Abstract
This section reviews the diagnostic criteria and pathogenesis of large granular lymphocyte (LGL) leukemia. There is a particular focus on the overlap of LGL leukemia and rheumatoid arthritis (Felty's syndrome). Current understanding of the mechanisms of neutropenia in these disorders is discussed. Finally, treatment indications and therapeutic recommendations are outlined.
Learning Objectives
Review diagnosis, pathogenesis, and treatment of LGL leukemia
Understand potential mechanisms of neutropenia in LGL leukemia and rheumatoid arthritis
Historic studies of large granular lymphocyte (LGL) leukemia have been faced with heterogeneous diagnostic criteria and variable duration of follow-up in part because LGL leukemia is a rare disease. Population-based studies have reported the overall age-standardized incidence as 0.72 per 1,000,000 person-per year and 0.2 cases per 1,000,000 individuals in the Netherlands and the United States, respectively.1,2 We present our summarized, current understanding here and refer the reader to more detailed analyses in recent multicenter studies.3,4 Ultimately, each patient will need to be managed individually, as this disease is still a chronic illness with no definitive cure.
Diagnosis of LGL leukemia
LGL leukemia is classified by the World Health Organization (WHO) under mature T-cell and NK-cell neoplasms in 3 categories: T-cell large granular lymphocytic leukemia (T-LGLL), chronic lymphoproliferative disorder of NK cells (referred to here as NK-LGLL for clarity), and aggressive NK-cell leukemia (ANKL) (Figure 1).5 The clinical and biological features of T-LGLL and NK-LGLL are similar. In contrast, ANKL is marked by multiorgan LGL infiltration and a drug-resistant, rapidly fatal course (median survival ∼1-2 months). ANKL is generally found only in Asia and is associated with Epstein-Barr virus infection. The other two are more indolent and are the focus of this section. Among these, the T-LGLL subtype accounts for ∼85% of the disease, and the NK-LGLL subtype accounts for ∼15%.
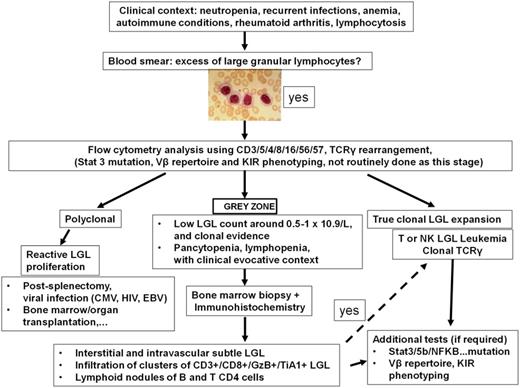
Clinical algorithm for the diagnosis of LGL leukemia. Adapted with permission from Lamy and Loughran.6
Clinical algorithm for the diagnosis of LGL leukemia. Adapted with permission from Lamy and Loughran.6
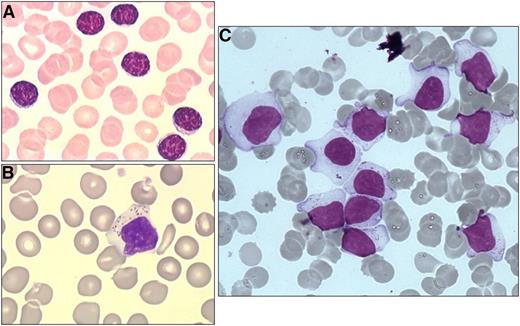
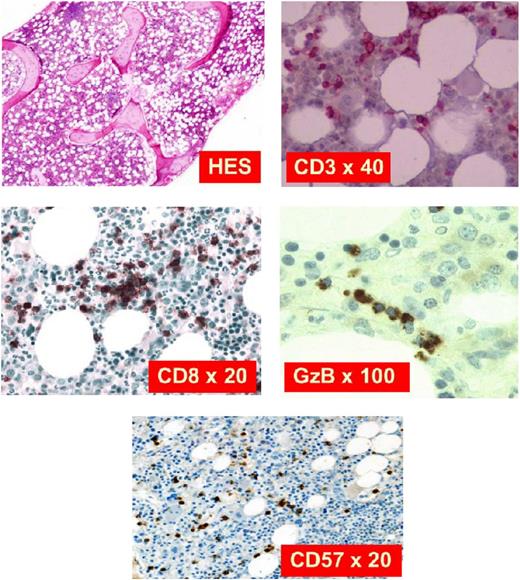
The diagnostic criteria for LGL leukemia has evolved over the years as understanding of the disease has improved with molecular findings.6 Currently, the diagnosis should be considered in the appropriate clinical context of presenting features such as cytopenias or autoimmune diseases. Next, a finding of excess large granular lymphocytes (>0.5 × 109/L) on peripheral blood smear is supportive (Figure 2). The LGL subtype is best determined through cell surface markers on flow cytometry. The T-cell form will usually show CD3+/CD8+/CD57+, whereas the NK-cell form will usually show CD3−/CD8+/CD16+/CD56+, although exceptions do occur. Finally, a diagnosis is confirmed by detection of clonality in the LGL population, which distinguishes the LGL expansion from a polyclonal, reactive lymphocytosis that may be seen following a viral infection. In the case of T-LGLL, this can be done with a polymerase chain reaction panel to assess clonal T-cell receptor γ (TCRγ) gene