Abstract
Although the seminal event in sickle cell disease is the polymerization of abnormal hemoglobin, the downstream pathophysiology of vasoocclusion results from heterotypic interactions between the altered, adhesive sickle cell red blood cells, neutrophils, endothelium, and platelets. Ischemia reperfusion injury, hemolysis, and oxidant damage all contribute to heightened inflammation and activation of the hemostatic system. These various pathways are the focus of emerging treatments with potential to ameliorate disease manifestations. This review summarizes the considerable progress in development of these agents despite challenges in selection of study end points and complex pathophysiology.
Learning Objectives
Describe the pathophysiology of sickle cell disease, including the roles of adhesion, oxidative stress, and inflammation
Summarize the current novel therapies for sickle cell disease
Introduction
Sickle cell disease (SCD), an inherited disease caused by a mutation in the β globin gene, was first described more than 100 years ago. Since then, there has been a steady increase in the understanding of the complex pathophysiology of the disease. It is only in recent years, however, that there has been a robust interest in the development of pharmacologic agents targeting the myriad pathways. Although there have been improvements in survival and quality of life for pediatric patients with SCD in the developed world, the adult disease burden and decreased life expectancy persist.1,2 There remains an urgent unmet need to develop novel treatments that will improve outcomes across the lifespan and also be applicable in low-resource settings. In this review, we aim to highlight updates in pathophysiology and novel agents currently under investigation in clinical trials. Barriers to the success of clinical trials include, but are not limited to, significant interpatient disease variability and appropriate choice of clinical and biomarker end points. Although the disease variability can theoretically be statistically accounted for by appropriate power calculations and a larger sample size, it remains a significant challenge. This is especially true for studies examining treatment of acute vasoocclusion crises (VOCs), which commonly use length of VOC and total opioid use as study end points. Variability in an individual’s opioid tolerance, coping abilities, and disease severity can greatly affect such end points. Studies targeted at preventing complications and acute events are increasingly incorporating patient-reported outcomes and diary reports rather than simply relying on acute health care use. This is crucial, because the PISCES study showed that adults accessed the health care system on 3.5% of diary days, although 29.3% of patients reported pain on >95% of the diary days.3 At the time of this review, there are 166 active or recruiting interventional trials for patients with SCD. We discuss the potential for further development of 33 pharmacologic agents currently in clinical trials based on positive or pending results. Of these, 10 agents are being studied for their impact during an acute VOC. Although clinical trials that reported negative results in the last 2 years are summarized, the discussion of other trials with negative results and promising pharmacologic agents in preclinical development is outside the scope of this manuscript.
Adhesion in SCD
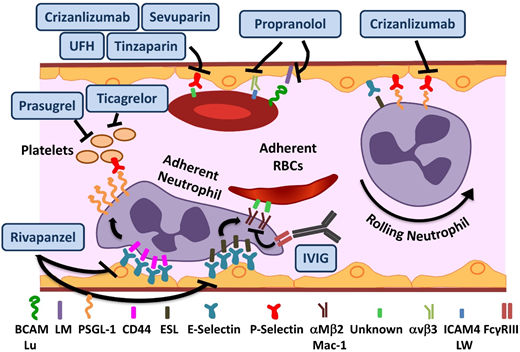
In addition to the deformation of red blood cells (RBCs) from polymerization, sickle hemoglobin (Hb) can undergo autooxidation, resulting in precipitation on the inner surface of the RBC membrane. This results in iron-mediated oxidant damage to the RBC membrane4 as well as alteration of the lipid profile. The damaged sickle RBCs (sRBCs) have a propensity to adhere to and interact with the endothelium via a myriad of adhesive molecules,5 leading to production of oxygen radicals by the endothelial cells. As detailed in “Oxidative stress, NO, and inflammation in SCD,” additional sources of reactive oxygen species (ROS) include damaged tissues, sRBCs, neutrophils, and platelets. The subsequent oxidant activation of NF-κB within the endothelium further results in upregulation of the endothelial adhesion molecules VCAM-1, ICAM-1, E, and E-selectin.6 Various studies have emphasized the pivotal and surprising role of neutrophils in a disease in which the seminal event occurs in the RBC. Both endothelial selectins (E and P) mediate leukocyte-endothelial binding, resulting in leukocyte recruitment to the vessel wall (Figure 1), predominantly in the postcapillary venules.7,8 Adherent neutrophils receive a secondary wave of signals transduced through E-selectin, leading to activation of Mac-1 integrin and resultant capture of sRBCs (Figure 1). The early success of agents targeting endothelial selectins is further validation of the important role of heterotypic adhesive interactions and neutrophils in mediating vasoocclusion. The adhesive interactions in SCD and drugs targeting them are summarized in Figure 1 and Table 1.
