Abstract
Primary mediastinal B-cell lymphoma (PMBCL) is recognized as a distinct clinicopathologic entity that predominantly affects adolescents and young adults and is more common in female subjects. Although PMBCL is considered to be a subtype of diffuse large B-cell lymphoma, its clinical, morphologic, and biological characteristics overlap significantly with those of nodular sclerosing Hodgkin lymphoma (NSHL). Over the past few years, the shared biology of these 2 entities has been highlighted in several studies, and mediastinal gray zone lymphoma, with features intermediate between PMBCL and NSHL, has been recognized as a unique molecular entity. Although there is a lack of consensus about the optimal therapeutic strategy for adolescent and young adult patients newly diagnosed with PMCBL, highly curative strategies that obviate the need for mediastinal radiation are favored by most. Progress in understanding the biology of PMBCL and its close relationship to NSHL have helped pave the way for the investigation of novel approaches such as immune checkpoint inhibition. Other strategies such as adoptive T-cell therapy and targeting CD30 are also being studied.
Learning Objectives
Understand recent novel advances in the biology of primary mediastinal B-cell lymphoma and implications with respect to novel treatments
Approach the treatment of primary mediastinal B-cell lymphoma optimally and understand the emerging role of novel agents
Introduction
Primary mediastinal B-cell lymphoma (PMBCL) represents ∼10% of all diffuse large B-cell lymphomas (DLBCL) but a much higher proportion of lymphomas in the adolescent and young adult population. Although categorized as a subtype of DLBCL, PMBCL is demographically, clinically, and biologically distinct from other DLBCL subtypes. Its clinical and biological features more closely resemble those of nodular sclerosing Hodgkin lymphoma (NSHL) arising in the mediastinum. In fact, PMBCL and NSHL share approximately one-third of their genes as well as common driver mutations, which have been identified.1,2 B-cell lymphomas arising in the mediastinum with features intermediate between PMBCL and NSHL have been termed mediastinal gray zone lymphomas (MGZL). These are exceedingly rare, affect male subjects more than female subjects (in contrast to PMBCL), and studies that have focused on their biology, albeit few, suggest that MGZL is a unique molecular entity, distinct from the parent entities.3
The optimal management of adolescent and young adult patients with PMBCL is controversial owing to the rarity of this disease and the relatively recent recognition that it represents a distinct entity. While historically, most approaches included consolidation mediastinal radiation, attempts have been made to move away from this paradigm and eliminate the risk of radiation’s long-term side effects. Recently, increased dose intensity approaches have obviated the need for radiation while maintaining high cure rates. Mediastinal B-cell lymphomas harbor several interesting targetable pathways, and studies are ongoing evaluating the role of specific small molecule inhibitors and strategies such as immune checkpoint blockade.
Recent biological insights in PMBCL
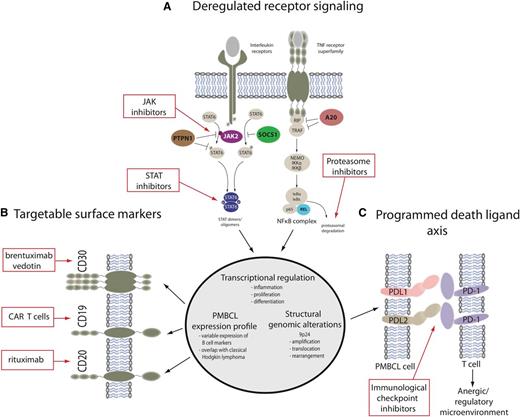
Although PMBCL has historically been considered a subtype of DLBCL, the World Health Organization’s classification of hematopoietic and lymphoid tumors recognizes it as a distinct entity based on its clinical and immunophenotypical presentation and a gene expression profile that is markedly different from the germinal center B-cell and activated B-cell subtypes of DLBCL.4 Mediastinal B-cell lymphomas, putatively derived from a thymic B cell, can be considered to lie on a pathobiologic spectrum of diseases, with NSHL (typically CD15- and CD30-positive) and PMBCL (CD20-positive) lying on either end and MGZL (with features intermediate and transitional between the parent entities) lying in between (Figure 1).5 Recently, many insights into the biology of PMBCL have paved the way for the investigation of novel agents and new approaches in this disease; some of the most helpful advances have been improved understanding of genetic alterations and perturbations in the Janus kinase/signal transducers and activators of transcription (JAK-STAT) and nuclear factor-κB (NF-κB) pathways, as well as the emerging recognition that mediastinal lymphomas are “immune privileged” with the ability to avoid immune destruction.6 These recently discovered genetic alterations underlie phenotypic characteristics of these diseases and are at play right across the aforementioned pathobiologic spectrum (Figure 2). They provide evidence that these entities are molecularly related and likely derived from a common cellular origin (thymic B cell).2,7
(A) Pathobiologic spectrum of mediastinal B-cell lymphomas. (B) Although NSHL is typically CD15 and CD30 positive, and PMBL is CD20 positive, there are mediastinal lymphomas in between these 2 entities with phenotypic and biologic features intermediate and transitional between NSHL and PMBL. These diseases are called mediastinal gray zone lymphomas (MGZLs).