rearrangement. Deep sequencing of the T-cell repertoire provides a more quantitative assessment of clonal size and could be useful in monitoring response to therapy.7 Alternatively, finding a restricted population using an antibody flow panel to assess TCR Vβ repertoire is presumptive evidence of clonality. In the NK subtypes, which do not typically express TCR, clonality is harder to establish but can be crudely assessed with antibodies that interrogate the killer immunoglobulin receptor expression repertoire. Marrow aspiration or biopsy studies are not usually needed at presentation, as the diagnosis of LGL leukemia is readily established using such blood tests. In situations where the diagnosis is not straightforward (eg, low LGL count), marrow examination can be helpful to aid in diagnosis. Formation of linear arrays seen by immunohistochemical staining with cytotoxic markers such as CD8, TIA-1, granzyme B, or perforin is considered quite characteristic in LGL leukemia (Figure 3).
Blood morphology. (A) Small lymphocytes for comparison. (B and C) Typical LGLs on blood smear. Adapted with permission from Lamy and Loughran.6
Blood morphology. (A) Small lymphocytes for comparison. (B and C) Typical LGLs on blood smear. Adapted with permission from Lamy and Loughran.6
Marrow biopsy for immunostaining. Hematoxylin and eosin stain shows slight hypercellularity with compensatory myeloid hyperplasia. CD3 is a pan T-cell marker expressed in all stages of T-cell development. CD8 is a marker for cytotoxic T cells. GzB is a component of cytotoxic granules. CD57 is a marker of terminal differentiation when expressed on CD8+ T cells. Adapted with permission from Lamy and Loughran.6
Marrow biopsy for immunostaining. Hematoxylin and eosin stain shows slight hypercellularity with compensatory myeloid hyperplasia. CD3 is a pan T-cell marker expressed in all stages of T-cell development. CD8 is a marker for cytotoxic T cells. GzB is a component of cytotoxic granules. CD57 is a marker of terminal differentiation when expressed on CD8+ T cells. Adapted with permission from Lamy and Loughran.6
Clinical features of LGL leukemia
Chronic neutropenia is the most common presenting symptom of LGL leukemia, although other hematologic abnormalities may also be present, with anemia being second most common.6,8 As a direct consequence of these cytopenias, the majority of LGL leukemia patients will require treatment at some point for neutropenia-related infections or, less commonly, transfusion-dependent or symptomatic anemias. Severe persistent neutropenia (absolute neutrophil count <500 cells/µL) is a major risk factor for pneumonia or sepsis in the LGL leukemia patient population, where the median age is approximately 60.4 LGL leukemia occurs very rarely in pediatric populations; anecdotal clinical experience (TPL) suggests that management of such patients may be more challenging. Other more common locations for typical bacterial infections include the skin, oropharynx, and perirectal regions. These symptoms contribute to morbidity in a majority of patients and disease-related deaths in a minority. Anemia secondary to LGL leukemia can have a number of mechanistic explanations, including hemolysis and pure red cell aplasia. In our experience, anemias are often macrocytic even in the absence of reticulocytosis.