Novel agents targeting adhesion and coagulation in SCD. Numerous adhesive interactions among sRBCs, neutrophils, and endothelial cells contribute to sickle cell vasoocclusion. Activation of endothelial cells leads to the recruitment of neutrophils, which is initiated by rolling of neutrophils on endothelial selectins, followed by adhesion. Adherent neutrophils receive a secondary wave of signals transduced through E-selectin, leading to the activation of αMβ2 (Mac-1) integrin on the leading edge. Activated Mac-1 on adherent neutrophils mediates the capture of circulating sRBCs. In addition, sRBCs express multiple adhesion molecules that interact with ligands on endothelial cells or the subendothelial matrix either directly or via bridging molecules in the plasma. Rivipansel targets E-selectin predominantly, whereas crizanlizumab and sevuparin inhibit P-selectin–mediated adhesive interactions. IV immunoglobulin (IVIG) interferes with neutrophil-mediated sRBC capture. Propranol blocks various sRBC-endothelial interactions that are stimulated by β-adrenergic signaling, such as LW (ICAM4)-αVβ3 and BCAM/lu-laminin (LM). Unfractionated heparin (UFH) and tinzaparin, in addition to their anticoagulant effects, target P-selectin. ESL, E-selectin ligand; PSGL-1, P-selectin ligand 1. Adapted from Morrone et al48 with permission.
Novel agents targeting adhesion and coagulation in SCD. Numerous adhesive interactions among sRBCs, neutrophils, and endothelial cells contribute to sickle cell vasoocclusion. Activation of endothelial cells leads to the recruitment of neutrophils, which is initiated by rolling of neutrophils on endothelial selectins, followed by adhesion. Adherent neutrophils receive a secondary wave of signals transduced through E-selectin, leading to the activation of αMβ2 (Mac-1) integrin on the leading edge. Activated Mac-1 on adherent neutrophils mediates the capture of circulating sRBCs. In addition, sRBCs express multiple adhesion molecules that interact with ligands on endothelial cells or the subendothelial matrix either directly or via bridging molecules in the plasma. Rivipansel targets E-selectin predominantly, whereas crizanlizumab and sevuparin inhibit P-selectin–mediated adhesive interactions. IV immunoglobulin (IVIG) interferes with neutrophil-mediated sRBC capture. Propranol blocks various sRBC-endothelial interactions that are stimulated by β-adrenergic signaling, such as LW (ICAM4)-αVβ3 and BCAM/lu-laminin (LM). Unfractionated heparin (UFH) and tinzaparin, in addition to their anticoagulant effects, target P-selectin. ESL, E-selectin ligand; PSGL-1, P-selectin ligand 1. Adapted from Morrone et al48 with permission.
