Abstract
Based on very high response rates in the relapsed and refractory setting, brentuximab vedotin and the programmed cell death protein 1 (PD-1) inhibitors, nivolumab and pembrolizumab, have quickly been incorporated into clinical trials for first- and second-line therapy of Hodgkin lymphoma. Preliminary data show that brentuximab vedotin alone is not adequate therapy for newly diagnosed Hodgkin lymphoma in older patients, but modestly decreases the risk of relapse when combined with adriamycin, vinblastine, and dacarbazine in patients with previously untreated advanced-stage disease. In second-line therapy, combining brentuximab vedotin with conventional chemotherapy or with PD-1 inhibitors as pretransplant salvage is associated with high overall and complete response rates, although further follow up is needed to assess whether posttransplant outcomes are improved. Although these new drugs are well tolerated when given as single agents, unexpected toxicities have been encountered with combination regimens, specifically severe pulmonary toxicity with the bleomycin and brentuximab vedotin combination and frequent infusion-related reactions. There is concern with the use of PD-1 inhibitors as first-line therapy due to the theoretical potential for more frequent or severe immune-mediated toxicities in patients who have not received prior chemotherapy. Aside from these concerns, these new agents have the potential to improve outcomes for patients even further, bringing us closer to eradicating recurrent Hodgkin lymphoma.
Learning Objectives
Recognize the approved indications for brentuximab vedotin and PD-1 inhibitors for relapsed or refractory Hodgkin lymphoma
Evaluate preliminary data using brentuximab vedotin and PD-1 inhibitors in first- and second-line regimens for Hodgkin lymphoma
Introduction
A new era in the treatment of Hodgkin lymphoma (HL) has begun. Approval of the antibody drug conjugate brentuximab vedotin in 2011, and the programmed cell death protein 1 (PD-1) inhibitors nivolumab and pembrolizumab in 2016 and 2017, respectively, has improved the outlook for patients with multiple relapsed and refractory HL. Studies incorporating these highly active agents into earlier lines of therapy in an effort to improve cure rates and avoid the toxicities associated with subsequent treatments are ongoing. Challenges include accurately identifying those most likely to benefit from new approaches and understanding how to safely combine these agents with conventional chemotherapy. Given the success of existing treatments in terms of both efficacy and safety, for early- and advanced-stage HL with nearly all patients still alive 5 years after initial diagnosis, incorporating new agents or replacing old agents should be done with caution in carefully monitored trials. Is there a role for these agents in all newly diagnosed patients with HL? Can new drugs be used in early-stage disease to avoid radiotherapy without adding substantial toxicity? Should new agents be used in first-line therapy only for those with a positive interim positron emission tomography (PET)? What is the best combination of new and old agents in the pretransplant salvage setting? These novel therapies hold great promise for improving outcomes in HL, but it will require substantial effort to clearly delineate the optimal use of these agents.
Approved indications for brentuximab vedotin, nivolumab, and pembrolizumab
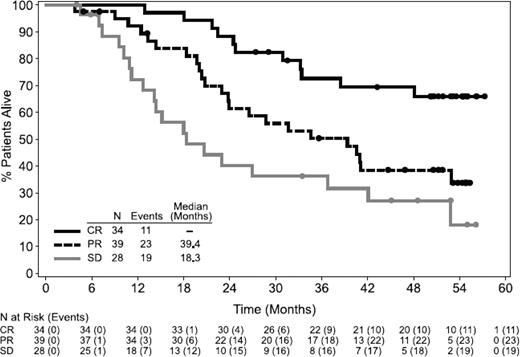