Other co-occurring symptoms are more heterogeneous and include splenomegaly, hepatomegaly, and, rather uncommonly, B symptoms (fever, night sweats, or weight loss). Chronic fatigue may be prominent, even in the absence of severe anemia. Approximately 10% to 40% of patients will also have a history of autoimmune disorders, most frequently rheumatoid arthritis (RA), although others such as Sjögren’s syndrome have been observed. With long-term observation, clonal B-cell disorders such as chronic lymphocytic leukemia, follicular lymphoma, hairy cell leukemia, mantle cell lymphoma, and Hodgkin lymphoma may develop.9 Benign monoclonal gammopathy is the most common of these clonal B-cell disorders, whereas myeloma is much less frequent. In 1 series, myeloid neoplasms and solid tumors preceded diagnosis of LGL leukemia in 5% and 15% of patients, respectively.10
Pathogenesis of LGL leukemia
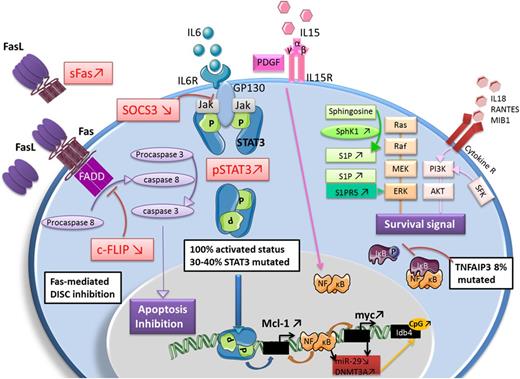
Most molecular insights in LGL leukemia come from the more common T-LGLL subtype. The NK subtype of LGL leukemia is rarer, making it hard to study. Recent genomic sequencing has identified a shared genetic basis in the form of activating mutations in the STAT3 transcription factor in approximately 30% to 40% of patients, regardless of the LGL subtype.11,12 Such mutations occur in the dimerization interface of the Src homology 2 (SH2) domain, with the most frequently observed mutations being Y640F and D661Y. Analogous activating mutations in the STAT5b transcription factor were subsequently found at a much lower frequency (∼2%), and appear to be more common in a rare CD4+ T-LGLL variant.13,14 Mutations in TNFAIP3 encoding the tumor suppressor A20, a negative regulator of NF-KB signaling, appear to be the next most frequent recurrent mutation, being observed in 3/39 patients in 1 series.15 Other mutations in the JAK/STAT (V995M mutation in protein tyrosine phosphatase receptor type T [PTPRT]) and T-cell activation/proliferation pathways (H126R mutation in BCLIIB, W674 stop mutation in Slit homolog 2 protein [SLIT2], and V391M in neuropilin-1 [NRP1] mutation) account for another minority of cases.16 Both STAT3 and STAT5 have major importance in lymphocyte function. Taken together, dysregulation of the STAT pathway likely contributes to the pathogenesis of LGL leukemia.
Constitutive STAT3 activation in all T-LGLL patients was discovered quite some time ago.17 Because an activating mutation is only present in less than half of these patients, the mechanism of activation in these other patients remains uncertain. In such cases, the overlap of LGL leukemia with frequently co-occurring autoimmune disorders may point to additional mechanisms. LGL leukemia patients have elevated serum levels of proinflammatory cytokines, many of which (interleukin-18 [IL-18], IL-8, IL-6, TRAIL, interferon-γ) may contribute to sustaining survival and activation of leukemic LGL in an autocrine fashion.4,18
In T-LGLL patients, the surface marker expression of LGL clones (CD3+/CD8+/CD57+/CD45RA+/CD62L−) is thought to represent a terminal effector memory phenotype and is suggestive of a chronic immune response.19 Therefore, it is speculated that a chronic antigen or autoantigen is the initial driver of LGL expansion. Subsequently, a defect in clearance of this cytotoxic lymphocyte population contributes to the excess accumulation of LGL cells. Mechanisms of dysregulated cell death include resistance to Fas-mediated apoptosis,19 dysregulated sphingolipid signaling,20,21 and platelet-derived growth factor and IL-15 survival signaling21,22 (Figure 4).
