Abstract
Genetic alterations of the PD-L1/PD-L2 locus on chromosome 9p24.1 are a defining biological feature of classical Hodgkin lymphoma (HL). The resulting programmed death-ligand 1 (PD-L1) expression on Hodgkin Reed-Sternberg cells as well as the PD-L1 expressed in the HL microenvironment result in an ineffective host antitumor immune response and make HL a ripe target for programmed cell death-1 (PD-1) blockade. Anti–PD-1 antibody monotherapy has been effective and well tolerated in patients with relapsed or refractory (rel/ref) HL, with the majority of patients experiencing an objective response (approximately two-thirds of patients) and a median duration of response of 16.6 months in the study with the longest follow-up. Based on these data, nivolumab and pembrolizumab were approved by the US Food and Drug Administration (FDA) for the treatment of advanced rel/ref HL. Evidence has emerged that patients with HL benefit from continued PD-1 blockade beyond disease progression according to traditionally defined response criteria, and that the addition of, or switch to, chemotherapy after anti–PD-1 antibody failure can potentially re-induce clinical response. Subsequent studies have evaluated novel anti–PD-1–based combination regimens as well as the use of anti–PD-1 antibody therapy earlier in the course of a HL patient’s therapy, including first salvage therapy for rel/ref disease (eg, nivolumab plus brentuximab vedotin) and even first-line treatment (eg, nivolumab added to doxorubicin, vinblastine, dacarbazine chemotherapy). The current role of PD-1 blockade in HL is as monotherapy in patients with advanced rel/ref disease, but the results of ongoing studies and the evolving treatment landscape in HL will determine the role of PD-1 blockade in the future.
Learning Objectives
Review the biologic rationale for PD-1 blockade in HL
Understand the current approved indications for anti–PD-1 antibody therapy for the treatment of relapsed or refractory HL
Examine available data on emerging anti–PD-1 antibody-based combination regimens for the treatment of HL
Evaluate the data on emerging biomarkers of response to PD-1 blockade
Introduction
The incorporation of novel, biologic therapies has ushered in a new era of treatment of classical Hodgkin lymphoma (HL). Although most patients with HL are cured with initial chemotherapy, 10% to 25% of patients will have relapsed or refractory (rel/ref) HL despite modern, risk-adapted approaches.1,2 The need to optimize initial therapy and improve outcomes in patients with rel/ref HL has led to the development of new drugs for HL that target its unique biology. In addition to the US Food and Drug Administration (FDA) approval of brentuximab vedotin (BV), an antibody-drug conjugate directed against CD30 on Hodgkin Reed-Sternberg (HRS) cells, the development of anti–programmed cell death-1 (PD-1) antibody therapy for the treatment of HL has been a major advance in the care of these patients. PD-1 blockade targets a pathway central to the pathogenesis of HL and has been a well-tolerated, highly effective treatment in patients with rel/ref HL. In this review, the underlying biological basis of PD-1 blockade in HL, the existing safety and efficacy data on single-agent and combination PD-1 blockade in HL, ongoing studies evaluating new combinations and settings for PD-1 blockade in HL, and biomarkers of response to PD-1 blockade will be described. With the evaluation of BV and PD-1 blockade earlier in the course of a patient’s treatment and the 2018 FDA approval of BV in the frontline setting in patients with advanced-stage HL, the role of PD-1 blockade in HL continues to evolve.
The rationale for PD-1 blockade in HL
HL is histologically defined by a small proportion of neoplastic HRS cells in a polymorphous inflammatory infiltrate. However, this inflammation does not appear to represent an effective host antitumor immune response.3 Nearly universal genetic alterations of chromosome 9p24.1, which include the PD-L1/PD-L2 loci, have been identified in HL, supporting the concept that the PD-1 pathway plays a key role in the host immune evasion that is central to HL pathogenesis.4 The genetic alterations in 9p24.1 are directly linked with increased expression of the PD-1 ligands, programmed death-ligand 1 (PD-L1) and PD-L2, on HRS cells.4,5 In addition, the JAK2 locus is also contained within the 9p24.1 region, and JAK2 activation upregulates PD-L1 transcription and expression.4 Furthermore, Epstein-Barr virus infection, which is frequently observed in HL, has also been identified as a mechanism of PD-L1 upregulation and expression in HL.6 In addition to the PD-1 ligand expression observed on HRS cells, tumor-associated macrophages (TAMs) in the HL tumor microenvironment (TME) frequently express PD-L1. In fact, as might be expected due to the rarity of HRS in the HL TME, TAMs express the majority of PD-L1 in the TME. The topology of PD-L1 expression in the HL TME suggests that TAMs may play an important role in the ineffective immune response observed in HL because PD-L1+ TAMs are geographically located in close proximity to PD-L1+ HRS cells as well as PD-1+ T cells (especially CD4+ cells) in the HL TME.7 Based on the multiple ways in which the PD-1 pathway appears to serve an important role in the pathogenesis of HL, there is a strong biologic rationale to use PD-1 blockade for the treatment of HL.