Brentuximab vedotin, an antibody drug conjugate targeting CD30, is currently approved for relapsed or refractory HL after an autologous stem cell transplant (ASCT) or following 2 prior lines of therapy. In the initial pivotal phase 2 study in 102 patients with relapsed HL, the overall response rate (ORR) was 75%, with a 34% complete response (CR) rate.1 Long-term follow-up of this study showed 47% of patients remain alive at a median follow-up of 3 years, and for the 34 patients who achieved a CR, the 3-year progression-free survival (PFS) was 58% and overall survival (OS) 73%.2 Remarkably, 47% of the patients who achieved complete remission remained in remission at a median follow-up of 53 months (Figure 1). Of the 16 CR patients who remained in long-term remission, 4 underwent allogeneic SCT in CR after brentuximab vedotin and 12 had no further therapy. For the 34% of patients who achieve CR with single-agent brentuximab vedotin after failing ASCT, delaying consideration of reduced-intensity allogeneic SCT until a subsequent relapse is a reasonable option, given the possibility that a substantial minority of these patients may be cured. Retreatment response rates to brentuximab vedotin are 60%, with 30% of patients achieving CR.3
OS following treatment with brentuximab vedotin. OS was analyzed using Kaplan–Meier methodology and is shown by best response. All censored patients are indicated by dots on the Kaplan–Meier curve. Reprinted from Gopal AK, Blood, 2015;125(8):1236-1243. Copyright (2015) The American Society of Hematology. Reprinted with permission.2
OS following treatment with brentuximab vedotin. OS was analyzed using Kaplan–Meier methodology and is shown by best response. All censored patients are indicated by dots on the Kaplan–Meier curve. Reprinted from Gopal AK, Blood, 2015;125(8):1236-1243. Copyright (2015) The American Society of Hematology. Reprinted with permission.2
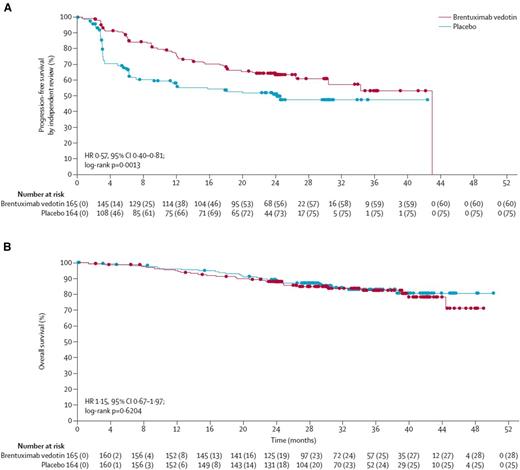
Brentuximab vedotin as adjuvant therapy following ASCT in high-risk patients is also an approved indication. The phase 3 AETHERA trial compared post-ASCT brentuximab vedotin (1.8 mg/kg every 3 weeks for 16 cycles) to observation in high-risk patients who had either not achieved a CR with initial chemotherapy, progressed within 1 year following initial treatment, or had extranodal involvement at relapse.4 The median PFS was 42.9 months for those receiving adjuvant brentuximab vedotin and 24.1 months for those who were observed posttransplant [hazard ratio (HR) 0.57, P = .0013] with 2-year PFS of 63% and 51%, respectively (Figure 2). There was significantly more neuropathy (56%) and neutropenia (35%) in the brentuximab vedotin arm. Approximately one-third of patients had to discontinue therapy prematurely for adverse events. Importantly, there was no difference in overall survival and the question has been raised as to whether similar outcomes could have been achieved if patients were treated with brentuximab vedotin at the time of recurrence post-ASCT. Questions also remain as to the benefit of maintenance brentuximab vedotin in patients who are considered high risk according to the eligibility criteria for the AETHERA trial, but who achieved a complete metabolic response (CR by PET) prior to ASCT. AETHERA did not require a PET prior to ASCT and the study was not powered to answer this question, but in the subgroup analysis of PET-negative patients the HR was not significant.
Progression-free and overall survival analyses Kaplan–Meier plots showing the primary endpoint of PFS by independent review (A), and interim analysis of overall survival (B). Filled circles show censored patients. Reprinted from Younes A, Lancet. 2015;385(9980):1853-1862. Copyright (2015) Elsevier Limited. Reprinted with permission.4
Progression-free and overall survival analyses Kaplan–Meier plots showing the primary endpoint of PFS by independent review (A), and interim analysis of overall survival (B). Filled circles show censored patients. Reprinted from Younes A, Lancet. 2015;385(9980):1853-1862. Copyright (2015) Elsevier Limited. Reprinted with permission.4
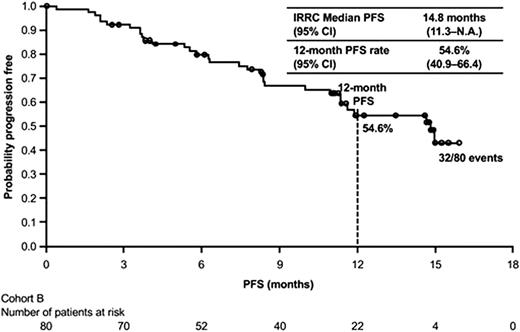