Summary of dysregulated cellular pathways in LGL leukemia. Adapted with permission from Lamy et al.8
Summary of dysregulated cellular pathways in LGL leukemia. Adapted with permission from Lamy et al.8
Overlap with RA and Felty's syndrome
Approximately 20% of T-LGLL patients will have RA. Historically, the triad of RA, splenomegaly, and neutropenia defines Felty's syndrome (FS).23 LGL leukemia patients with RA may also have splenomegaly and neutropenia, and may be clinically indistinguishable from FS patients. This clinical overlap, along with a shared predilection for HLA-DR4 in these 2 groups, has led to proposals that the 2 disorders represent a spectrum of a similar disease process.24,25
Pathogenic role of neutrophil lysis in RA
RA is a systemic autoimmune disease characterized by synovial inflammation, progressive joint destruction, and extra-articular manifestations, including ocular, skin, and pulmonary involvement. Whereas patients with RA do not typically suffer from neutropenia unless they develop FS or LGL leukemia, neutrophil lysis is increasingly recognized as playing a pivotal role in underlying RA immunopathogenesis. The importance of neutrophil lysis as a driver of RA immunopathogenesis stems from the role played by neutrophils in driving citrullination, the posttranslational protein modification catalyzed by peptidylarginine deiminase enzymes (PADs) converting arginine to citrulline, a key mechanism in RA pathogenesis. Recent RA research has thus focused on how neutrophil cell death leads to citrullination in RA, with 2 specific hypotheses coming to the forefront: neutrophil lysis via neutrophil extracellular trap (NET) formation versus neutrophil lysis via membranolytic, pore-forming methods, as putative drivers of RA immunopathogenesis.26
NETosis is a specialized form of programmed necrosis of neutrophils in which the dying neutrophil extrudes an extracellular fibril matrix composed of chromatin fibers decorated with azurophilic granule-derived antimicrobial peptides and enzymes. During most forms of NETosis, histone H3 as well as other important intracellular proteins, such as vimentin and α-enolase, undergo peptidylarginine deiminase enzyme–dependent citrullination, which has been suggested to contribute to anticitrullinated protein antibody (ACPA) formation in RA.27-31
Protein citrullination occurs not only in solution, but also on cell-bound targets, including synovial fluid platelet-derived microparticles.32 Those citrullinated proteins, either cell-bound or in solution, may be targeted by autoantibodies to induce immune complex (IC) formation and subsequent FcγR-mediated inflammatory responses.28,32 Indeed, Lood et al31 recently showed that ICs have the capacity to induce NETosis, causing prominent inflammation and autoimmunity. Of relevance to RA are data demonstrating that ICs containing NET-derived citrullinated H2B from the RA synovium activate macrophage tumor necrosis factor α production and propagate NETosis in vitro, as well as induce ACPA-associated arthritis in a mouse model in vivo.31 Neutrophil-bound ICs are more prevalent in FS than in RA patients, so that immunoglobulin G (IgG)–containing ICs may induce spontaneous activation and accelerated clearance of circulating neutrophils via NETosis in FS.27
Despite convincing research linking NETosis with RA disease pathogenesis, the notion of NET formation being sufficient to explain the broad range of citrullinated autoantigens to which ACPAs react to in RA has been questioned. In an attempt to explore the range of citrullinated targets and the mode of cell death relevant to citrullination, Romero et al29 examined a process that they called “hypercitrullination,” the citrullination of multiple intracellular proteins, which the authors showed to occur in cells obtained from RA synovial fluid. Using in vitro reagents, Romero et al29 and, more recently, Zhou et al30 demonstrated that hypercitrullination depends on 2 specific immune-mediated membranolytic pathways (one mediated by complement activation and formation of the membrane attack complex, and the other by activated cytotoxic cells [similar to leukemic LGL] through the perforin-granzyme pathway), and that this pattern of activation was absent in NETosis. Indeed, antineutrophil-targeting antibodies and/or ICs are frequently found in RA synovial fluid33,34 and may participate in activating complement causing membrane attack complex–mediated hypercitrullination. However, precisely how antineutrophil-binding IgG leads to complement-mediated neutrophil lysis and hypercitrullination in RA has yet to be elucidated.
Mechanisms of neutropenia
In general terms, neutropenia may arise from decreased marrow production, increased tissue sequestration or “margination,” and/or increased peripheral destruction (reviewed by Lazaro and Morel35 ).
There are probably several mechanisms of neutropenia in T-LGLL. Bone marrow biopsies in T-LGLL patients show varying degrees of LGL infiltration. Myeloid hypoplasia with paucity of mature forms is sometimes observed, suggestive of a production defect.25 Inflammatory cytokines such as interferon-γ, tumor necrosis factor α, and FAS ligand have been shown to exert inhibitory effects on myelopoiesis,36,37 and can be produced by leukemic LGL. Chronic neutropenia in LGL leukemia may also arise in part due to elevated serum levels of soluble Fas ligand (sFasL). sFasL can trigger apoptosis of neutrophils through the abundant Fas receptors expressed by neutrophils.38 The selectivity of Fas ligand–mediated neutropenia is not clear, as the Fas receptor is expressed on many other cell types. It is conceivable that T-cell receptor recognition of an unknown antigenic peptide on neutrophil lineage cells could cause such specificity. Nevertheless, serum levels of sFasL are a good biomarker of disease activity, and such levels decrease with response to therapy and amelioration of neutropenia.