In fact, not only is PD-1 pathway alteration a key facet of the pathogenesis of HL, more significant PD-1 pathway derangement in an HL tumor confers a negative prognosis in patients treated with standard therapies. In a cohort of 108 newly diagnosed patients with HL treated according to the Stanford V regimen, an increasing degree of PD-L1/PD-L2 genetic alteration assessed by fluorescence in situ hybridization (9p24.1 amplification > copy gain > polysomy vs disomy) was associated with inferior progression-free survival (PFS).5 Taken together with the near universal PD-1 pathway alterations observed in HL, these data suggest 2 enticing hypotheses for the potential role of PD-1 blockade in HL: the high levels of PD-1 pathway derangement in HL may predict increased susceptibility to PD-1 blockade and perhaps the use of anti–PD-1 therapy could abrogate the negative prognostic impact of PD-1 pathway derangement seen in patients treated with standard therapies.
Anti–PD-1 antibody monotherapy in rel/ref HL
Anti–PD-1 antibody monotherapy has been evaluated in patients with rel/ref HL and produced a high rate of objective responses in early-phase studies (Table 1). A phase 1 study of nivolumab in patients with rel/ref hematologic malignancies demonstrated an 87% overall response rate (ORR), 17% complete response (CR) rate, and 100% clinical benefit rate in 23 patients with rel/ref HL.8 The majority of patients enrolled in the study (78%) had failed prior autologous stem cell transplantation (autoSCT) and prior BV. A subsequent phase 2 study (CheckMate 205) of nivolumab for rel/ref HL treated 243 patients who had failed prior autoSCT into 3 cohorts of patients: patients in cohort A were BV naive (n = 63), patients in cohort B had failed BV that was administered after autoSCT (n = 80), and patients in cohort C had received prior BV before and/or after autoSCT failure (n = 100). The ORR with nivolumab therapy among all treated patients was 69% with a CR rate of 16% as assessed by an independent review committee. Response rates were similar across cohorts and were similar if a patient was refractory to first-line therapy, their last line of therapy, or to BV after autoSCT. The median time to response was 2.1 months, and the median duration of response (DOR) across cohorts was 16.6 months. The median PFS according to best response was 22.2 months in patients who achieved CR, 15.1 months in patients with a partial response (PR), and 11.2 months in patients with a best response of stable disease, suggesting that even patients without an objective response benefitted from therapy (Figure 1).9,10
Studies of anti–PD-1 or PD-L1 antibody monotherapy in rel/ref HL
| Drug . | Phase . | N . | ORR, % . | CR, % . | DOR . | Median PFS, unless specified . | Reference . |
|---|---|---|---|---|---|---|---|
| Nivolumab | 1 | 23 | 87 | 17 | * | 86% at 24 wk | 8 |
| Nivolumab | 2 | 10 | |||||
| Overall | 243 | 69 | 16 | 16.6 mo | 14.7 mo | ||
| Cohort A | 63 | 65 | 29 | 20.3 mo | 18.3 mo | ||
| Cohort B | 80 | 68 | 13 | 15.9 mo | 14.7 mo | ||
| Cohort C | 100 | 73 | 12 | 14.5 mo | 11.9 mo | ||
| Pembroliuzmab | 1 | 31 | 65 | 16 | 70% with DOR ≥24 wk | 46% at 52 wk | 11 |
| Pembroliuzmab | 2 | 12 | |||||
| Overall | 210 | 69 | 22 | Not reached | 63% at 9 mo | ||
| Cohort A | 69 | 74 | 22 | Not reached | |||
| Cohort B | 81 | 64 | 25 | Not reached | |||
| Cohort C | 60 | 70 | 20 | Not reached | |||
| Avelumab | 1 | 31 | 42 | 16 | * | * | 13 |
| Drug . | Phase . | N . | ORR, % . | CR, % . | DOR . | Median PFS, unless specified . | Reference . |
|---|---|---|---|---|---|---|---|
| Nivolumab | 1 | 23 | 87 | 17 | * | 86% at 24 wk | 8 |
| Nivolumab | 2 | 10 | |||||
| Overall | 243 | 69 | 16 | 16.6 mo | 14.7 mo | ||
| Cohort A | 63 | 65 | 29 | 20.3 mo | 18.3 mo | ||
| Cohort B | 80 | 68 | 13 | 15.9 mo | 14.7 mo | ||
| Cohort C | 100 | 73 | 12 | 14.5 mo | 11.9 mo | ||
| Pembroliuzmab | 1 | 31 | 65 | 16 | 70% with DOR ≥24 wk | 46% at 52 wk | 11 |
| Pembroliuzmab | 2 | 12 | |||||
| Overall | 210 | 69 | 22 | Not reached | 63% at 9 mo | ||
| Cohort A | 69 | 74 | 22 | Not reached | |||
| Cohort B | 81 | 64 | 25 | Not reached | |||
| Cohort C | 60 | 70 | 20 | Not reached | |||
| Avelumab | 1 | 31 | 42 | 16 | * | * | 13 |
Not reported.
