
| . | Preeclampsia/HELLP . | TTP . | HUS . | AFLP . |
|---|---|---|---|---|
| Elevated blood pressure | +++ | + | + | ++ (50% of cases) |
| Neurological symptoms | +/++ (headache) | +++ (numbness, weakness, aphasia, mental status) | + | + |
| Abdominal symptoms | + (RUQ pain) | ++ (unspecific/diffuse) | + | +++ (unspecific/diffuse) |
| Fever | − | −/+ | −/+ | − |
| Easy bruising | − | −/+ | − | − |
| Thrombocytopenia | +/++ (>50 × 109/L) | +++ (<20 × 109/L) | + (<100 × 109/L) | + |
| Renal impairment (elevated creatinine; > ∼2 mg/dL) | +/++ | +/++ | +++ | ++/+++ |
| Hepatic dysfunction and inflammation (AST/ALT) | + | −/+ | −/+ | +++ (and bilirubin) |
| Coagulopathy | −/+ | − | − | +++ |
| LDH | + | +/+++ | +/++ | +++ |
| Microangiopathic hemolytic anemia | + | +/+++ | +/++ | + |
| Hypoglycemia | − | − | − | + |
| ADAMTS13 activity | Normal | <10%* | >20%-30%† | >30% |
| . | Preeclampsia/HELLP . | TTP . | HUS . | AFLP . |
|---|---|---|---|---|
| Elevated blood pressure | +++ | + | + | ++ (50% of cases) |
| Neurological symptoms | +/++ (headache) | +++ (numbness, weakness, aphasia, mental status) | + | + |
| Abdominal symptoms | + (RUQ pain) | ++ (unspecific/diffuse) | + | +++ (unspecific/diffuse) |
| Fever | − | −/+ | −/+ | − |
| Easy bruising | − | −/+ | − | − |
| Thrombocytopenia | +/++ (>50 × 109/L) | +++ (<20 × 109/L) | + (<100 × 109/L) | + |
| Renal impairment (elevated creatinine; > ∼2 mg/dL) | +/++ | +/++ | +++ | ++/+++ |
| Hepatic dysfunction and inflammation (AST/ALT) | + | −/+ | −/+ | +++ (and bilirubin) |
| Coagulopathy | −/+ | − | − | +++ |
| LDH | + | +/+++ | +/++ | +++ |
| Microangiopathic hemolytic anemia | + | +/+++ | +/++ | + |
| Hypoglycemia | − | − | − | + |
| ADAMTS13 activity | Normal | <10%* | >20%-30%† | >30% |
Estimated prevalence of clinical signs and symptoms and laboratory features in women with TMAs during pregnancy. Reference ranges in healthy pregnancy must be taken into consideration.
+, prevalence; −, not usually present; ALT, alanine aminotransferase; AST, aspartate transaminase; LDH, lactate dehydrogenase; RUQ, right upper quadrant.
Some investigators require that the ADAMTS13 activity level in plasma be below 10% of normal to make the diagnosis of TTP, whereas others use this solely as providing confirmation of a clinical diagnosis.
ADAMTS13 activity is generally above 30% of normal in patients with a clinical diagnosis of aHUS, but there are no guidelines that exclude this diagnosis based on activity levels per se.
Pregnancy-specific TMA
Preeclampsia and hemolysis, elevated liver enzymes, low platelets.
Incidence and presentation.
Preeclampsia (PEC) is by far the most common cause of thrombocytopenia associated with evidence of TMA presenting in the late second or in the third trimester of pregnancy (Figure 1). Infrequently, PEC with associated thrombocytopenia develops during the first week postpartum, although even more delayed presentations have been reported. Approximately 50% of women with PEC develop thrombocytopenia with platelet counts generally above 100 × 109/L and not <50 × 109/L unless there are superimposed complications.4,25 Rarely, thrombocytopenia precedes other manifestations. Although the pathogenesis of thrombocytopenia is uncertain, it is reported that extracellular vesicles shed by syncytiotrophoblasts from PEC placentas may augment platelet activation,26 which, in turn, release soluble factors and extracellular vesicles that might contribute to placental and systemic microvascular ischemia.27
Diagnosis.