Novel agents targeting adhesion
| Name . | Mechanism . | Trial phase, type, NCT# . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| Rivipansel (GMI-1070)* | Small-molecule pan-selectin antagonist; maximal activity against E-selectin | 1/2, single arm open label, NCT00911495 | Safety | Completed | Well tolerated; increase in total WBC, without associated symptoms76 |
| 2, RDBPC, NCT01119833 | Time to resolution of VOC | Completed | Trend in expected direction in primary end point | ||
| Cumulative opioid use significantly decreased (by 83%; P = .010)10 | |||||
| 3, RDBPC, NCT02187003 | Time to readiness for discharge | Recruiting | All SCD genotypes; age ≥6 y | ||
| IV infusion every 12 h during VOC | |||||
| SEG101 (crizanlizumab) | Humanized mAb against P-selectin | 2, RDBPC, NCT1895361 | Annual rate of sickle cell–related pain crises with high-dose crizanlizumab vs placebo | Completed | All SCD genotypes; reduction in pain crisis rate by 45% in high-dose arm; improvement in various secondary end points; no improvement in markers of hemolysis77 |
| 2, single arm open label, NCT03264989 | PKs and PDs | Recruiting | All SCD genotypes | ||
| 2, single arm open label, NCT03474965 | PKs and PDs | Not yet recruiting | All SCD genotypes; age 6 mo to 17 y | ||
| IVIG* | Disrupts neutrophil-mediated sRBC capture | 1, RDBPC, NCT01757418 | Safety, dose finding, and neutrophil activation | Completed | Low-dose IVIG was safe, well tolerated, and stabilized Mac-1 activity14 |
| 2, RDBPC, NCT01757418 | Length of VOC | Recruiting | Single low-dose during VOC | ||
| Sevuparin* | Heparinoid with anti–P- and anti–L-selectin activity | 2, RDBPC, NCT02515838 | Time to resolution of VOC | Recruiting | All SCD genotypes; continuous IV infusion |
| Name . | Mechanism . | Trial phase, type, NCT# . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| Rivipansel (GMI-1070)* | Small-molecule pan-selectin antagonist; maximal activity against E-selectin | 1/2, single arm open label, NCT00911495 | Safety | Completed | Well tolerated; increase in total WBC, without associated symptoms76 |
| 2, RDBPC, NCT01119833 | Time to resolution of VOC | Completed | Trend in expected direction in primary end point | ||
| Cumulative opioid use significantly decreased (by 83%; P = .010)10 | |||||
| 3, RDBPC, NCT02187003 | Time to readiness for discharge | Recruiting | All SCD genotypes; age ≥6 y | ||
| IV infusion every 12 h during VOC | |||||
| SEG101 (crizanlizumab) | Humanized mAb against P-selectin | 2, RDBPC, NCT1895361 | Annual rate of sickle cell–related pain crises with high-dose crizanlizumab vs placebo | Completed | All SCD genotypes; reduction in pain crisis rate by 45% in high-dose arm; improvement in various secondary end points; no improvement in markers of hemolysis77 |
| 2, single arm open label, NCT03264989 | PKs and PDs | Recruiting | All SCD genotypes | ||
| 2, single arm open label, NCT03474965 | PKs and PDs | Not yet recruiting | All SCD genotypes; age 6 mo to 17 y | ||
| IVIG* | Disrupts neutrophil-mediated sRBC capture | 1, RDBPC, NCT01757418 | Safety, dose finding, and neutrophil activation | Completed | Low-dose IVIG was safe, well tolerated, and stabilized Mac-1 activity14 |
| 2, RDBPC, NCT01757418 | Length of VOC | Recruiting | Single low-dose during VOC | ||
| Sevuparin* | Heparinoid with anti–P- and anti–L-selectin activity | 2, RDBPC, NCT02515838 | Time to resolution of VOC | Recruiting | All SCD genotypes; continuous IV infusion |
Unless otherwise specified, studies included only adult patients with HbSS or HbSβ0.
mAb, monoclonal antibody; PD, pharmacodynamic; RDBPC, randomized double blind placebo controlled; WBC, white blood cell count.
Study drug administered during acute VOC.
In preclinical SCD mouse models, rivipansel (GMI-1070), a small-molecule panselectin antagonist with greatest activity against endothelial E-selectin,9 resulted in reversal of VOCs by decreased leukocyte adhesion and red cell capture (Figure 1) and improved blood flow and survival.9 The multicenter randomized placebo-controlled double-blind phase 2 study of 76 patients age 12 to 60 years demonstrated that it was well tolerated, with a clinically but not statistically significant reduction in the primary end point of time to resolution of VOC (41 vs 63 hours).10 The mean cumulative IV opioid analgesic use, a prespecified secondary end point, was reduced by 83% compared with placebo. These results are extremely promising, given the challenge of modifying outcomes in the setting of an established VOC. The time to transition to oral analgesics is an important and robust end point. It reached statistical significance in the pediatric GMI-1070 cohort, suggesting a greater potential for reversibility in younger patients. Patients receiving long-acting opioids, and therefore presumably with chronic pain, reported higher pain scores but responded to the study drug similarly to the rest of the cohort, suggesting that although these patients should be analyzed separately, they should not be excluded a priori from trials. Currently, the multicenter phase 3 trial for patients with SCD age >6 years with pain crisis is recruiting patients.