PFS according to best response to nivolumab in patients with rel/ref HL enrolled on the CheckMate 205 study. Reprinted from Armand et al10 with permission.
PFS according to best response to nivolumab in patients with rel/ref HL enrolled on the CheckMate 205 study. Reprinted from Armand et al10 with permission.
Pembrolizumab was evaluated in a phase 1b study (KEYNOTE-013) of patients with rel/ref HL who failed prior BV (71% with prior autoSCT). More than half of patients (55%) had received 5 or more lines of prior therapy. Among 31 patients enrolled, the ORR was 65% and CR rate was 16%. The response duration was at least 24 weeks in 70% of responding patients, and, in the overall cohort, the PFS at 52 weeks after initiation of pembrolizumab was 46%.11 A phase 2 study (KEYNOTE-087) of pembrolizumab in patients with rel/ref HL enrolled 210 patients into 3 cohorts: cohort 1 included patients who failed autoSCT and post-autoSCT BV, cohort 2 included patients who received prior salvage chemotherapy and BV but were refractory and ineligible for autoSCT, and cohort 3 included patients who failed autoSCT but did not receive BV after autoSCT. The ORR and CR rates across all cohorts by independent review were 69% and 22%, respectively. Similar to the phase 2 nivolumab study, the response rates across the cohorts were similar and were similar regardless of number of prior therapy lines or whether the patient had received prior BV. Patients who had been refractory to their frontline therapy had an 80% ORR, whereas patients who were refractory to all prior lines of therapy still had an ORR of 56.5%. The majority of patients (76%) had a duration of response of 6 months or greater, and the 9-month PFS was 63%.12 Based on these results, an ongoing randomized, open-label phase 3 study is being conducted in patients with rel/ref HL evaluating the PFS and overall survival (OS) after pembrolizumab compared with BV (NCT02684292).
Although there appears to be a small proportion of patients with solid tumors treated with anti–PD-1 antibodies who have a long-term durable remission (eg, ∼15% CR rate in melanoma with ∼90% patients remaining in CR at median 30 months13 ), there appears to be a continuing risk of relapse or disease progression in patients with rel/ref HL who respond to PD-1 blockade. In the study with the longest follow-up available, even among patients with CR to nivolumab, there appear to be late relapses.10 At the present time, it is too soon to determine whether there will be a small proportion of patients with HL who will experience durable remission after PD-1 blockade.
Checkpoint blockade, including anti–PD-1 antibody therapy, is associated with characteristic immune-related adverse events (irAEs) triggered by reversing the immunosuppressive effects of inhibitory checkpoints. irAEs significant enough to require high-dose corticosteroids and preclude prevent further administration of anti–PD-1 antibodies are uncommon, but treating physicians should be aware of these potentially dangerous toxicities and how they should be managed.14,15 In the phase 1 and 2 studies of pembrolizumab and nivolumab in patients with rel/ref HL, treatment has been well tolerated, with very few grade 3 or 4 adverse events (AEs) reported and only 5% to 7% of patients discontinuing treatment due to treatment-related AEs. The most common irAEs were hypo/hyperthyroidism (12%-16%), rash (9%), hepatitis (5%), pneumonitis (3%-4%), but most were grade 1 or 2, and grade 3 or higher irAEs were rare.10,12
A notable feature of treatment with anti–PD-1 antibody therapy in patients with HL is the clinical benefit observed with continued treatment beyond disease progression. In the phase 2 nivolumab study, 70 of 105 patients who experienced disease progression continued nivolumab treatment beyond progression (TBP) for a median of 5.2 months. Fifty-one patients had evaluable postprogression data and 61% had decreased or stable target tumor burden with TBP. The median time between initial progressive disease (PD) and next systemic therapy was 8.8 months after TBP compared with 1.5 months in patients who did not receive TBP, the median OS after date of initial progression was not reached, and the 1-year OS with TBP was 84%, which was higher than patients who did not receive TBP (61%).10
