Abstract
Allogeneic hematopoietic cell transplantation is a potentially curative therapy for many malignant and nonmalignant hematologic diseases. Graft-versus-host disease (GVHD) is a common complication after transplantation and remains a major cause of morbidity and mortality, limiting the success of a potentially curative transplant. This paper reviews the current and emerging strategies in GVHD prevention and treatment. New insights are leading the way to the development of novel targeted approaches to minimize the risk of disease relapse and infection. Continued collaborative efforts to conduct high-quality, multicenter clinical trials with standard end points and risk stratification are needed to determine the optimal approach to minimize GVHD and limit toxicities.
Learning Objectives
Review current standard graft-versus-host disease (GVHD) prophylaxis regimens and treatment
Describe novel approaches and biologic insights currently under investigation for GVHD prevention
Understand the need for high-quality, multicenter, randomized trials with standard end points and risk stratification to determine the optimal GVHD prevention and treatment strategies
Introduction
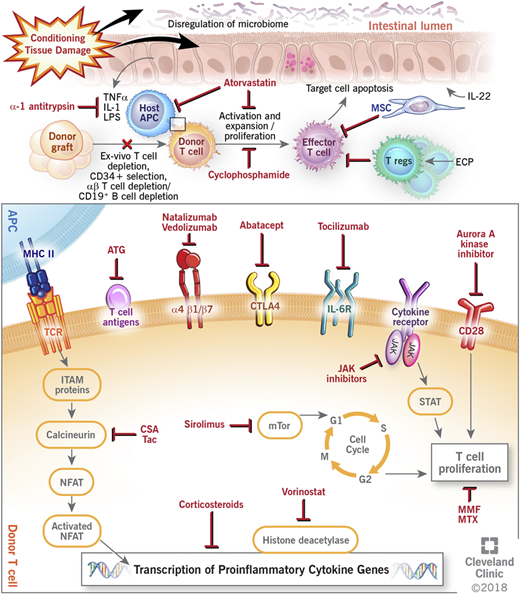
Graft-versus-host disease (GVHD) remains a challenge after allogeneic hematopoietic cell transplantation (HCT). GVHD occurs when immunocompetent donor T cells recognize the recipient host as foreign and mount an immune response to allogeneic antigen-bearing cells with subsequent destruction of host tissues. Despite current prophylactic strategies, morbidity and mortality remain high, and treatment of established GVHD can be difficult, with only about 40% of patients having a durable response to corticosteroid therapy.1 Recent experimental models and biologic insights, however, have greatly improved the understanding of the pathogenesis of GVHD, and newer approaches targeting different components of immune dysregulation are currently being used and further investigated in improving GVHD outcomes. This paper reviews the current and novel approaches in GVHD prevention and treatment (Figure 1).
Mechanisms of action of current and novel approaches to prevent and treat GVHD. APC, antigen-presenting cell; ATG, antithymocyte globulin; CSA, cyclosporine; CTLA4, cytotoxic T lymphocyte antigen 4; ECP, extracorporeal photopheresis; IL, interleukin; IL-6R, interleukin-6 receptor; ITAM, immunoreceptor tyrosine-based activation motif; JAK, janus kinase; LPS, lipopolysaccharides; MHC II, major histocompatibility complex II; MMF, mycophenolate; MSC, mesenchymal stem cell; mTOR, mammalian target of rapamycin complex; MTX, methotrexate; NFAT, nuclear factor of activated T cell; STAT, signal transducer and activator of transcription protein; Tac, tacrolimus; TCR, T-cell receptor; TNFα, tumor necrosis factor-α; Treg, regulatory T cell.
Mechanisms of action of current and novel approaches to prevent and treat GVHD. APC, antigen-presenting cell; ATG, antithymocyte globulin; CSA, cyclosporine; CTLA4, cytotoxic T lymphocyte antigen 4; ECP, extracorporeal photopheresis; IL, interleukin; IL-6R, interleukin-6 receptor; ITAM, immunoreceptor tyrosine-based activation motif; JAK, janus kinase; LPS, lipopolysaccharides; MHC II, major histocompatibility complex II; MMF, mycophenolate; MSC, mesenchymal stem cell; mTOR, mammalian target of rapamycin complex; MTX, methotrexate; NFAT, nuclear factor of activated T cell; STAT, signal transducer and activator of transcription protein; Tac, tacrolimus; TCR, T-cell receptor; TNFα, tumor necrosis factor-α; Treg, regulatory T cell.