Crizanlizumab is a humanized monoclonal antibody against P-selectin expressed on the endothelium and platelets (Figure 1). Endothelial P-selectin mediates direct sRBC adhesion to endothelium as well as leukocyte recruitment and rolling (Figure 1). Crizanlizumab demonstrated a tolerable adverse effect profile, with a decrease in the frequency of pain crises in the high-dose arm in a phase 2 study (SUSTAIN trial) in adults.11 In this successful study, a total of 198 patients were randomized to receive 14 doses of study drug vs placebo administered IV over 52 weeks. This resulted in a 45% reduction in VOC events in the high-dose treatment arm compared with placebo. There was a statistically significant improvement in numerous secondary end points, including a significant increase in time to first and second crises. Although the study drug used in this phase 2 study was produced from the SelG1 cell line, a new cell line (SEG101) has now been developed. Additional pharmacokinetic (PK) and pharmacodynamic data are required to assess crizanlizumab produced using the SEG101 cell line and confirm the recommended dose. Currently, there is a phase 2 multicenter trial recruiting patients to evaluate pharmacokinetics (PK) and PD in adults and another registered study for dose confirmation and safety evaluation in pediatrics of the SEG101 product. On the basis of the clearly positive results, a phase 3 study will undoubtedly follow, but such a study is not currently registered at www.clinicaltrials.gov.
IVIG binds to FcγRIII receptors expressed on neutrophils (Figure 1), leading to SHP-1–mediated inhibition of Src kinase pathway activity and reduction in Mac-1 integrin activation on the leading edge of the adherent neutrophils.12 In preclinical SCD mouse models, VOC reversal resulted from decreased neutrophil adhesion and sRBC capture.13 IVIG was well tolerated in the phase 1 trial, with a reduction in neutrophil Mac-1 activity.14 The phase 2 trial of IVIG for use in acute VOCs is currently recruiting patients.
Sevuparin, a chemically modified heparin derivative, has low anticoagulant activity becaause of elimination of antithrombin binding domains, thus reducing the risks of hemorrhagic complications. It retains its antiadhesive properties, with inhibition of P- and L-selectins (Figure 1). The reduction in adhesion of RBCs to endothelial cells in vitro15 as well as other targets results in improvement of VOCs in preclinical models.16 A phase 2 multicenter trial is currently recruiting patients to evaluate its role in acute VOCs.
Oxidative stress, NO, and inflammation in SCD
The damaged sRBCs and activated endothelial cells lead to ongoing intermittent vasoocclusion with subsequent ischemia-reperfusion injury, a potent trigger for inflammation.17 Hemolysis of sRBCs further exacerbates the proinflammatory microenvironment with the release of cell-free Hb, ROS, and arginase.18 Cell-free Hb scavenges nitric oxide (NO), causing vasoconstriction, upregulation of endothelial adhesion molecules, and activation of platelets, and heme contributes to the activation of the innate immune inflammatory pathways via toll-like receptor 4.19 ROS elevation within RBCs and the intravascular space in SCD originates from numerous sources. sRBCs generate increased amounts of ROS and have an impaired ability to neutralize the ROS because of reduction of endogenous antioxidants. Ischemia-reperfusion injury and NO depletion contribute to further ROS production from damaged tissues, activated neutrophils, and platelets.18 Transfusion-associated iron overload leads to further ROS production. ROS activates monocytes and polymorphonuclear leukocytes in a feed-forward loop, leading to further exacerbation of inflammation, endothelial damage, and adhesion. Recent years have seen a significant expansion in the number of emerging therapies targeting oxidative stress and the NO pathway, with promising early results. These are discussed here and summarized in Table 2 and Figures 2 and 3.