Avelumab, which blocks PD-L1 rather than PD-1, is also being evaluated in a phase 1 study (JAVELIN, NCT02603419) of patients with rel/ref HL who have failed prior autoSCT, allogeneic SCT (alloSCT), or are transplant ineligible. Preliminary safety and efficacy data on 31 enrolled patients showed that safety appeared to be similar across avelumab-dosing regimens with an ORR of 42% and a 16% CR rate. Notably, the ORR in the 8 patients who had undergone prior alloSCT was 62.5%, though 2 patients developed grade 3 liver graft-versus-host disease (GVHD) that resolved with avelumab discontinuation and immunosuppression. With only these limited data available to date, additional studies will be necessary to determine whether the lower response rate observed in the JAVELIN study truly represents decreased efficacy of PD-L1 blockade relative to anti–PD-1 antibodies that block the interaction between PD-1 and both PD-L1 and PD-L2.16
Similar to the JAVELIN study, responses to single-agent PD-1 blockade have been observed in patients with HL who have relapsed after alloSCT. A retrospective study described a 79% ORR and 52% CR rate to single-agent anti–PD-1 antibody therapy (usually standard doses of nivolumab or pembrolizumab) in 29 evaluable patients with relapsed HL after alloSCT. The median PFS following post-alloSCT anti–PD-1 antibody administration was 19.4 months with a median OS that was not reached. GVHD was common (51%), typically occurred quickly after initiation of anti–PD-1 therapy (1-2 cycles), and was usually difficult to treat, frequently resulting in GVHD-related death (8 of 17 patients with GVHD).17 A phase 1 study that is prospectively evaluating nivolumab in patients with relapsed hematologic malignancies after alloSCT has demonstrated responses (all PR) in 3 of the 4 patients with HL enrolled. Notably, the initial dose of nivolumab evaluated on the study was below the standard dose at 1 mg/kg and a deescalation cohort was enrolled at 0.5 mg/kg due to dose-limiting immune-related toxicities (though only 3 of 14 patients have developed mild chronic GVHD).18 Other ongoing studies are evaluating the use of PD-1 blockade for relapsed HL after alloSCT (NCT02981914) and the JAVELIN study (NCT02603419) is now limiting enrollment to patients with relapsed HL after alloSCT. A separate but related issue is the concern for increased toxicity after alloSCT in patients with HL who received an anti–PD-1 antibody prior to alloSCT. Although the overall rates of transplant-related mortality are similar to historical data, a possible signal of anti–PD-1–related hyperacute GVHD and steroid-requiring febrile syndromes has arisen in patients who received anti–PD-1 therapy prior to alloSCT.19 No clear temporal relationship between post-alloSCT toxicity and proximity to prior anti–PD-1 therapy has been established. Because the relapse rate and nonrelapse mortality after alloSCT in HL patients who received prior anti–PD-1 therapy are low, there are not sufficient data to suggest that alloSCT after PD-1 blockade should be avoided. Consensus guidelines for the management of PD-1 blockade in the setting of alloSCT for lymphoma provide recommendations regarding alloSCT decision-making in patients who have received prior PD-1 blockade and the use of PD-1 blockade in patients with prior alloSCT.20
Combination therapy incorporating PD-1 blockade in rel/ref HL
PD-1 blockade in patients with rel/ref HL has been an important advance; however, there are ongoing efforts to optimize the efficacy of these agents in HL. Patients who achieve a CR appear to derive the longest duration of benefit from PD-1 blockade and only a minority of patients will have a CR.10,12 The addition of other agents in combination with PD-1 blockade is a logical next step to try and increase the CR rate. Initial anti–PD-1 antibody-based combinations have been focused on patients with rel/ref HL. There are several agents that are being combined with anti–PD-1 antibodies based on possible biological synergies with the goal of augmenting the effectiveness of PD-1 blockade (Table 2).