The PD-1 inhibitors, nivolumab and pembrolizumab, are also Food and Drug Administration (FDA)-approved for refractory or multiple relapsed HL. The nivolumab approval is specifically for patients who have failed both ASCT and posttransplantation brentuximab vedotin, and the pembrolizumab approval is for patients with refractory disease or those who have failed at least 3 prior lines of therapy. The initial report on the phase 1 study of nivolumab in 23 patients with recurrent or refractory HL showed a remarkable 87% ORR by investigator assessment, with 17% complete remissions.5 An 80-patient phase 2 study, CheckMate 205, of single-agent nivolumab dosed at 3 mg/kg every 2 weeks confirmed an ORR by central review of 66.3% with 9% CRs.6 The initial results of CheckMate 205 (median follow-up 8.9 months) showed a median remission duration of 7.8 months, which improved to 13.1 months with longer follow-up. Figure 3 shows the updated PFS curve for this study.6,7 The most recent update of CheckMate 205 showed similar response rates and duration of response (DOR) for patients who were brentuximab vedotin naïve (65%, median DOR 20 months), patients who had prior brentuximab vedotin following ASCT (68%, median DOR 16 mo), and patients who received brentuximab vedotin before or after ASCT (73%, median DOR 15 months).8 Response rates are similar for pembrolizumab, 200 mg every 3 weeks, at 69% with 22% complete remissions and median remission duration of 11 months.9 The subset of patients with primary refractory HL had an ORR of 79% with 23% CRs and in the 81 patients who were transplant-ineligible, who had failed prior brentuximab vedotin, the ORR was 64.2% with 24.7% CRs . The PD-1 inhibitors have been very well tolerated in the relapsed setting, with rare reports of grade 3 or 4 toxicities, including immune-mediated events, and represent an excellent option for patients failing ASCT and brentuximab vedotin. An ongoing randomized phase 3 trial is comparing pembrolizumab to brentuximab vedotin in patients with relapsed or refractory HL, including patients who have failed ASCT, are ASCT-ineligible due to refractory disease following at least 2 lines of therapy, or aged ≥65 years (NCT02684292). A phase 3 confirmatory study for nivolumab is also ongoing comparing nivolumab plus brentuximab vedotin to brentuximab vedotin alone in patients with relapsed or refractory HL following ASCT or who are transplant ineligible (CheckMate 812, NCT03138499).
PFS Kaplan–Meier plot showing the primary endpoint of PFS by independent review. Reprinted from Timmerman J, Blood. 2016;128(22):1110. Copyright (2016) The American Society of Hematology. Reprinted with permission.7
PFS Kaplan–Meier plot showing the primary endpoint of PFS by independent review. Reprinted from Timmerman J, Blood. 2016;128(22):1110. Copyright (2016) The American Society of Hematology. Reprinted with permission.7
Brentuximab vedotin and PD-1 inhibitors at first relapse
Given the remarkably high single-agent response rates in the multiple relapsed and refractory setting, there has been significant interest in incorporating these active agents into earlier lines of therapy. Although not yet an FDA-approved indication, preliminary results of several studies using brentuximab vedotin either as a single agent or in combination as pretransplant salvage have been reported. Both the City of Hope and Memorial Sloan-Kettering Cancer Center have explored single-agent brentuximab vedotin at first relapse, followed by standard salvage chemotherapy, typically ifosfamide, carboplatin, and etoposide (ICE) or augmented ICE, for patients not achieving complete remission prior to ASCT.10,11 In the phase 2 study by Moskowitz et al, 27% of patients with first relapse of HL achieved a complete remission with 2 cycles of brentuximab vedotin, where a CR was defined as a Deauville score of 1 or 2, and went on to receive an ASCT without further salvage.10 The 73% of patients who did not achieve a CR received 2 cycles of augmented ICE prior to SCT. Overall, the PET-negative rate with either brentuximab vedotin alone or brentuximab vedotin followed by augmented ICE was 73%, and the 2-year event-free survival following ASCT was 90%.