(A) Pathobiologic spectrum of mediastinal B-cell lymphomas. (B) Although NSHL is typically CD15 and CD30 positive, and PMBL is CD20 positive, there are mediastinal lymphomas in between these 2 entities with phenotypic and biologic features intermediate and transitional between NSHL and PMBL. These diseases are called mediastinal gray zone lymphomas (MGZLs).
Targetable molecular features of primary mediastinal large B-cell lymphoma and mediastinal gray zone lymphoma. (A) The main activation cascades of JAK-STAT and NF-κB signaling are shown leading to altered transcriptional regulation. Only representative pathway molecules are displayed. Known gene alterations in the pathway are highlighted in color, and pathway inhibitors are shown in the red boxes. (B) A selection of surface markers as part of a PMBCL-specific expression profile is depicted alongside targeted therapeutic approaches. (C) Structural genomic alterations of chromosome 9p24 lead to overexpression of programmed death ligands that are potentially amenable to immunological checkpoint inhibition. Dunleavy and Steidl.7
Targetable molecular features of primary mediastinal large B-cell lymphoma and mediastinal gray zone lymphoma. (A) The main activation cascades of JAK-STAT and NF-κB signaling are shown leading to altered transcriptional regulation. Only representative pathway molecules are displayed. Known gene alterations in the pathway are highlighted in color, and pathway inhibitors are shown in the red boxes. (B) A selection of surface markers as part of a PMBCL-specific expression profile is depicted alongside targeted therapeutic approaches. (C) Structural genomic alterations of chromosome 9p24 lead to overexpression of programmed death ligands that are potentially amenable to immunological checkpoint inhibition. Dunleavy and Steidl.7
JAK-STAT pathway
Several functional genomic studies in PMBCL and NSHL have identified that JAK-STAT signaling activation is a characteristic feature of these 2 entities.8 In PMBCL, JAK-STAT signaling likely depends on both interleukin-13 receptor-mediated signaling, as well as constitutive activation resulting from various somatic gene mutations; among these are amplifications of JAK2, deletions or inactivating mutations of the negative regulators SOCS1 and PTPN1, and mutations of STAT6.9 More than 50% of PMBCL cases have genomic gains of chromosome 9p containing a locus for JAK2, and the minimally amplified region contains multiple genes, including JAK2 and the programmed death ligands CD274 (PDL1), PDCD1LG2 (PDL2), and JMJD2C,10 which contribute synergistically to the pathogenesis of PMBCL.6 Somatic mutations of SOCS1 have a similar frequency in PMBCL and are also found in a significant proportion of classic Hodgkin lymphoma cases, highlighting the biologic overlap of these entities.11-13 PTPN1 is another negative regulator of JAK-STAT signaling, and mutations in this gene have been identified in ∼20% of PMBCL and classic Hodgkin lymphoma.2 Evidence suggests that point mutations in STAT6 may transcriptionally contribute to the pathogenesis of PMBCL, and these have been reported in 36% of PMBCL cases.14 NF-κB genes are typically activated in PMBCL with nuclear translocation of c-REL in most cases.15
Tumor microenvironment in PMBCL
The role of nontumor cells in cross-talk and signaling to tumor cells is well recognized in the lymphoma microenvironment. In PMBCL, this microenvironment can be highly variable, closely resembling NSHL (high cell diversity and sparse tumor cells) at one end and DLBCL (high tumor cell content with sheeting out of these cells) at the other end of a spectrum.16 Immune privilege in PMBCL likely results from downregulation of major histocompatibility complex class I and II molecules (which frequently occurs in PMBCL), as well as increased expression of programmed death ligands; this scenario results in reduced immunogenicity and T-cell anergy.17,18 Recently, the genetic basis of these expression phenotypes has been partly elucidated. Many of these genetic features are also present in DLBCL arising in classic immune privilege sites such as primary testicular DLBCL and primary central nervous system lymphoma, which suggests biologic overlap between PMBCL and classic immune-privileged DLBCL.