Studies of anti–PD-1 antibody-based combinations in HL
| Drugs . | Phase . | Population . | N . | ORR, % . | CR, % . | DOR . | PFS . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Nivolumab + AVD | 2 | Newly diagnosed | 51 | 84 | 67 | * | 94% at 9 mo (mPFS) | 40 |
| Nivolumab + BV | 1/2 | rel/ref (first salvage) | 61 | 82 | 61 | Not reached | 89% at 6 mo | 37 |
| Nivolumab + BV | 1 | rel/ref | 19 | 89 | 50 | * | 91% at 6 mo | 18 |
| Nivolumab + ipilimumab | 1 | rel/ref | 31 | 74 | 19 | Not reached | Not reached | 22 |
| Nivolumab or pembrolizumab + chemo (various) | Retrospective | rel/ref | 30 | * | 8.4 mo (median) | 36 | ||
| Anti–PD-1 + chemo | 11 | 90 | 45 | |||||
| Chemo after PD-1 | 19 | 61 | 32 |
| Drugs . | Phase . | Population . | N . | ORR, % . | CR, % . | DOR . | PFS . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Nivolumab + AVD | 2 | Newly diagnosed | 51 | 84 | 67 | * | 94% at 9 mo (mPFS) | 40 |
| Nivolumab + BV | 1/2 | rel/ref (first salvage) | 61 | 82 | 61 | Not reached | 89% at 6 mo | 37 |
| Nivolumab + BV | 1 | rel/ref | 19 | 89 | 50 | * | 91% at 6 mo | 18 |
| Nivolumab + ipilimumab | 1 | rel/ref | 31 | 74 | 19 | Not reached | Not reached | 22 |
| Nivolumab or pembrolizumab + chemo (various) | Retrospective | rel/ref | 30 | * | 8.4 mo (median) | 36 | ||
| Anti–PD-1 + chemo | 11 | 90 | 45 | |||||
| Chemo after PD-1 | 19 | 61 | 32 |
chemo, chemotherapy; mPFS, modified progression-free survival.
Not reported.
In addition to delivering a potent microtubule cytotoxin into HRS cells, there is evidence that BV can induce immunogenic cell death in HL.21 Based on the possibility of immunogenic effects that may cooperate with PD-1 blockade, the combination of BV and nivolumab has been evaluated in patients with rel/ref HL. A study in 18 patients with rel/ref HL including heavily treated patients and 4 patients with prior BV exposure has preliminarily demonstrated a high ORR of 89% with 50% of patients experiencing CR. Among the patients with prior BV exposure, 2 achieved CR and 1 had PR. There was no clear signal for augmented toxicity with the combination, though 2 severe episodes of treatment-related pneumonitis were observed.22 This study is ongoing and currently evaluating triplet therapy with BV, nivolumab, and the CTLA4 inhibitor, ipilimumab (NCT01896999). There is also an ongoing randomized phase 3 trial evaluating BV alone compared with BV plus nivolumab in patients with rel/ref HL who have failed autoSCT or are transplant-ineligible (CheckMate 812, NCT03138499).
Combination checkpoint blockade has been highly effective in patients with solid tumors,23,24 and there is evidence that multiple immune checkpoints are expressed in the HL TME and represent a possible therapeutic target.25 The anti–CTLA-4 antibody, ipilimumab (1 mg/kg), has been evaluated in combination with nivolumab (3 mg/kg) in patients with rel/ref hematologic malignancies, including 31 patients with HL. The response rates to combination nivolumab/ipilimumab in rel/ref HL (ORR, 74%; CR, 19%) were similar to those observed with single-agent PD-1 blockade but grade 3 or higher AEs were higher (29%) than is typically seen with nivolumab.26 The expression of other inhibitory checkpoints like lymphocyte-activation gene 3 (LAG-3) that suppress T-cell function is common in HL and combination anti–PD-1/anti–LAG-3 studies are ongoing (NCT02061761).25 Several studies are under way aimed at targeting immune checkpoints that impact other immune cells in the HL TME. With major histocompatibility complex class I (MHC-I) and β2-microglobulin (B2M) absent in the TME of most patients with HL,27 there may be a role to coax natural killer cells (not dependent on MHC-I) into producing antitumor responses in HL and multiple studies are ongoing to test this hypothesis (NCT02665650, NCT02061761, NCT01592370). Based on the abundance of TAMs in the HL TME and their possible immunosuppressive effects,7 targeting molecules like CD47, a macrophage-specific inhibitory checkpoint, may be useful; a study is evaluating this strategy in hematologic malignancies including HL (NCT02663518).