Prevention
The most common GVHD prophylaxis has historically been based on a calcineurin inhibitor and a short course of methotrexate (MTX). MTX, an antimetabolite and folate antagonist, attenuates T-cell activation at low noncytotoxic doses and has had a long history in the prevention of GVHD. The first calcineurin inhibitor, cyclosporine (CSA), was introduced, and studies showed that the combination of CSA and a short course of MTX was significantly better at preventing GVHD, improving survival compared with either drug alone.2 Tacrolimus (Tac) subsequently was also found to be effective in combination with a short course of MTX for prevention of GVHD.3 Although structurally distinct, both CSA and Tac have similar mechanism of actions—CSA complexes with cyclophilin and Tac complexes with FKBP12 to inhibit calcineurin and block the dephosphorylation, nuclear translocation, and transcriptional function of nuclear factor of activated T cells, thus reducing T-cell function. Two large multicenter phase 3 prospective trials were performed to compare CSA in combination with MTX and Tac with MTX.4,5 These trials showed superiority of Tac in reducing acute GVHD but no difference in overall and relapse-free survival rates compared with CSA and MTX. Therefore, both regimens are considered standard backbones to most GVHD prevention strategies for patients undergoing allogeneic HCT.
Mycophenolate mofetil (MMF) is an ester prodrug of the immunosuppressant mycophenolic acid (MPA), a selective inhibitor of inosine monophosphate dehydrogenase that is a key enzyme in the de novo synthesis of guanine nucleotides. T and B lymphocytes are extremely dependent on this pathway, and thus, MPA has a potent cytostatic effect on lymphocytes. Given its favorable toxicity profile, the combination of a calcineurin inhibitor and MMF is also commonly used in patients undergoing allogeneic HCT6 ; however, several studies have raised the question of MMF’s efficacy compared with MTX with findings of more severe acute GVHD and higher nonrelapse mortality.7 A recent Center for International Blood and Marrow Transplant Research study of 3979 matched sibling donors and 4163 unrelated donors showed significantly inferior GVHD and survival outcomes with CSA + MMF compared with Tac + MTX, CSA + MTX, and Tac + MMF in myeloablative transplantation,8 suggesting an advantage of MTX over MMF for GVHD prevention.
Other current approaches to GVHD prophylaxis
Sirolimus binds to FKBP12 and inhibits the mammalian target of the rapamycin inhibitor to block interleukin-2 (IL-2)–mediated signal transduction, leading to cell cycle arrest in naïve T cells. Its immunomodulatory properties include inhibition of antigen presentation and dendritic cell maturation as well as preservation of regulatory T-cell (Treg) subsets after transplantation. A phase 3 multicenter, prospective, randomized clinical trial conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) comparing Tac + sirolimus with Tac + MTX showed no difference in rates of grades 2 to 4 acute GVHD or GVHD-free survival.9 Hematopoietic recovery was more rapid with less mucositis in the Tac + sirolimus arm, although it was also associated with increased rates of endothelial injury syndromes, elevations in cholesterol and triglycerides, and increase in creatinine. Tac + sirolimus is thus considered an important alternative for patients undergoing total body irradiation-based transplantation, particularly for those who may be at higher risk for developing severe mucositis or require faster engraftment for risk of infection.
Posttransplant cyclophosphamide (PTCy) alone or in combination with other immunosuppressive agents has also emerged as an effective pharmacologic approach to GVHD prevention. This strategy was pioneered by investigators at Johns Hopkins in the haploidentical setting based on experimental models showing cyclophosphamide’s potent and selective activity against alloreactive donor T cells, resulting in low incidences of GVHD and transplant-related mortality (TRM). This approach has revolutionized our ability to cross the human leukocyte antigen (HLA) barrier by performing mismatched transplants, greatly expanding donor availability. The success and widespread use of PTCy in T cell–replete haploidentical and mismatched transplants have now prompted its use as a single agent in recipients of transplant from matched sibling or unrelated donors. Although initial reports using bone marrow showed encouraging results with comparable rates of GVHD,10 subsequent studies using peripheral blood stem cells reported higher rates of GVHD and poor outcomes.11 The addition of a calcineurin inhibitor or other immunosuppressive drugs to PTCy seems to further mitigate GVHD, and it is a viable prophylactic strategy to promote tolerance and minimize longer-term immunosuppressive therapy in high-risk patients.12 Results from the BMT-CTN trial evaluating novel approaches for GVHD prevention using reduced intensity conditioning (PROGRESS I trial NCT02208037) have shown that PTCy + Tac + MMF is associated with lower rates of severe acute GVHD and chronic GVHD requiring immunosuppression without a significant impact on overall survival or relapse compared with Tac + MTX.13
T-cell depletion as an approach to GVHD prophylaxis