PDL2 and its close paralog (PDL1) are both critical target genes of chromosome 9p gains and amplifications that are found in >50% of PMBCL cases.19,20 Next-generation sequencing and fluorescence in situ hybridization analysis showed that 20% of PMBCL cases harbor recurrent genomic rearrangements involving the 9p locus resulting in PDL1 and PDL2 gene fusions.20,21 PDL1/PDL2 expression is higher in the rearranged cases compared with cases with gains or amplifications.20 In PMBCL, the co-amplification of JAK2 and the programmed death ligand locus on chromosome 9p24 suggest that JAK-STAT signaling and acquired immune privilege are synergistic in lymphomagenesis. Therefore, strategies that combine JAK-STAT and immune checkpoint inhibition may be particularly helpful in these diseases.
Management of PMBCL and MGZL
Because these mediastinal lymphomas are rare and have only been described relatively recently, there are very few studies and no prospective randomized comparisons to inform on optimal up-front therapy (Table 1). Early studies in PMBCL suggested that consolidation mediastinal radiation was a critical component of curative therapy and for this reason, radiation remains part of standard up-front approaches in a significant proportion of cases. This approach is problematic, however, given that this disease has a predilection for young female subjects and hence, after mediastinal radiation, there is a high long-term risk of secondary malignancies, particularly breast cancer, as well as ischemic heart disease.22 Although some proponents of radiation argue that secondary malignancies and other morbidities after radiation will be less with more focused radiation fields and lower doses, this theory has not been definitively proven and may only be assessed with longer time and follow-up.23 Therefore, at this point, it is critical to develop approaches that maintain very high cure rates but obviate the need for radiation in the majority of patients.
Selected recent studies treating PMBCL
| Study . | Treatment . | Study type . | Patient characteristics . | Outcome . | |
|---|---|---|---|---|---|
| Chemotherapy . | RT± . | ||||
| Savage et al26 | CHOP/R-CHOP /MACOP- B/VACOP-B | Variable | Retrospective study, N = 153 | All IPI groups | At 5 y, PFS was 69%. Only MACOP-B/VACOP-B vs CHOP-like regimens were significantly different |
| Rieger et al24 | CHOP/R-CHOP | Variable, RT intended in 87% | Retrospective analysis, N = 87 | Confined to AA-IPI of 0-1 | At 3 y, EFS was 78% for R-CHOP and 52% for CHOP |
| Soumerai et al25 | R-CHOP | Yes, 77% of responding patients | Retrospective study, N = 63 | All IPI groups | At 5 y, PFS was 68% |
| Dunleavy et al28 | DA-EPOCH-R | No | Prospective study, N = 51 | All IPI groups | At 5 y, EFS was 93% |
| Martelli et al31 | R-MACOP-B, R-VACOP-B, R-CHOP | Yes | Prospective study, N = 125 | All IPI | Estimated 5 y PFS is 86% |
| Gleeson et al27 | R-CHOP-14 vs R- CHOP-21 | Yes | Retrospective analysis, N = 50 | Confined to stage I and II | 5 y PFS, 79.8% |
| Study . | Treatment . | Study type . | Patient characteristics . | Outcome . | |
|---|---|---|---|---|---|
| Chemotherapy . | RT± . | ||||
| Savage et al26 | CHOP/R-CHOP /MACOP- B/VACOP-B | Variable | Retrospective study, N = 153 | All IPI groups | At 5 y, PFS was 69%. Only MACOP-B/VACOP-B vs CHOP-like regimens were significantly different |
| Rieger et al24 | CHOP/R-CHOP | Variable, RT intended in 87% | Retrospective analysis, N = 87 | Confined to AA-IPI of 0-1 | At 3 y, EFS was 78% for R-CHOP and 52% for CHOP |
| Soumerai et al25 | R-CHOP | Yes, 77% of responding patients | Retrospective study, N = 63 | All IPI groups | At 5 y, PFS was 68% |
| Dunleavy et al28 | DA-EPOCH-R | No | Prospective study, N = 51 | All IPI groups | At 5 y, EFS was 93% |
| Martelli et al31 | R-MACOP-B, R-VACOP-B, R-CHOP | Yes | Prospective study, N = 125 | All IPI | Estimated 5 y PFS is 86% |
| Gleeson et al27 | R-CHOP-14 vs R- CHOP-21 | Yes | Retrospective analysis, N = 50 | Confined to stage I and II | 5 y PFS, 79.8% |
DA-EPOCH-R, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin, plus rituximab; EFS, event-free survival; IPI, international prognostic index; OS, overall survival; R-MACOP-B, rituximab and methotrexate with leucovorin rescue, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin; R-VACOP-B, rituximab with etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin.