In addition to combination checkpoint blockade, the addition of other immunomodulatory agents to PD-1 blockade is being evaluated in patients with rel/ref HL. Ibrutinib is a Bruton tyrosine kinase inhibitor that also inhibits interleukin-2–inducible kinase, which can shift helper T-cell (Th) polarity toward Th1 activation and cell-mediated immunity and away from immunosuppressive Th2 activation,28,29 which may augment cell-mediated immune attack triggered by PD-1 blockade. Multiple ongoing studies are evaluating this strategy in rel/ref HL (NCT02950220, NCT02940301). Phosphatidylinositol 3-kinase (PI3K) pathway signaling plays a role in T-cell differentiation and function, and inhibition of PI3K can suppress regulatory T-cell and myeloid-derived suppressor cell function.30 PI3K inhibition with idelalisib has produced antitumor responses in patients with rel/ref HL,31 and studies of combination PI3K inhibition and PD-1 blockade in rel/ref HL are ongoing (NCT03471351). Similarly, lenalidomide is an immunomodulatory drug with myriad immune effects including modulation of T-cell responses and is being evaluated in combination with anti–PD-1 antibody therapy in rel/ref HL (NCT03015896, NCT02875067).32 Of note, PD-1 blockade in combination with immunomodulatory drugs like lenalidomide in patients with multiple myeloma has been associated with an increased risk of death.33 No similar signal has yet been observed in HL, but further study will be necessary to determine whether the risks are particular to multiple myeloma or are observed across diseases. Histone deacetylase (HDAC) inhibitors also have immunomodulatory effects both on tumor cells (eg, promoting release of mediators of immunogenic cell death) as well as on immune cells (eg, decreasing regulatory T-cell numbers and function).34 Notably, there is preclinical evidence that HDAC inhibitors can induce PD-L1 expression in certain preclinical tumor models and can also increase T-cell infiltration into tumors.35-37 Multiple studies are evaluating combination HDAC inhibition and PD-1 blockade in rel/ref HL (NCT03150329, NCT03179930).
Conventional cytotoxic agents like chemotherapy and radiation therapy are also being studied in combination with PD-1 blockade. There is evidence that radiotherapy and certain chemotherapies may have immunogenic properties, and killing of tumor cells with conventional agents may release tumor antigens that could prime an anti–PD-1–directed antitumor immune response.36,38,39 Alternatively, it is possible that PD-1 blockade may improve responses to concurrent or subsequent chemotherapies. A retrospective study conducted in the LYSA network evaluated 30 patients with heavily treated rel/ref HL (median 6 prior therapy lines) who had insufficient response to an anti–PD-1 antibody and were subsequently treated with concurrent chemotherapy and PD-1 blockade or chemotherapy alone as their next therapy. The chemotherapy administered in combination with or after the anti–PD-1 antibody was a range of single-agent (eg, vinblastine) and combination chemotherapies (eg, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone [BEACOPP]). In patients who received concurrent anti–PD-1/chemotherapy, the ORR was 90% with a 45% CR rate, whereas patients who received chemotherapy after PD-1 blockade had a 61% ORR with 32% achieving CR.40 Multiple prospective studies are evaluating the combination of chemotherapy or radiation therapy with PD-1 blockade in patients with rel/ref HL (NCT03343652, NCT03179917, NCT03480334, NCT03495713).
PD-1 blockade earlier in the course of therapy of patients with rel/ref HL
In parallel with the studies evaluating therapeutic combinations with anti–PD-1 antibodies to improve upon their efficacy in patients with advanced rel/ref HL, multiple clinical trials are evaluating the administration of anti–PD-1 antibodies earlier in the course of HL therapy. Similar to the development path of BV, PD-1 blockade is being evaluated as post-autoSCT consolidation/maintenance therapy aimed at preventing relapse. Phase 2 studies of pembrolizumab or nivolumab consolidation in patients with rel/ref HL who have undergone autoSCT are ongoing (NCT02362997, NCT03436862). Another phase 2 study is evaluating the addition of nivolumab to BV post-autoSCT consolidation therapy in a similar patient population to that enrolled in the AETHERA trial; however, patients with prior exposure to BV and/or PD-1 blockade are eligible for inclusion (NCT03057795).
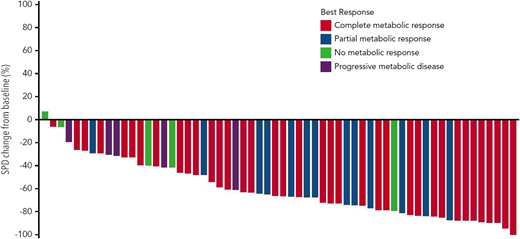
PD-1 blockade has been studied as part of first salvage therapy in transplant-eligible patients with rel/ref HL. A phase 1/2 study of the combination of BV and nivolumab as initial salvage therapy enrolled 62 patients with rel/ref HL who had failed standard upfront therapy. Patients received BV (1.8 mg/kg) with nivolumab (3 mg/kg) for up to 4 × 21-day cycles. During cycle 1, BV was administered on day 1 and nivolumab was administered on day 8. The ORR to the combination was 82% with a CR rate of 61% (Figure 2). At the time of presentation, 90% of patients had proceeded to an autoSCT, with 70% of the overall cohort proceeding to autoSCT directly after BV plus nivolumab. The combination was well tolerated with only 8% of patients requiring systemic corticosteroids for treatment of irAEs. Infusion-related reactions were common (44%) but nearly always mild. BV and nivolumab therapy did not impact the yield of stem cell mobilization and collection.41 Longer follow-up data are necessary to assess the durability of remission offered by nivolumab plus BV followed by autoSCT. A study (CheckMate 744) is evaluating the combination of BV and nivolumab as a first salvage strategy in children, adolescents, and young adults (NCT02927769). Additional trials are evaluating the addition of nivolumab or pembrolizumab (NCT03016871, NCT03077828) to combination chemotherapy with ifosfamide, carboplatin, and etoposide as a first salvage strategy in patients with rel/ref HL.