In vivo T-cell depletion with antithymocyte globulin (ATG) products has been associated with decreased GVHD but also, still debated effects on relapse, infection risk, and overall survival. ATGs are polyclonal immunoglobulins directed against antigens expressed on human T lymphocytes. Several randomized trials have reported a significant benefit of ATG for the prevention of GVHD, in particular chronic GVHD, with subsequent superior quality of life and survival free of immunosuppression and GVHD.14-17 Although 2 prospective, randomized studies from Canada and Europe demonstrated significantly lower rates of chronic GVHD and similar survival and relapse with ATG compared with non-ATG groups,16,17 a subsequent prospective, randomized, double-blind trial comparing ATG with no ATG conducted in the United States showed inferior progression-free and overall survival due to increase in relapse and nonrelapse mortality.18 The discrepancies in these findings are not completely clear, but findings in the latter study suggest that ATG effects may be dependent on lymphocyte count at the time of ATG administration. Differences in dosages and formulations of ATG also remain unknown factors. Although it is clear that the use of ATG as GVHD prophylaxis leads to significantly decreased acute and chronic GVHD, it remains to be seen whether it is the optimal approach compared with other strategies given increased infection as well as possibly relapse-related deaths.
Ex vivo T-cell depletion has been investigated since the 1980s by a variety of methods.19,20 Initial trials using pan–T-cell depletion showed significant reduction in risk of GVHD even without the use of standard posttransplant pharmacologic GVHD prophylaxis; however, it was also associated with an increased incidence of disease relapse, rejection, and infections.21 Additional exploration of T-cell depletion and graft manipulation includes CD34+ selection,22 T-cell depletion with subsequent T-cell add back,23,24 and selective CD3+ (αβ T cell) and CD19+ B-cell depletion.25 Selective elimination of αβ T cells, which are implicated in GVHD, with preservation of γδ T cells and natural killer (NK) cells, which mediate antitumor activity and immune reconstitution, is an area of ongoing active investigation (NCT02323867, NCT02600208, NCT03301168, and NCT03047746). Studies investigating selective T-cell depletion via CD34+ cell selection without other GVHD pharmacologic prophylaxis have also shown promise, with successful engraftment, improved chronic GVHD, and no differences in relapse mortality, nonrelapse mortality, or overall survival.22 Given the success of these recent strategies, an ongoing BMT-CTN trial is further evaluating calcineurin inhibitor-free GVHD prophylaxis with a 3-arm trial comparing PTCy using bone marrow grafts, mobilized CD34-selected peripheral blood stem cell graft, and a control cohort of Tac + MTX (PROGRESS 2; NCT02345850).
Development of novel end points
The evaluation of novel GVHD prophylactic approaches is complex. As reviewed above, although many current GVHD prevention strategies that either increase immunosuppression or manipulate the graft may reduce GVHD, they also have an effect on disease relapse, infection, or graft failure. The success of allogeneic HCT is thus dependent not only on the development of GVHD but also on the risk of disease relapse and infection. In addition, not all GVHD is considered to be a detriment—the development of grade 2 GVHD has been shown to be not predictive of treatment failure (death or relapse),26 and can be associated with improved outcomes.27 In contrast, the development of grades 3 and 4 GVHD is significantly associated with treatment failure, and it may be a more appropriate end point to consider in evaluating GVHD prophylaxis. Given additional effects on relapse and infection, however, evaluating the true effectiveness of GVHD prophylaxis ideally incorporates multiple outcomes. The BMT-CTN has thus developed composite GVHD end points to better characterize posttransplant recovery and the effectiveness of different GVHD prophylactic strategies.26 In an analysis of 6 different promising GVHD prophylactic approaches, the BMT-CTN evaluated several composite GVHD end points: graft-versus-host disease relapse-free survival (GRFS)—survival without acute grades 3 to 4 GVHD plus chronic GVHD plus disease relapse or progression or death; off immunosuppression relapse-free survival—withdrawal of all immunosuppression or other systemic intervention for treatment or prophylaxis of GVHD and without primary disease progression or death; and chronic graft-versus-host disease relapse-free survival (CRFS)—survival without development of chronic GVHD plus disease relapse, progression, or death. The results of this analysis subsequently resulted in the 2 large multicenter trials evaluating GVHD prophylaxis—PROGRESS I (BMT-CTN 1203; NCT02208037) and PROGRESS 2 (BMT-CTN 1301; NCT02345850)—using GRFS and CRFS as the primary outcomes, respectively. GRFS has thus become a popular primary end point in many studies to better reflect HCT recovery without ongoing morbidity. It is important to consider, however, that the evaluation of novel GVHD prophylaxis strategies remains complex. In using GRFS as a primary end point in evaluating novel approaches, severe GVHD consequently represents the smallest proportion of this composite end point.28
Other experimental approaches to acute GVHD prevention
Many novel GVHD prophylaxis approaches (including agents such as bortezomib, maraviroc,13 etancercept,29 infliximab,30 and daclizumab and basiliximab31 ) have shown promise in preclinical and single-institution studies; however, they have subsequently shown no benefit in larger multicenter clinical trials. Smaller numbers, selective patient population, differences in GVHD scoring, and end points analyzed account for some of the lack of reproducibility, and they underscore the importance of large multicenter clinical trials using uniform and standard end points. Several newer approaches, many of which move away from a broad-based immunosuppressive approach, are being evaluated in early studies, and they are further reviewed here and summarized in Figure 1 and Table 1.