Because the sequential approach still results in the majority of the patients undergoing intensive salvage therapy prior to ASCT, additional studies are exploring combination therapy with new agents. Preliminary results of the combination of brentuximab vedotin and bendamustine as pretransplant salvage showed an ORR of 93% with a CR rate of 74% and a 1-year PFS of 80%.12 The complete remission rates were 84% in those with relapsed disease and 64% in those with primary refractory disease. Surprisingly, 56% of patients had infusion related reactions (IRR), including fevers, chills, dyspnea, nausea, flushing, and hypotension, a significantly higher rate than seen with either agent individually. Premedication with corticosteroids and antihistamines decreased the incidence and severity of IRR. Phase 1/2 studies are also ongoing with brentuximab vedotin and ICE chemotherapy in the Lymphoma Study Association (brentuximab vedotin 1.2 to 1.8 mg/kg D1 with standard ICE) and at the University of Washington (brentuximab vedotin 1.5 mg/kg D1, 8 with standard ICE) with a 94% ORR and 88% CR rate reported in the first 16 patients treated13,14 ). Preliminary results of a phase 2 trial of brentuximab vedotin (1.8 mg/kg D1) and etoposide, methylprednisolone (Solu-Medrol), cytarabine (Ara-C), cisplatin (platinum) (ESAHP) showed an ORR of 96% and a 70% CR rate.15 Given the limited number of cycles of therapy pre-ASCT, peripheral neuropathy has not been dose-limiting despite the combination of brentuximab vedotin and platinum agents. A phase 2 study of nivolumab in combination with ICE as pretransplant salvage is also ongoing (NCT03016871).
The combination of brentuximab vedotin and nivolumab at first relapse has also been studied in a phase 1/2 study of 62 patients with an ORR of 85% and a CR rate of 64% (Deauville 1–3).16 Four of 62 patients required steroids for immune-mediated toxicities, including colitis and pneumonitis, although the incidence of grade 3 and 4 toxicity was low at 7%. As was seen with the brentuximab vedotin and bendamustine combination, there was a higher than expected rate of IRRs (41%), which happened most frequently with the cycle 2 brentuximab vedotin infusion. Interestingly, the incidence of IRRs with single-agent nivolumab (20%) or single-agent brentuximab vedotin (<10%) is substantially lower than the combination.1,6 A phase 1 study of the brentuximab vedotin and nivolumab combination was also reported by Eastern Cooperative Oncology Group (ECOG) investigators.17 In this more heavily pretreated group of patients (8 of 19 with prior ASCT), the ORR was 89% with 50% complete remissions and a 6-month PFS of 91%. There was one treatment-related death and one patient with grade 3 pneumonitis. Interestingly, in contrast to the prior study, only one patient had a grade 1 IRR and was retreated with adequate premedications. Close follow-up of patients treated on trials incorporating new agents into pretransplant salvage is needed to assess for both posttransplant efficacy and toxicity, as these newer combinations could potentially increase posttransplant risks, such as pneumonitis.
Incorporation of brentuximab vedotin and PD-1 inhibitors into front-line therapy in HL
Older patients
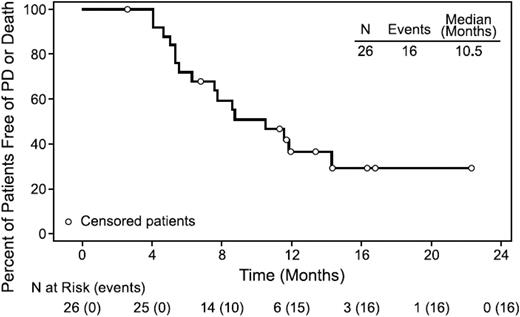
Given the high response rates of brentuximab vedotin in relapsed and refractory disease and the increased toxicity of standard ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) chemotherapy in older patients, a phase 2 study was initiated to evaluate single-agent brentuximab vedotin as front-line therapy for newly diagnosed HL patients aged 60 or older.18 The ORR was 92% with a 73% CR. Disappointingly, the median remission duration was 9.1 months for all patients and 11.8 months for those achieving CR (Figure 4). Toxicity was more severe than noted previously in younger patients, with 78% of patients experiencing peripheral sensory neuropathy, including 30% with grade 3 neuropathy. This short remission duration was confirmed in a second study of 38 patients who were felt to be unfit for standard chemotherapy despite an ORR of 84%. The complete metabolic response rate following cycle 4 was only 26%, the median PFS was 7 months, and 28 of 31 evaluable patients had progressed at the time of the report.19
PFS for all patients. Median PFS was 10.5 months (range, 2.6+ to 22.3+ months) in all efficacy-evaluable patients (n = 26). Reprinted from Forero-Torres A, Blood. 2015;126(26):2798-2804. Copyright (2015) The American Society of Hematology. Reprinted with permission.18
PFS for all patients. Median PFS was 10.5 months (range, 2.6+ to 22.3+ months) in all efficacy-evaluable patients (n = 26). Reprinted from Forero-Torres A, Blood. 2015;126(26):2798-2804. Copyright (2015) The American Society of Hematology. Reprinted with permission.18
Given the high relapse rates of single-agent brentuximab vedotin, other investigators have explored the combination of brentuximab vedotin and dacarbazine and brentuximab vedotin and bendamustine in older patients as front-line therapy.20,21 The combination of brentuximab vedotin and dacarbazine was relatively well tolerated in 22 older patients with an ORR of 100% and a CR rate of 62%.20 The median PFS had not been reached at 9.8 months. The combination of brentuximab vedotin and bendamustine was only explored in 11 patients because of unacceptable toxicity, despite a dose reduction in the bendamustine from 90 mg/m2 to 70 mg/m2 on D1, 2.20 However, in the phase 1/2 HALO study of bendamustine and brentuximab vedotin in older patients (62 to 79 years), the first 15 patients treated were able to complete treatment with a CR rate of 87%.21 Sixty-seven percent of patients remained in remission at 9 months and there were no deaths.22 The combination of brentuximab vedotin and nivolumab is also being explored as front-line therapy in patients over 60 years of age in a phase 2 study conducted by Academic and Community Cancer Research United (NCT02758717). Preliminary results are not yet available. A phase 3 study of new combinations for first-line therapy in older patients compared with ABVD would be needed to ensure that outcomes are not compromised and toxicity is acceptable with alternative approaches.