Change in tumor size in patients with rel/ref HL treated with BV plus nivolumab as first salvage therapy. Reprinted from Herrera et al41 with permission.
Change in tumor size in patients with rel/ref HL treated with BV plus nivolumab as first salvage therapy. Reprinted from Herrera et al41 with permission.
Incorporation of PD-1 blockade into frontline therapy for HL
Higher degrees of PD-1 pathway derangement (ie, 9p24.1 amplification or copy gain) are associated with a higher likelihood of treatment failure with standard initial therapy for HL.5 Therefore, the incorporation of PD-1 blockade into initial therapy of HL is a logical strategy to attempt to overcome the negative prognostic impact of PD-1 pathway alteration. The preliminary findings of a study evaluating the addition of nivolumab to doxorubicin, vinblastine, dacarbazine (AVD) as initial therapy in patients with newly diagnosed advanced-stage (stage IIB, III, IV) HL (CheckMate 205, cohort D) were reported at the 2017 American Society of Hematology (ASH) annual meeting. Fifty-one patients were enrolled and treated with nivolumab monotherapy for 4 doses every 2 weeks followed by combination nivolumab plus AVD (N-AVD) for 12 doses every 2 weeks. Forty-nine patients completed nivolumab monotherapy and ultimately 44 patients completed the full course of N-AVD. Aside from neutropenia (49%) and febrile neutropenia (10%), grade 3 or higher AEs were uncommon. There were few patients who discontinued treatment due to toxicity (4 patients, 8%) and the profile of irAEs was similar to what has been observed with single-agent PD-1 blockade, with endocrinopathy (all thyroid-related) being common (26%, all grade 1-2) and only 1 patient requiring high-dose corticosteroids (for hepatitis). At the end of nivolumab monotherapy, the ORR and CR rates were similar to what has been observed in patients with advanced rel/ref HL (ORR, 69%; CR, 18% by independent review). In the intent-to-treat population, at the end of combination therapy, the ORR was 84% and the CR rate was 67% (ORR, 93%; CR, 74% in evaluable patients). Of note, there were moderate discrepancies between investigator and independently assessed responses: the end of treatment CR rate per investigators was 80%. Only 2 patients not in CR at the end of treatment received subsequent therapy. These data may speak to the difficulty of assessing responses to PD-1 blockade using standard criteria and the potential utility of newer response criteria that account for indeterminate findings in patients receiving immunotherapy.42,43 The modified PFS in the overall cohort at 9 months was 94%, but follow-up is too short to draw any definitive conclusions about the durability of responses as of yet.44
Additional clinical trials are evaluating the incorporation of PD-1 blockade into standard first-line HL therapy (NCT03331341), including some studies using positron emission tomography–adapted strategies (NCT03033914, NCT03226249). Other studies are specifically evaluating the incorporation of PD-1 blockade into initial therapy for patients with early-stage HL (NCT03004833, NCT03233347). Finally, a series of clinical trials are studying anti–PD-1 antibody therapy alone (NCT03331731) or the combination of BV and nivolumab (NCT01716806, NCT02758717) as initial treatment of patients with previously untreated HL who are elderly or have comorbidities precluding the use of standard chemotherapy.
Biomarkers of response to PD-1 blockade and potential for personalized medicine?
Anti–PD-1 antibodies are highly active in the treatment of HL, but it is clear that there are differences in the depth and durability of response among patients. In addition to using combination therapies to improve upon the antitumor efficacy of these agents, another potential approach to maximizing the utility of PD-1 blockade is to use biomarkers of response to identify the patients most likely to benefit from their use. To date, available data suggest that biomarkers that can quantify the degree of PD-1 pathway derangement in a patient’s tumor or the particular pattern of antigen-presentation machinery abnormalities present are associated with outcomes in HL patients treated with anti–PD-1 antibodies.