Although rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is considered to be the de facto standard for other subtypes (ie, germinal center B-cell and activated B-cell subtypes) of DLBCL, published experience with this regimen in PMBCL is limited to retrospective studies or retrospective evaluations of prospective studies.24-27 The largest evaluation thus far of R-CHOP in PMBCL was a subgroup analysis of the randomized MInT (MabThera International Trial), in which most patients received R-CHOP.24 Although the 3-year event-free survival was 78%, 73% of patients received preplanned radiation therapy and, importantly, the analysis was confined to patients aged <60 years with an age-adjusted IPI score of 0 to 1. Recently, a British subset analysis looked at the outcome of 50 patients with PMBCL treated in a randomized study of R-CHOP-21 vs R-CHOP-14; all the patients included in this analysis with PMBCL had stage I or II disease and although the 5-year progression-free survival was 79.8%, 58% of patents received radiation therapy.27 One other retrospective analysis of 58 patients with PMBCL who received R-CHOP at a single institution reported a progression-free survival of 68% at 5 years, with 77% of responders receiving radiation. This study was not restricted to any particular risk group, and patients with high IPI scores and extra-mediastinal disease fared significantly worse than those with low IPI scores and localized involvement.25
Many “dose-intense” approaches have been tested in this disease, and considering the close clinical and biological relationship of NSHL and PMBCL, more dose-intense regimens than R-CHOP may be more effective and could reduce the reliance on mediastinal radiation for curative outcome. DA-EPOCH-R, without radiation, demonstrated an EFS and OS of 93% and 97%, respectively, in an initial prospective study of 53 patients; with long-term follow-up of a larger cohort, these results were maintained.28 The critical feature with this approach is the nonreliance on radiation while maintaining high cure rates, and appreciating that no prospective randomized comparison of treatments in PMBCL exists (for the aforementioned reasons), DA-EPOCH-R is a promising and effective standard in this disease. This regimen was studied in a German series of pediatric patients with PMBCL and, albeit early results of that series are available, the approach was highly effective with no need for radiation.29 A prospective, international study of the regimen in pediatrics was recently completed, and results from this study should soon be available.
MGZLs are exceedingly rare, and their treatment has not been well studied; previously, many of these were identified as “anaplastic large-cell lymphoma Hodgkin-like” and had a poor outcome. Their indeterminate pathobiology has led to uncertainty about how they should be optimally approached. A prospective study using the DA-EPOCH-R regimen in MGZL cases that were CD20 positive reported an EFS and OS of 62% and 74%, respectively, which were inferior compared with the outcome of PMBCL.30 Studies assessing the unique molecular characteristics and biology of MGZL are in progress.
End-of-therapy FDG-PET
End-of-treatment response assessment is 1 of the most challenging steps in the management of PMBCL.31 It is common to have a residual mediastinal mass on computed tomography imaging after completion of curative therapy, and although fluorodeoxyglucose–positron emission tomography (FDG-PET) imaging may be helpful in further assessing these cases (and has a high negative predictive value), it has a high rate of false-positive results. After DA-EPOCH-R in PMBCL, a recent long-term follow-up showed that 33% of cases had a positive end-of therapy (EOT) FDG-PET (Deauville score 4 or 5), but just 20% of these cases had disease present. How best to approach EOT FDG-PET–positive cases is unclear, and the proportion of true-positive cases may differ according to the regimen used. After DA-EPOCH-R, cases that had a Deauville score of 5 had the highest positivity for disease, and thus one should have a low threshold to perform a biopsy in these cases. Other positive cases (Deauville score of 4) should at least be monitored closely, with a repeat FDG-PET scan soon after the positive EOT FDG-PET scan; if the standardized uptake values are increasing, there should be a low threshold for performing a biopsy. An ongoing randomized PMBCL study by the International Extranodal Lymphoma Study Group is investigating if an EOT-negative FDG-PET can identify patients who do not require radiation. The high rate of false-positive EOT PET suggests that alternative modalities to FDG-PET could be helpful in managing this disease and should be explored in future trials.