AVD and brentuximab vedotin
In a phase 1 study of ABVD plus brentuximab vedotin in patients with advanced-stage HL, this combination was found to be intolerable with a 44% incidence of pulmonary toxicity, including 2 deaths among the 25 patients treated.23 A second cohort tested the combination of AVD plus brentuximab vedotin in an additional 26 patients.23 Importantly, there was no pulmonary toxicity. The incidence of grade 3 neutropenia was 77% and febrile neutropenia 8%. Growth factors were administered at the treating physician’s discretion but were given to the majority of patients. In this cohort of 26 patients, 7 had bulky stage II or IIB disease and 19 had stage III or IV disease. The 3-year failure-free survival for this cohort of patients was 96% with a 3-year OS of 100%.24 Based on these encouraging results, an international study (ECHELON-1) of 1334 patients with stage III or IV previously untreated HL were randomized to ABVD versus AVD plus brentuximab vedotin. The preliminary results of ECHELON-1 were reported on June 26, 2017 in a press release with a 2-year modified PFS (defined as relapse, death, or initiation of a new line of therapy for patients who were not in CR) of 82.1% in the AVD plus brentuximab vedotin arm and 77.2% in the ABVD arm (HR of 0.77, P = .035).25 There was a significantly increased incidence of febrile neutropenia and peripheral neuropathy in the experimental arm and a significantly increased rate of severe pulmonary toxicity in the control arm. Preliminary results are expected at the 2017 meeting of the American Society of Hematology. The press release also described an overall survival “trend” in favor of the brentuximab vedotin arm. The final data presentation will determine whether this combination, which comes with a substantial sticker price and increased toxicity, becomes a standard option for some patients. Whereas there is no role for growth factors with standard ABVD chemotherapy, prophylactic growth factors are highly recommended for all patients with the combination of AVD plus brentuximab vedotin. In addition to the added cost, the complication of how to administer growth factors with every 2-week chemotherapy dosing remains complicated, as pegfilgrastim is not approved for use within 14 days prior to chemotherapy. Given the results of the RATHL (response-adapted therapy for advanced HL) trial showing equivalent outcomes for patients treated with 6 cycles of ABVD versus 2 cycles of ABVD followed by 4 cycles of AVD in the setting of a negative interim PET, the finding of increased pulmonary toxicity in the ABVD arm of the ECHELON-1 study may be less relevant, as most patients will no longer receive 6 cycles of bleomycin.
Brentuximab vedotin has also been incorporated into therapy for early-stage previously untreated HL. Although many patients with early favorable or unfavorable disease are currently treated with chemotherapy alone, especially in the setting of a negative interim PET, the recent phase 3 European Organization for Research and Treatment of Cancer (EORTC) H10 study showed an advantage in PFS for those receiving consolidative radiotherapy, even with a negative interim PET.26 The 5-year PFS was 99% [ABVD + involved node radiotherapy (INRT)] versus 87% (ABVD) in the favorable subgroup (HR 15.8) and 92.1% (ABVD + INRT) vs 89.6% (ABVD) (HR 1.45) in the unfavorable subgroup. The question is whether the addition of new agents, such as brentuximab vedotin or the PD-1 inhibitors, either concurrently or sequentially with AVD, could replace consolidative radiotherapy and result in similar outstanding results. A definitive answer would require a very large phase 3 study. A recent phase 2 study of 41 patients treated with ABVD for 2 to 6 cycles followed by 6 cycles of brentuximab vedotin consolidation reported 90% of patients achieved a complete remission following ABVD and 95% following the consolidative brentuximab vedotin. Two-year PFS was 92% and 2-year OS was 97%.27 Abramson et al treated 34 patients with newly diagnosed early stage HL with 2 doses of lead-in brentuximab vedotin followed by 4 to 6 cycles of AVD plus brentuximab vedotin and reported a 97% CR rate and 90% 2-year PFS.28 One older patient died due to neutropenic sepsis and future patients were treated with prophylactic growth factors. The overall excellent prognosis for the majority of early-stage patients with a limited number of cycles of ABVD alone sets a high bar for future studies aimed at making very small improvements.