PEC is now defined as the onset of hypertension beginning after 20 weeks of gestation that may or may not be accompanied by 1 or more of the “severe features” listed in Table 2. PEC can also develop in women with preexisting hypertension. HELLP may be a variant of PEC characterized by more severe thrombocytopenia, more fulminant microangiopathic hemolytic anemia (MAHA), and more profoundly elevated liver function tests. Overt clinical disseminated intravascular coagulation (DIC) is rare, but biochemical changes consistent with DIC may be present in up to 10% of women and can be a marker of disease progression.28
Criteria for diagnosis of PEC
| Criteria . |
|---|
| PEC is defined by the onset of hypertension beginning after 20 wk of gestation, which may or may not be accompanied by 1 or more of the following “severe features”: |
| (1) Thrombocytopenia (<100 × 109/L) |
| (2) Impaired liver function or injury (liver transaminases elevated to twice the normal concentration) |
| (3) New-onset renal insufficiency (serum creatinine >1.1 mg/dL or doubling of the baseline creatinine in the absence of known renal disease) |
| (4) Pulmonary edema |
| (5) New-onset cerebral or visual disturbances |
| (6) Persistent systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 110 mm Hg |
| Criteria . |
|---|
| PEC is defined by the onset of hypertension beginning after 20 wk of gestation, which may or may not be accompanied by 1 or more of the following “severe features”: |
| (1) Thrombocytopenia (<100 × 109/L) |
| (2) Impaired liver function or injury (liver transaminases elevated to twice the normal concentration) |
| (3) New-onset renal insufficiency (serum creatinine >1.1 mg/dL or doubling of the baseline creatinine in the absence of known renal disease) |
| (4) Pulmonary edema |
| (5) New-onset cerebral or visual disturbances |
| (6) Persistent systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 110 mm Hg |
These criteria are based on recommendations by the American College of Obstetricians and Gynecologists and the Task Force on Hypertension in Pregnancy.25
Management.
The diagnosis of PEC and HELLP, and their distinction from TTP and HUS, is critical because the only treatment is delivery (Table 1). Results of randomized studies do not support use of corticosteroids to reduce maternal bleeding or other morbidity.29 Expectant management might be appropriate for some women who are <34 weeks gestation depending on the clinical status of the mother. However, women managed expectantly remain at risk for sudden and severe progression of disease, including deterioration in mental status and development of DIC with as-sociated hemorrhage, in which case emergent delivery and supportive therapy with transfusion of packed red cells, platelets, and coagulation factors may be needed. Serial monitoring of platelet counts and other laboratory studies may be useful in guiding timing and mode of delivery. Although abnormalities in complement regulatory pathways have been described in some patients with HELLP, there is as yet only anecdotal experience with the use of eculizumab.30,31 Most women improve clinically soon after delivery, although improvement in laboratory parameters may lag. The diagnosis of TTP or HUS should be considered in any woman who does not show clinical and laboratory improvement within 48 to 72 hours postdelivery or in women whose clinical situation decompensates after delivery.
Neonatal outcome.
Neonatal thrombocytopenia is uncommon in the absence of prematurity or when neonates are born small for gestational age. Neonatal morbidity and mortality varies from 7% to 20%, driven primarily by the complications of prematurity that are directly related to any deterioration in maternal status and the gestational age at which HELLP develops.32
Considerations for subsequent pregnancies.
Estimated risks of recurrence in a subsequent pregnancy vary from 5% to 94% based on several variables, including number of prior affected pregnancies, presence of chronic hypertension, severity of previous preeclampsia, and early gestational age of onset, among others.28 Low-dose aspirin (60-150 mg per day) modestly reduces the risk of developing PEC, the incidence of preterm birth, and fetal growth restriction in women who are at increased risk33 ; treatment (typically 81 mg per day) is initiated at 12 to 16 weeks’ gestation, but benefit may be seen even if aspirin is started by 20 weeks’ gestation.33
Acute fatty liver of pregnancy.
Incidence and presentation.
AFLP occurs in 1 in 5000 to 10 000 pregnancies and is more common with multiple gestations than in singletons. Up to 75% of women present with nausea or vomiting and 50% have abdominal pain or signs and symptoms consistent with PEC.34 Diagnosis is based on impaired renal and hepatic function, including impaired synthesis of clotting factors, accompanied by abdominal pain, nausea, and vomiting. Reduction in plasma antithrombin III may be an early marker of AFLP, and, in many women, the coagulopathy is disproportionately severe relative to other evidence of liver dysfunction, although marked elevations in transaminases and bilirubin can develop. Hypoglycemia, present in severe cases, is a key diagnostic feature not seen in related conditions28 (Table 1). Although it is often difficult to differentiate HELLP from AFLP, evidence of hepatic insufficiency including hypoglycemia, DIC, or encephalopathy is seen more often in AFLP. Imaging modalities are not helpful in differentiation. Maternal thrombocytopenia is not uncommon, but severe thrombocytopenia is infrequent.
Management.
Treatment consists of supportive management and resuscitation of the mother with aggressive replacement with packed red cells and fresh-frozen plasma to maintain adequate perfusion to vital organs. Antithrombin III concentrate and platelets may be useful in severely affected mothers.28 Stabilization of the mother and prompt delivery of the fetus is indicated, irrespective of gestational age.
Considerations for subsequent pregnancies.