Novel approaches to GVHD
| Therapies . | Mechanisms of action . | Data . | Ongoing clinical trials . |
|---|---|---|---|
| Prevention | |||
| Tocilizumab | Human monoclonal antibody against IL-6R | Phase 2 study of tocilizumab + Tac + MTX: 14% grade 2-4 acute GVHD, 3% grades 3 and 4 acute GVHD at 100 d39 | NCT03434730 |
| Abatacept | Costimulation blockade of CD28:CD80/86 to inhibit T cells | 2 of 10 patients with grade 2-4 acute GVHD, no day 100 TRM40 | NCT01743131 |
| NCT02867800 | |||
| Tregs | Regulate self-tolerance, limit GVHD while maintaining GVL effect | Modified expanded umbilical cord blood–derived Tregs: grade 2-4 acute GVHD 9% at 100 d42 | NCT01660607 |
| NCT00602693 | |||
| NCT01818479 | |||
| NCT01795573 | |||
| T-cell depletion (CD34 selection and selective ex vivo T-cell depletion) | Depletion of alloreactive T cells and selective αβ T-cell depletion, with preservation of γδ T cells and NK cells | CD34+ selection: grade 2-4 acute GVHD 22.7%, chronic GVHD 6.8%29 | NCT02323867 |
| NCT02600208 | |||
| NCT03301168 | |||
| NCT03047746 | |||
| NCT02345850 | |||
| Statins | Inhibit proinflammatory Th-1 differentiation, induce Treg expansion, and downregulate APCs | Phase 2 study of statin to both donors and recipients with Tac + MTX—grade 2-4 3.3%; chronic GVHD 52.3%46 | NCT03066466 |
| Vorinostat | Histone deacetylase inhibitor decreases inflammatory cytokines, enhances Treg function, and reduces GVHD while preserving GVL | Phase 2 study of vorinostat + Tac + MTX: grade 2-4 acute GVHD 22%, grades 3 and 4 acute GVHD 8%; chronic GVHD 29%51 | NCT01790568 |
| JAK inhibitors (itacitinib, ruxolitinib) | Reduction of proinflammatory cytokines, T-cell activation and function, preserves Tregs, GVL effect | Preclinical studies and use in treatment setting | NCT03320642 |
| Manipulation of the microbiome | Association of loss of diversity with increased GVHD and TRM; mediate anti-inflammatory cytokines and Tregs | Pilot study of fecal microbiota transfer early posttransplant: 2 of 13 developed acute GI GVHD66 | NCT02763033 |
| NCT02641236 | |||
| NCT03102060 | |||
| NCT03529825 | |||
| Treatment | |||
| Sirolimus | Inhibition of mTOR impairs T-cell signaling | Retrospective study of sirolimus as primary therapy for acute GVHD: 50% achieved CR vs 59% (matched historical control using 1 mg/kg prednisone)67 | NCT02806947 |
| JAK inhibitors (ruxolitinib, itacitinib) | Reduction of proinflammatory cytokines, T-cell activation and function, preserves Tregs, GVL effect | Retrospective study of steroid refractory acute GVHD with ruxolitinib, ORR 81.5%, and CR 46.3%68 | NCT03139604 |
| Phase 1 study of itacitinib in acute GVHD, ORR 88.3% first line and 64.7% steroid-refractory GVHD69 | |||
| α-1 antitrypsin | Serine protease inhibitor that modulates immune and inflammatory function through cytokine profiles | Phase 1/2 study of 12 patients with steroid-refractory GVHD: ORR 8 of 12, CR 4 of 1270 | NCT01700036 |
| NCT02953122 | |||
| NCT03172455 | |||
| IL-22 | Acts on intestinal stem cells to strengthen epithelial barrier function; tissue repair | Preclinical murine models show reduced mortality and improved intestinal pathology from GVHD with in vivo IL-2271 | NCT02406651 |
| Monoclonal antibodies (natalizumab, vedolizumab) | Targeting α4-integrins on activated lymphocytes mediating adhesion and trafficking | Used in inflammatory bowel diseases; case series of 5 patients with grade 4 GI GVHD, with responses in all patients72 | NCT02176031 |
| NCT02133924 | |||
| NCT02993783 | |||
| Extracorporeal photopheresis | Induction of Tregs(?), unknown | Retrospective studies of steroid refractory acute GHVD, ORR of ∼60%73 | NCT02524847 |
| NCT02151539 | |||
| Mesenchymal stromal cells | Inhibition of B- and T-cell activation, APCs, NK cells and increase Tregs | Several early phase studies with ORR 60-75%74 | NCT00603330 |