The marrow pathology in FS patients is most often characterized by myeloid hyperplasia with immature granulocytic precursors, suggestive of compensatory production due to a peripheral survival defect.25 Additionally, splenic pathology can reveal increased plasma cells, likely contributing to the immune complexes observed in patient sera.39 ICs, whether IgM or rheumatoid factor driven, can induce neutrophil apoptosis and increase adherence to endothelial cells, both of which contribute to decreased circulating neutrophils.40,41 Consequently, up to 80% of FS patients experience improvement with splenectomy.42 Manifestations of FS are more often driven by humoral mechanisms with ICs as the dominant contributor to neutropenia.
Treatment of LGL leukemia
Because pathogenesis of LGL leukemia is attributed to sustained activation of cytotoxic cells, immunosuppressive regimens are the mainstay of treatment. Indications for treatment include persistent severe neutropenia (<500/µL), symptomatic or transfusion-dependent anemia, and RA requiring immunomodulatory therapy. Current first-line therapeutic options include methotrexate (MTX), cyclophosphamide (CP), and cyclosporine A (CyA). As such therapies work slowly, they should be continued if tolerated for at least 4 months to determine response (Table 1). In certain circumstances, prednisone can be added if a faster response is needed (eg, severe neutropenia <100/μL) or to help control brisk hemolytic anemia. We prescribe prednisone at 1 mg/kg by mouth daily for 30 days followed by taper to off at the end of the next 24 days. Overall response rates for any of these first-line therapies are approximately 50% to 60% in retrospective studies.6,10 Complete remission appears to be highest with CP, with reports of up to half of patients.43 However, due to concerns of mutagenic potential, the cumulative treatment should be limited to no more than 12 months. In contrast, MTX and CyA are generally safer and can be maintained indefinitely, as long as they are well tolerated. In the only prospective trial to date, the response rate to first-line MTX was 38%, whereas the response rate to second-line treatment with CP was 64%.4 This indicates that many patients may respond to CP even after failing MTX. In that study, MTX was discontinued in responding patients after 1 year of therapy. Almost all of these patients had slow recurrence of disease after MTX was discontinued. Of note, a randomized prospective trial of MTX versus CP for initial therapy is being conducted in France.
Evaluation of treatment response
| Complete clinical remission . | Parameter . |
|---|---|
| Hemoglobin, g/dL | >12 |
| Platelets | >150 × 109/L |
| Absolute neutrophil count | >1.5 × 109/L |
| Lymphocyte count | <4 × 109/L |
| LGL count | In the normal range |
| Complete molecular remission | For T-LGLL, disappearance of T-cell clone using polymerase chain reaction |
| Partial response | Any improvement in blood counts that do not satisfy complete remission |
| Treatment failure | No improvement within 4 months after start of treatment |
| Complete clinical remission . | Parameter . |
|---|---|
| Hemoglobin, g/dL | >12 |
| Platelets | >150 × 109/L |
| Absolute neutrophil count | >1.5 × 109/L |
| Lymphocyte count | <4 × 109/L |
| LGL count | In the normal range |
| Complete molecular remission | For T-LGLL, disappearance of T-cell clone using polymerase chain reaction |
| Partial response | Any improvement in blood counts that do not satisfy complete remission |
| Treatment failure | No improvement within 4 months after start of treatment |
In the prospective Eastern Cooperative Oncology Group trial, the median overall survival for patients with anemia was 69 months, whereas median overall survival for patients with neutropenia had not been reached 13 years after study activation. In the largest single-institution experience, median overall survival for all patients was 166 months.10 Of interest, there was a longer overall median survival in patients with STAT3 mutation. In that study, patients who required more than 3 lines of therapy had poorer survival. Salvage therapy for patients not responding to sequential single-agent MTX, CP, and CyA was needed in 10% to 20% of patients. Various salvage regimens in small numbers of patients were described, including anti-thymocyte globulin, Campath, tofacitinib, abatacept, and splenectomy. A series of 15 patients with refractory cytopenias showed hematologic response in all patients undergoing splenectomy, although long-term outcomes remain undefined.44
Appropriate physician judgment is required to tailor an individualized clinical assessment and treatment plan. Active and ongoing research in LGL leukemia is made possible by the establishment of a growing national registry for LGL leukemia. Clinicians with patients suspected to have this disorder are encouraged to contact us (tploughran@virginia.edu).
Acknowledgments
The authors are grateful for assistance from Jeffrey Xing and Dr. David Feith in the preparation and revision of this manuscript.
Correspondence
Thomas P. Loughran, Jr., University of Virginia Cancer Center, PO Box 800334, Charlottesville, VA 22908-0334; e-mail: tploughran@virginia.edu.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.