An inherited defect in mitochondrial β-oxidation of fatty acids, long-chain-3-hydroxyacyl CoA dehydrogenase (LCHAD) deficiency, is identified in up to 20% of affected fetuses. Accumulation of LCHAD metabolites produced by the fetus or placenta is hepatotoxic for the mother.28 This is an autosomal-recessive fetal inborn error of mitochondrial fatty acid oxidation that carries a 25% chance of recurrence in a subsequent pregnancy. However, placing the mother on a diet effective for treating LCHAD in the newborn has not been shown to reduce the risk of recurrent AFLP.
TMA not specific to pregnancy
Thrombotic thrombocytopenic purpura.
Incidence and presentation.
Serologically documented acquired, antibody-induced TTP (aTTP) is estimated to occur in 1 in 200 000 pregnancies.35,36 Approximately 10% of women with aTTP24,37 and a quarter to half of those with congenital TTP (cTTP)35,38,39 present for the first time during pregnancy, often the first pregnancy,36 or postpartum.35,40 This predisposition may reflect the fall in ADAMTS13 and rise in von Willebrand factor that occurs normally during gestation.
Diagnosis.
Prompt diagnosis is important because maternal mortality can be reduced by 80% to 90% with timely recognition and treatment. TTP presents more commonly during the latter half of gestation or postpartum.35,41 Diagnosis is most straightforward when a previously healthy mother presents in the first trimester with severe MAHA, thrombocytopenia, and neurologic dysfunction, which may be accompanied by renal insufficiency and fever (Figure 1; Table 1). Clinical certainty becomes progressively more difficult as term approaches in view of the overlap between key features of TTP with severe PEC. TTP should be a prominent consideration when a woman with TMA does not meet criteria for severe PEC or HELLP, when the platelet count falls below 20 × 109/L, or in the presence of neurologic findings such as weakness, numbness, aphasia, or an overt change in mental status.38,41,42 TTP confers an increased risk of PEC,43 which can complicate early recognition and increase morbidity. An abundance of schistocytes and nucleated red blood cells on the peripheral blood smear and a marked elevation in serum LDH favors the diagnosis of TTP, but is not invariably found on presentation. The clinical distinction of TTP from atypical HUS (aHUS) is also favored by a platelet count below 20 × 109/L, by the absence of severe and progressive renal insufficiency (creatinine above 2.2 mg/dL), and by neurologic complications unexplained by the severity of the renal failure. The diagnosis of TTP should become a prominent consideration when clinical signs and symptoms do not resolve and the platelet count does not increase above 100 × 109/L by 48 to 72 hours postdelivery.42 cTTP, which comprises up to 50% of all cases of gestational TTP in some series,36 should be suspected when severe ADAMTS13 is detected in the absence of an IgG inhibitor even in the absence of a suggestive family history; gene sequencing is required for definitive diagnosis and to guide management of subsequent pregnancies.44
Management.
Clinical acumen is critical because the decision to institute plasmapheresis for aTTP, plasma infusion for cTTP, or a complement inhibitor for aHUS vs early delivery for PEC/HELLP often precedes confirmation of the diagnosis of TTP based on an ADAMTS13 level below 10% of normal. Pregnancy does not impair response to plasma infusion in cTTP or plasmapheresis35 and corticosteroids39 in aTTP. Nor is there evidence that termination of pregnancy improves maternal outcome.40 Fetal loss caused by widespread placental ischemia is frequent when TTP occurs in the first and second trimester, but the incidence of healthy live births approaches 75% to 90% when TTP develops closer to term and when maternal treatment has been successful.35,39,43 Transplacental passage of anti-ADAMTS13 antibodies has been documented in the absence of clinical sequelae. There is little published guidance on the use of ritixumab,45 azathioprine,36 or other modalities in women with aTTP who do not respond to plasmapheresis. An intermediate purity factor VIII preparation containing ADAMTS13 has been used; data on use of recombinant ADAMTS13 are needed. Use of low-dose aspirin and low-molecular-weight heparin has been advocated without high-grade evidence.36
Considerations for subsequent pregnancies.
The risk of recurrence in a subsequent pregnancy exceeds 50%40,41 for women with cTTP or those with aTTP who have persistent severely reduced ADAMTS13 activity. Therefore, ADAMTS13 activity and antibody should be measured prior to or early in the first trimester to identify women at highest risk who require close surveillance.43 Prophylaxis with serial plasma infusions should be strongly considered throughout subsequent gestations and postpartum periods in women with cTTP, and plasmapheresis has been advocated for women with aTTP when plasma levels of ADAMTS13 fall below 5% to 10%, but supporting data are limited.45 The frequency of exchange is based on platelet count and LDH39 with generally excellent maternal outcomes.36 Platelet counts should be followed monthly in women with a prior history of aTTP but normal levels of ADAMTS13 at the onset of pregnancy.36 Women with pregnancy-associated TTP may also have an increased risk of PEC during subsequent pregnancies.43
Atypical hemolytic uremic syndrome.