| NCT02687646 | |||
| NCT02336230 | |||
| NCT02770430 | |||
| NCT02359929 | |||
| Fecal microbiota transplant | Association of loss of diversity with increased GVHD and TRM; mediate anti-inflammatory cytokines and Tregs | Case series of fecal microbiota transplant: 3 out of 4 patients with CR48 | NCT03359980 |
| NCT03214289 | |||
| NCT03148743 |
| Therapies . | Mechanisms of action . | Data . | Ongoing clinical trials . |
|---|---|---|---|
| Prevention | |||
| Tocilizumab | Human monoclonal antibody against IL-6R | Phase 2 study of tocilizumab + Tac + MTX: 14% grade 2-4 acute GVHD, 3% grades 3 and 4 acute GVHD at 100 d39 | NCT03434730 |
| Abatacept | Costimulation blockade of CD28:CD80/86 to inhibit T cells | 2 of 10 patients with grade 2-4 acute GVHD, no day 100 TRM40 | NCT01743131 |
| NCT02867800 | |||
| Tregs | Regulate self-tolerance, limit GVHD while maintaining GVL effect | Modified expanded umbilical cord blood–derived Tregs: grade 2-4 acute GVHD 9% at 100 d42 | NCT01660607 |
| NCT00602693 | |||
| NCT01818479 | |||
| NCT01795573 | |||
| T-cell depletion (CD34 selection and selective ex vivo T-cell depletion) | Depletion of alloreactive T cells and selective αβ T-cell depletion, with preservation of γδ T cells and NK cells | CD34+ selection: grade 2-4 acute GVHD 22.7%, chronic GVHD 6.8%29 | NCT02323867 |
| NCT02600208 | |||
| NCT03301168 | |||
| NCT03047746 | |||
| NCT02345850 | |||
| Statins | Inhibit proinflammatory Th-1 differentiation, induce Treg expansion, and downregulate APCs | Phase 2 study of statin to both donors and recipients with Tac + MTX—grade 2-4 3.3%; chronic GVHD 52.3%46 | NCT03066466 |
| Vorinostat | Histone deacetylase inhibitor decreases inflammatory cytokines, enhances Treg function, and reduces GVHD while preserving GVL | Phase 2 study of vorinostat + Tac + MTX: grade 2-4 acute GVHD 22%, grades 3 and 4 acute GVHD 8%; chronic GVHD 29%51 | NCT01790568 |
| JAK inhibitors (itacitinib, ruxolitinib) | Reduction of proinflammatory cytokines, T-cell activation and function, preserves Tregs, GVL effect | Preclinical studies and use in treatment setting | NCT03320642 |
| Manipulation of the microbiome | Association of loss of diversity with increased GVHD and TRM; mediate anti-inflammatory cytokines and Tregs | Pilot study of fecal microbiota transfer early posttransplant: 2 of 13 developed acute GI GVHD66 | NCT02763033 |
| NCT02641236 | |||
| NCT03102060 | |||
| NCT03529825 | |||
| Treatment | |||
| Sirolimus | Inhibition of mTOR impairs T-cell signaling | Retrospective study of sirolimus as primary therapy for acute GVHD: 50% achieved CR vs 59% (matched historical control using 1 mg/kg prednisone)67 | NCT02806947 |
| JAK inhibitors (ruxolitinib, itacitinib) | Reduction of proinflammatory cytokines, T-cell activation and function, preserves Tregs, GVL effect | Retrospective study of steroid refractory acute GVHD with ruxolitinib, ORR 81.5%, and CR 46.3%68 | NCT03139604 |
| Phase 1 study of itacitinib in acute GVHD, ORR 88.3% first line and 64.7% steroid-refractory GVHD69 | |||
| α-1 antitrypsin | Serine protease inhibitor that modulates immune and inflammatory function through cytokine profiles | Phase 1/2 study of 12 patients with steroid-refractory GVHD: ORR 8 of 12, CR 4 of 1270 | NCT01700036 |
| NCT02953122 | |||
| NCT03172455 | |||
| IL-22 | Acts on intestinal stem cells to strengthen epithelial barrier function; tissue repair | Preclinical murine models show reduced mortality and improved intestinal pathology from GVHD with in vivo IL-2271 | NCT02406651 |
| Monoclonal antibodies (natalizumab, vedolizumab) | Targeting α4-integrins on activated lymphocytes mediating adhesion and trafficking | Used in inflammatory bowel diseases; case series of 5 patients with grade 4 GI GVHD, with responses in all patients72 | NCT02176031 |
| NCT02133924 | |||