In patients with rel/ref HL treated with nivolumab, a higher degree of 9p24.1 molecular alteration was associated with improved response and PFS. Notably, as compared with patients with 9p24.1 polysomy and copy gain, no patients with 9p24.1 amplification had primary PD after nivolumab.45 Similar to 9p24.1 alterations, a higher degree of PD-L1 expression by immunohistochemistry was associated with improved response and PFS in rel/ref HL patients treated with nivolumab. Patients who experienced primary PD with nivolumab treatment all had the lowest degree of PD-L1 expression whereas nearly all patients who achieved CR had higher degrees of PD-L1 expression (Figure 3).45 These data suggest that a higher degree of PD-1 pathway derangement in a particular HL patient’s tumor may be predictive of response and DOR with anti–PD-1 antibody therapy.
Response to nivolumab in patients with rel/ref HL according to PD-L1 H-score. Reprinted from Roemer et al45 with permission.
Response to nivolumab in patients with rel/ref HL according to PD-L1 H-score. Reprinted from Roemer et al45 with permission.
In addition to PD-L1/PD-L2 genetic alteration and PD-L1 expression on HRS and in the TME, another unique biologic feature of HL is altered antigen-presentation machinery on HRS cells associated with molecular alterations in B2M and CIITA. There is no expression of MHC-I on HRS cells in about half of HL tumors, and MHC-II expression on HRS cells is absent in a sizable minority (30%), whereas the majority of HL tumors have either absence or significant decrease of MHC-I and MHC-II expression. The absence of or decrease of MHC-I (and B2M) expression on HRS cells is a negative prognostic factor in HL patients treated with standard initial therapy.27 With MHC-I and B2M absent or decreased on HRS cells in most cases of HL, it appears that the mechanism of action of anti–PD-1 antibodies in HL does not rely on CD8+ cytotoxic T-cell–based responses. As expected, response to nivolumab and postnivolumab PFS in patients with rel/ref HL are not associated with the presence of MHC-I or B2M expression on HRS cells. Rather, response to nivolumab and PFS after nivolumab (in patients more distant from prior autoSCT) are associated with at least some degree of expression (positive or decreased vs negative) of MHC-II on HRS cells.45 This suggests that intact MHC-II and CD4+-mediated T-cell responses play an important role in anti–PD-1 antibody responses in HL.
These biomarkers should not yet be used to determine an HL patient’s candidacy for anti–PD-1 antibody therapy: the majority of patients with low-level 9p24.1 derangement, low PD-L1 expression, and no MHC-II expression will still have an objective response to PD-1 blockade. However, these data provide hope that with further refinement and validation, biomarkers may be able to be used in the future to personalize anti–PD-1 antibody therapy (eg, sequencing with other therapies) in HL.
Conclusions: where does PD-1 blockade currently fit?
At the present time, anti–PD-1 antibodies are FDA approved for the treatment of patients with rel/ref HL with slight differences in the specific indications for each agent. Nivolumab is approved for use in patients with HL who have failed autoSCT and post-SCT BV. Pembrolizumab is approved for use in patients with HL who are refractory or who have relapsed after 3 or more lines of therapy. In practice, there is no evidence that there are significant differences between the agents in terms of efficacy or safety profile in patients with rel/ref HL. Unlike what is observed with traditional chemotherapy, patients who have disease progression after PD-1 blockade often derive benefit from continued treatment beyond progression. As long as patients are tolerating therapy and continue to benefit clinically, treatment should generally be continued until more significant progression and/or symptoms develop. There is evidence to suggest that, in patients with insufficient response to or disease progression after PD-1 blockade, the addition of chemotherapy concurrent with an anti–PD-1 antibody or use of chemotherapy after the anti–PD-1 antibody can be effective. AlloSCT remains an option for patients with rel/ref HL who have received prior PD-1 blockade; however, both agents carry warnings on their FDA labels about possible complications early after alloSCT, and treating physicians should proceed with caution.
Although use of anti–PD-1 antibodies earlier in a patient’s disease course (eg, first salvage) remains investigational, there is emerging evidence that PD-1 blockade can be safely and effectively administered as part of second-line and possibly even initial therapy. Especially with FDA approval of BV as part of initial therapy in patients with advanced-stage HL, the evolving treatment landscape of HL necessitates ongoing reassessment of the role of PD-1 blockade in HL. The results of ongoing clinical trials evaluating novel anti–PD-1 antibody-based combinations in newly diagnosed and rel/ref patients with HL will determine where PD-1 blockade will fit in the future.
Correspondence
Alex F. Herrera, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: aherrera@coh.org.
References
Competing Interests
Conflict-of-interest disclosure: A.F.H. has received research funding from, and consulted for, Bristol-Myers Squibb, Genentech, Merck, Seattle Genetics, Pharmacyclics, and KiTE Pharma. A.F.H. has received research funding from AstraZeneca and Immune Design.
Author notes
Off-label drug use: This manuscript describes use of nivolumab and pembrolizumab.