Incidence and presentation.
aHUS is estimated to occur in 1 in 25 000 pregnancies.36 Ten percent to 20% of all women present with aHUS for the first time during pregnancy,44 perhaps reflecting the stress of complement activation that develops normally during gestation. In 1 series, 25% to 40% of women who developed pregnancy-associated aHUS did so during the initial pregnancy44 ; however, many women have had previous unaffected gestations. Eighty percent of affected women develop aHUS postpartum, with the remainder distributed throughout all trimesters. The diagnosis of aHUS becomes preeminent when a woman presents with progressive renal failure, thrombocytopenia with platelet counts above 50 × 109/L, MAHA, and, occasionally, evidence of ischemic tissue injury elsewhere in the absence of meeting criteria for PEC/HELLP (Table 1).
Diagnosis.
Differentiation from TTP may be difficult before renal failure becomes the predominant feature, especially when thrombocytopenia is severe or when extrarenal manifestations develop. Consumption of plasma C3 and C4 and generation of soluble C5b-9 complexes are seen in some forms of the disease, but are not diagnostic. The diagnosis is supported in about two-thirds of patients by identifying a mutation that impairs expression or function of proteins that regulate the alternative pathway C3 convertase,44 less commonly by a gain-of-function mutation in C3 or factor B, autoantibodies against factor H, or, rarely, a mutation in thrombomodulin, plasminogen, or diacyglycerol kinase-ε. However, because turnaround times for these tests are long and some mutations might be classified as “variants of unknown significance,”46 the greatest value of genetic testing is to affirm the diagnosis for the purposes of justifying long-term costly management, developing an approach to subsequent pregnancies and consideration of family members.
Management and subsequent pregnancies.
Plasmapheresis should be initiated upon diagnosis and continued until TTP is excluded based on marked progressive renal failure and ADAMTS13 activity above 30%. Fifty-five percent to 80% of episodes of MAHA due to congenital aHUS respond to plasma infusion outside of the setting of pregnancy, but patients with factor I deficiency are often recalcitrant. Moreover, the evidence is less compelling that plasma infusion or plasmapheresis prevents development of end-stage renal disease when aHUS develops during pregnancy (other than in the subset of patients with anti–factor H antibodies).44,47 Historically, 80% of mothers with aHUS required dialysis and 60% to 70% developed end-stage renal disease. It is hoped that early intervention with the anti-C5 antibody eculizumab will improve outcome.48 Eculizumab has been used safely during pregnancy in women with paroxysmal nocturnal hemoglobinemia49 and in a more limited number of women with aHUS.50 Fetal levels of this IgFcγ2/4 hybrid antibody are low and no fetal harm has been reported, nor has the drug been detected in breast milk.49 Pregnancy and apheresis increase drug requirements.49 Patients should be vaccinated against meningococcus, and it is generally recommend that angiotensin-converting enzyme inhibitors should be discontinued. There is no evidence that delivery alters outcome.42 The risk of fetal loss is 10% to 20%, which may reflect confinement of microthrombi to the maternal renal microvasculature in contrast to more widespread maternal and placental microvascular injury in TTP.36,44 Limited available evidence indicates there is a 10% to 30% risk of recurrence in subsequent pregnancies depending on the underlying mutation.51
Other causes of TMA in pregnancy.
Laboratory and/or clinically overt TMA can develop in patients with active systemic lupus,52,53 other forms of vasculitis or scleroderma,54 antiphospholipid syndrome,55 and in other settings, such as allogeneic bone marrow transplantation, allograft rejection, and graft-versus-host disease. There is insufficient information to determine whether pregnancy poses an increased risk for TMA in these settings, but management of the underlying disorders is generally required for effective control of the superimposed microangiopathy.
This article was selected by the Blood and Hematology 2017 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2017. It is reprinted with permission from Blood 2017, Volume 130.
Acknowledgments
This work was supported in part by National Institutes of Health, National Heart, Lung, and Blood Institute grants P01HL110860 and HL07971-07 (D.B.C.).
Correspondence
Douglas B. Cines, Perelman School of Medicine, University of Pennsylvania, 513 A Stellar-Chance, 422 Curie Blvd, Philadelphia, PA 19104-4274; e-mail: dcines@mail.med.upenn.edu.
References
Competing Interests
Conflict-of-interest disclosure: D.B.C. has served as a consultant for Amgen, Rigel, Astellas, Juno, and Ionis, and has received research funding from Syntimmune, Momenta, and T2 Biosystems. L.D.L. declares no competing financial interests.
Author notes
Off-label drug use: Prednisone, rituximab, dapsone, other immunosuppressants, and eculizumab for use in pregnancy, which is considered off-label.