| NCT02993783 | |||
| Extracorporeal photopheresis | Induction of Tregs(?), unknown | Retrospective studies of steroid refractory acute GHVD, ORR of ∼60%73 | NCT02524847 |
| NCT02151539 | |||
| Mesenchymal stromal cells | Inhibition of B- and T-cell activation, APCs, NK cells and increase Tregs | Several early phase studies with ORR 60-75%74 | NCT00603330 |
| NCT02687646 | |||
| NCT02336230 | |||
| NCT02770430 | |||
| NCT02359929 | |||
| Fecal microbiota transplant | Association of loss of diversity with increased GVHD and TRM; mediate anti-inflammatory cytokines and Tregs | Case series of fecal microbiota transplant: 3 out of 4 patients with CR48 | NCT03359980 |
| NCT03214289 | |||
| NCT03148743 |
APC, antigen-presenting cell; CR, complete response; GI, gastrointestinal; GVL, graft-versus-leukemia; IL-6R, interleukin-6 receptor; JAK, janus kinase; mTOR, mammalian target of rapamycin complex; NK, natural killer; ORR, overall response rate.
IL-6 plays an important role in inflammation and immune regulation and has been implicated in a variety of immune-mediated inflammatory diseases. Experimental models show increased IL-6 receptor levels during GVHD, with reduction in GVHD with blockade of IL-6. Tocilizumab, a human monoclonal antibody against IL-6R, in combination with standard GVHD prophylaxis has resulted in encouragingly low rates of GVHD, warranting additional investigation in a randomized trial.32,33
Studies targeting in vivo T-cell costimulation blockade as a way to inhibit T cells and prevent GVHD have identified abatacept or cytotoxic T lymphocyte antigen 4-immunoglobin, a selective inhibitor of CD28:CD80/86, as a potential strategy for GVHD prophylaxis. Abatacept is approved for the use of autoimmune arthritis, and a small first-in disease trial in GVHD has shown promising low rates of GVHD and no TRM at day 100, although higher rates of viral reactivation.34 A phase 2 multicenter, randomized, double-blind trial of abatacept in combination with standard GVHD prophylaxis is currently being conducted (NCT01743131).
Tregs are important regulators of self-tolerance and have been found in preclinical models to suppress the expansion of alloreactive donor T cells and limit GHVD while maintaining the graft-versus-leukemia effect.35 The safety and feasibility of Treg infusion were initially shown in 2 clinical trials using Tregs isolated and expanded from partially HLA-matched umbilical cord units and donor Tregs in the haploidentical setting, showing favorable GVHD outcomes.36,37 Challenges continue to remain in Treg purity and expansion on a larger scale; however, this continues to be a promising GVHD prophylaxis approach and target,38 with several ongoing studies (NCT 01660607, NCT00602693, NCT01818479, and NCT01795573).
3-Hydroxy-3-methyl-coenzyme A reductase inhibitors or “statins” have been shown to have immunomodulatory and anti-inflammatory properties by inhibiting proinflammtory TH-1 differentiation, inducing Treg expansion, and downregulating antigen-presenting cells.39 Preclinical studies of atorvastatin showed protective effects against GVHD. A prospective phase 2 clinical study modeling murine experiments of atorvastatin administration to both donors and recipients showed a promisingly low incidence of GVHD (3.3%) in matched sibling donors40 ; however, a subsequent second single-institution phase 2 study did not show any difference in incidence of GVHD but favorable overall survival compared with historical controls.41 A second study by Hamadani et al40 using atorvastatin in recipients only of matched sibling and unrelated donor transplants also showed safety and a favorably low incidence of acute GVHD.42 A randomized, open label, phase 3 study of Tac + MTX with or without atorvastatin administration in matched unrelated donor transplants is currently ongoing (NCT03066466).