Novel agents and emerging strategies
Although removing radiation from the equation in up-front therapy is an important goal in PMBCL therapeutics, for both (radiation-naive) refractory and relapsed cases with disease confined to the mediastinum and amenable to a radiation therapeutic field, radiation (alone or as part of combined modality therapy) should usually be considered as a curative strategy. Radiation boosts may occasionally be helpful in patients in whom disease has recurred at a site of previous irradiation. For advanced-stage refractory/relapsed PMBCL, there is no standard approach, but transplantation-based treatments or novel strategies/approaches (summarized here) should be considered.
With recent strides (as discussed earlier) in understanding the molecular biology of PMBCL and MGZL, several new targets that are amenable to novel agents and strategies have been identified. One should keep in mind the close clinical and biological relationship of PMBL and MGZL to NSHL because new agents that are effective in Hodgkin lymphoma may be helpful in these related mediastinal lymphomas. Recently, a phase 2 study of brentuximab vedotin, an antibody drug conjugate consisting of a chimeric anti-CD30 monoclonal antibody, was performed in patients with relapsed and refractory PMBCL.32 Although this agent has a high response rate in Hodgkin lymphoma, its response rate in PMBCL was just 13.3%.
A high proportion of PMBCL cases have amplification on a region of chromosome 9p24 where targets such as the PD1 ligands programmed cell death ligand 1 (PD-L1) and PD-L2 and Janus kinase 2 (JAK2)10,19,33 are located. The PD1 checkpoint pathway is likely to enable evasion of the antitumor immune response, and immune checkpoint inhibitors have shown exciting activity in a wide range of cancers, including Hodgkin lymphoma.34 Recently, pembrolizumab was tested in patients with relapsed and refractory PMBCL.35 In 18 patients, in whom the median number of previous therapies was 3, the overall response rate to the drug was 41%, with 2 patients reaching the maximum 2-year treatment duration and remaining in remission. Pidilizumab, a humanized monoclonal antibody to PD1, was tested in a phase 2 study in relapsed/refractory aggressive B-cell lymphoma, where it was given as consolidation therapy after autologous transplantation in high-risk patients.36 Although patients with PMBCL were included, the primary end point of the study (to prevent disease relapse) makes assessing the activity of pidilizumab difficult. Other immune checkpoint inhibitors are in development in PMBCL.37 JAK-STAT signaling is important in both PMBCL and Hodgkin lymphoma, and inhibitors of this pathway can decrease PMBL and Hodgkin lymphoma growth in vitro and in vivo.38 As discussed earlier, combining JAK-STAT inhibition with immune checkpoint inhibition may be a very promising strategy in these diseases.
There are emerging data that T cells genetically modified to express chimeric antigen receptors targeting CD19 have promising activity in diffuse large B-cell lymphoma, including PMBCL, and final results of multicenter studies are awaited.39 Recently, in an interim analysis of >100 patients with relapsed/refractory aggressive B-cell lymphoma who received 1 of the chimeric antigen receptor–CD19 platforms, response was seen in >75%, with almost 50% achieving a complete response.40 A sizeable proportion of these cases had PMBCL, and similar activity has been observed with other anti CD19 platforms.41
Conclusions
PMBCL (and MGZL) represent distinct clinicopathologic entities that should have a distinct management approach, compared with the other subtypes of DLBCL. These diseases have a high cure rate with current standard strategies, but because these diseases predominantly affect young female subjects, it is critical to continue to develop strategies that are highly effective but eliminate the need for mediastinal radiation. There have been many advances in understanding the molecular biology of these lymphomas and, in particular, realizing that the role of PD1 ligands and JAK-STAT pathways has paved the way for the investigation of novel therapies and approaches in PMBCL and MGZL.
Correspondence
Kieron Dunleavy, George Washington University Cancer Center, 2300 I St NW, Washington, DC 20037; e-mail: kdunleavy@mfa.gwu.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.