Novel agents in early development
In addition to brentuximab vedotin and PD-1 inhibitors, a number of other agents are in early development. Based on exciting results in refractory B-acute lymphoblastic leukemia and diffuse large B-cell lymphoma, chimeric antigen receptor (CAR) T-cell therapy is also being explored in HL. A phase 1 trial of a CD30-specific CAR T-cell approach treated 18 patients with relapsed or refractory HL and reported an ORR of 39%, with 7 of 18 patients achieving partial remission.29 The treatment was well tolerated without evidence of cytokine release syndrome. It was also seen that lymph nodes responded better than extranodal lesions. Additional phase 1 studies evaluating anti-CD 30 CAR T-cells in relapsed HL are currently ongoing (NCT02690545). In preclinical studies Epstein-Barr virus-cytotoxic T lymphocytes that retained the antitumor activity conferred by their native receptor, while expressing a CAR specific for CD30, were active in mouse xenografts of Epstein-Barr virus+ tumors.30 Other novel targets, such as CD123, which is expressed on Reed Sternberg cells in approximately 50% of patients with HL, have shown promise in preclinical studies, with CAR-T 123 inducing complete and durable eradication of disseminated HL tumors engrafted into nonobese diabetic-SKID mice.30
Phase 1 studies are also ongoing with AFM13, a tetravalent bispecific chimeric recombinant antibody targeting CD30 and CD16A (the Fc-γ RIIIA receptor is expressed on the surface of natural killer cells, macrophages, and activated monocytes) in combination with pembrolizumab. The response rate to single-agent AFM13 in relapsed HL was low (11.5%), but preclinical rationale for the combination with PD1 inhibitors led to an ongoing phase 1 study of this combination in relapsed and refractory HL (NCT02665650).
In conclusion, brentuximab vedotin and the PD1 inhibitors, nivolumab and pembrolizumab, have been critical additions to the HL armamentarium. The high-response rate and favorable toxicity profile has led to rapid incorporation of these agents into first- and second-line therapy trials. These efforts should be applauded and will potentially improve outcomes for patients with HL both in terms of improved PFS and fewer late effects if radiotherapy or ASCT can be avoided. Signals from many of these trials do emphasize the need for caution when adding new agents to earlier lines of therapy. The severe lung toxicity seen with the combination of bleomycin and brentuximab vedotin in a first-line phase 1 trial was unexpected. In addition, the increased incidence of significant infusion-related reactions with both the brentuximab vedotin and bendamustine, as well as brentuximab vedotin and nivolumab combinations, was not something that would have been predicted based on single-agent data. There is a theoretical concern administering PD1 inhibitors as part of front-line therapy to patients who have an intact immune system and the possibility of a higher incidence and grade of immune-related side effects. Adding brentuximab vedotin to AVD chemotherapy resulted in a modestly improved PFS, but this did not come without a price, including the need for growth factors and increased neurotoxicity.
Correspondence
Nancy L. Bartlett, Washington University School of Medicine, Siteman Cancer Center, St. Louis, MO 63110; e-mail: nbartlet@wustl.edu.
References
Competing Interests
Conflict-of-interest disclosure: N.L.B. is on the Board of Directors or an advisory committee and has consulted for Pfizer, KITE, Gilead, and Seattle Genetics, and has received research funding from Pfizer, KITE, Merck & Co, Bristol-Myers Squibb, Immune Design, Forty Seven, Affimed, Janssen, Pharmacyclics, Millennium, Astra Zeneca, ImaginAB, Novartis, Genentech, Seattle Genetics, and Celgene.
Author notes
Off-label drug use: Brentuximab vedotin, nivolumab, and pembrolizumab in clinical trials in earlier lines of Hodgkin lymphoma therapy.