Vorinostat is a histone deacetylase inhibitor used in the treatment of cutaneous T-cell lymphoma. At low and noncytotoxic concentrations, vorinostat has been shown to possess anti-inflammatory and immunoregulatory effects. Experimental GVHD models have shown that vorinostat decreases inflammatory cytokines, enhances Treg function, and reduces GVHD while preserving a graft-versus-leukemia effect.43 A phase 1/2 clinical trial of vorinostat with standard GVHD prophylaxis showed safety and feasibility and resulted in relatively low rates of GVHD in matched sibling donor transplants.44 A subsequent phase 2 trial of vorinostat with Tac + MTX in the myeloablative unrelated donor setting again showed safe administration along with encouragingly low rates of acute GVHD (22%) and favorable survival (76%).45
Alteration in the gastrointestinal microbiome has also become an active area of study in the prevention and treatment of GVHD.46 Recent small studies have shown the success of fecal microbiota transplantation in the treatment of steroid-refractory gastrointestinal GVHD.47 Subsequently, a pilot study of third-party fecal microbiota transplantation administered early after neutrophil engraftment post-HCT has shown proof of principle for expansion of recipient microbiome diversity. Although too early to correlate with GVHD outcomes, this approach to repopulate intestinal microbiota was shown to be feasible, safe, and a potential novel strategy in GVHD prevention.48
Novel preclinical GVHD preventative strategies
There are several novel strategies being studied in the preclinical setting for the prevention of GVHD. These approaches focus on targeting cytokine receptors, proinflammatory pathways, and the intestinal microbiome among many others. New targets include the inhibition of Aurora kinase A and the Janus Kinase/signal transducer and activator of transcription pathway,49,50 inhibition of mitogen-activated protein kinase,51 and improving the expansion and suppressive capabilities of Tregs,52,53 with the goal of suppressing GVHD without affecting the graft-versus-leukemia effect. Additional investigations into the gastrointestinal microbiome also show that dysbiosis and loss of Paneth cells have been shown to be critical in the development of GVHD. Recent murine models have shown that R-spondin-1, a Wnt agonist, protects intestinal stem cells from injury by expanding Paneth cells and enhancing secretion of antimicrobial α-defensins, preventing GVHD-mediated dysbiosis.54 Although most of these approaches remain in the laboratory, the preferential targeting of the microbiome, cytokine, and inflammatory pathways holds the promise of improved GVHD prevention, moving away from our standard broad-based immunosuppressive approaches that are often limited by increased disease relapse and infection.
Treatment
Glucocorticoids remain the only standard initial treatment of acute GVHD, despite response rates of only 40% to 60%.1,55 Studies have shown no advantage to initial treatment with corticosteroid (prednisone-equivalent) doses >2.5 mg/kg per day,56 and in patients with grade 2 GVHD, studies have shown no disadvantage to starting doses of 1 mg/kg per day.55 Patients with severe GVHD tend to be less responsive to steroids, leading to high TRM. Although several agents have been evaluated in the upfront and second-line setting, no proven therapy other than corticosteroids has been shown to be more effective or uniformly adopted to date.1,57
Risk stratification
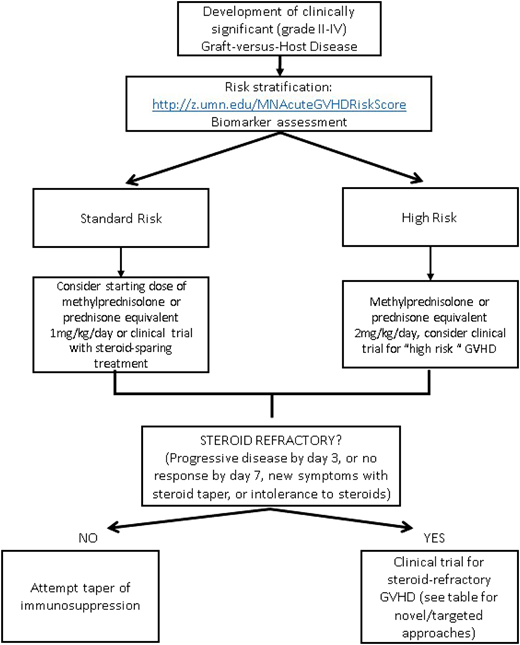
The identification and risk stratification of patients for treatment based on clinical staging and blood biomarkers have thus been proposed as a new treatment paradigm to identify those who are at greatest risk and require more aggressive upfront therapy while sparing those who are likely to respond from excess toxicity. The refined Minnesota acute GVHD risk score was developed to stratify patients into standard risk, which is defined as single-organ involvement (stage 1-3 skin or stage 1 or 2 gastrointestinal) or 2-organ involvement (stage 1-3 skin plus stage 1 gastrointestinal or stage 1-3 skin plus stage 1-4 liver), and high risk (all others). Patients identified as high-risk GVHD are less likely to respond to therapy and have an increased risk of TRM.58 The Ann Arbor biomarker risk score based on plasma levels of tumor necrosis factor receptor-1, regenerating islet-derived 3-α (REG3α), and suppression of tumorigenicity 2 (ST2) has also been developed and validated to identify patients less likely to respond to treatment.59 This has been further refined (MAGIC biomarkers) to include just 2 biomarkers (REG3α and ST2)60 that have prognostic utility at diagnosis as well as time of clinical response. Data on using microRNA (miRNA), which regulates proinflammatory genes and signaling,61 as a biomarker are also emerging. The detection of multiple miRNAs in the serum has been strongly associated with GVHD, and an miRNA signature may serve as a specific independent biomarker to diagnose and predict severity of GVHD.62 By evaluating novel therapies based on risk stratification models, we may be able to improve outcomes for the patients at the highest risk of treatment failure while minimizing toxicities.
Upfront GVHD therapy
Although several past studies have combined the use of immunosuppressive agents (eg, ATG,63 infliximab,64 MMF, etanercept, and pentostatin65 ) with corticosteroids as frontline therapy, no strategy has been identified to be beneficial beyond steroids alone.1,57 These studies underscore not only the importance of risk stratification approaches to identify the highest-risk patients but also, the critical need for GVHD approaches beyond blanket immunosuppression.
More recently, sirolimus as a single agent has been studied as upfront treatment in patients with newly diagnosed acute GVHD, showing safety and efficacy.66 This has led to an ongoing BMT-CTN (1501) phase 2 trial using sirolimus vs prednisone for first-line treatment of Minnesota standard risk acute GVHD, with additional stratification by the Ann Arbor biomarker risk score (NCT02806947). This study recently completed accrual, and results are eagerly awaited.
Second-line therapy for GVHD
There is no standard indication or timing for the initiation of second-line therapy for acute GVHD. Steroid-refractory GVHD is typically defined by progressive symptoms after 3 days of therapy or lack of improvement after 1 to 2 weeks, depending on severity of symptoms. Poor tolerance of high-dose steroids may also be an indication to start second-line therapy. Although many prospective and retrospective analyses have been done evaluating second-line treatments, no effective adjunctive therapy has been identified, and it is unfortunately often characterized by lack of response, significant toxicities, and subsequent high TRM.1 Given the lack of a superior second-line therapy and Food and Drug Administration–approved agent for the treatment of acute GVHD, enrollment in a well-designed clinical trial should always be encouraged. In the absence of a trial, there are several novel therapeutic options available; however, the choice of any secondary agent is acknowledged as “off label” and guided by side effect profile, cost/availability, and physician preference and discretion. Table 1 provides a brief summary of some of the current novel second-line strategies for steroid-refractory acute GVHD,47,66-73 some of which are reviewed in more detail in a subsequent accompanying paper. Novel targets for the treatment of chronic GVHD are not included and are beyond the scope of this paper. An algorithm for a proposed treatment approach is shown in Figure 2.
Summary and conclusions
GVHD remains a significant complication after allogeneic HCT, limiting its success as a curative therapy. Additional understanding of the biology and pathogenesis of GVHD has improved our approaches to safer and more targeted strategies. Continued effective translation of preclinical experimental models into clinical implementation will be needed, and as we continue to identify more therapies for prevention and treatment, planning of well-designed, multicenter, randomized clinical trials will be critical to identify the most optimal approaches to GVHD.
Correspondence
Betty Ky Hamilton, Blood and Marrow Transplant Program, Hematology and Medical Oncology, Taussig Cancer Institute, Cleveland Clinic, 9500 Euclid Ave, CA60, Cleveland, OH; e-mail: hamiltb2@ccf.org.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.