Novel agents targeting inflammation
| Name . | Mechanism . | Trial phase, type, NTC # . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| Glutamine | NADH producer | 3, RDBPC, NCT01179217 | N of crises | FDA approved | Age >5 y; reduction in VOCs77 |
| Omega-3 fatty acids | Reduces endothelial activation; improved sRBC membrane and NO production | 2, RDBPC, ISRCTN80844630 | Annualized rate of VOC hospitalizations | Completed | Age 2-24 y; improved rates of VOC, anemia, and number of transfusions23 |
| 1/2, open label single arm, NCT02947100 | Safety in dose escalation, reduction in thermal sensitivity | Recruiting | Age 8-25 y | ||
| 3, RPC triple blind, NCT02525107 | Frequency and severity of VOCs, duration of hospitalization | Not yet recruiting | Age 13-70 y | ||
| 2, RDBPC, NCT02973360 | Change in baseline blood cells omega-3 fatty acid index | Completed | All SCD genotypes; age 5-17 y; advanced lipid technologies | ||
| Well tolerated with reduction in diary-reported home-managed SCD pain78 | |||||
| 3, RPC triple blind, NCT02604368 | Annualized VOC rate | Not yet recruiting | All SCD genotypes; using advanced lipid technologies for improved bioavailability | ||
| N-acetylcysteine | Reduces oxidative stress and increases glutathione | 3, RDBPC, NCT01849016 | Rate of pain d per y (diary) | Completed | All SCD genotypes; trends toward lower rates of pain, VOCs, did not reach significance; study limited by poor adherence28 |
| 1/2, single arm open label, NCT01800526 | VWF activity | Enrolling by invitation | |||
| NKTT120 | 1, open label single arm, NCT01783691 | Safety | Completed | Well tolerated; depleted iNKTs for 2-5 mo33 | |
| Arginine* | NO substrate | 2, RDBPC, NCT01796678 | Length of stay | Completed | All SCD genotypes; single center; trend toward decreased length of stay |
| Reduction in total parenteral opioid use by 54% (P = .02)37 | |||||
| 2, RDBPC, NCT02536170 | Total parenteral opioid use | Recruiting | All SCD genotypes; age 3-21 y; multicenter | ||
| 1/2, open label randomized crossover design, NCT02447874 | PK and change in NO metabolites | Enrollingby invitation | Age 7-21 y | ||
| 2, randomized open label, NCT00004412 | Leg ulcer healing | Completed | All SCD genotypes; improved leg ulcer healing79 | ||
| Citrulline* | Precursor to arginine | 1, open label, NCT02314689/NCT02697240 | AEs and citrulline level | Completed | All SCD genotypes; age 6-50 y; IV formulation; well tolerated40 |
| Riociguat | Stimulates soluble guanylate cyclase | 2, RDCPC, NCT02633397 | Treatment-related SAEs | Recruiting | All SCD genotypes |
| IW-1701 | Stimulates soluble guanylate cyclase | 2, RDBPC, NCT03285178 | Treatment-emergent AEs | Recruiting | All SCD genotypes; age 16-70 y |
| IMR-687 | PDE9 inhibitor | 2a, RDBPC, NCT03401112 | Treatment-related AEs | Recruiting | All SCD genotypes |
| Simvastatin | Upregulates NOS, reduces endothelial dysfunction | 1/2, open label single arm, NCT00508027 | Change in baseline labs | Completed | All SCD genotypes; increased NO metabolites, decreased CRP and IL-645 |
| 1/2, open label single arm, NCT01702246 | Change in frequency of vasoocclusive pain events | Completed | Decreased rates of pain and analgesic use; improved inflammatory markers46 | ||
| Atorvastatin | Upregulates NOS | 2, RDBPC crossover, NCT01732718 | Change in endothelial function on USG imaging | Completed | |
| Montelukast | Cysteinyl leukotriene receptor antagonist | 2, RDBPC, NCT01960413 | Improvement in soluble VCAM | Completed | Age 16-70 y |
| Zileuton | 5-lipoxygenase inhibitor | 1, open label single arm, NCT01136941 | AEs | Completed | All SCD genotypes; age >12 y; well tolerated49 |
| Mometasone | Inhaled corticosteroid | 2, RDBPC, NCT02061202 | Feasibility | Completed | Age ≥15 y; decreased soluble VCAM and daily pain50 |
| Ambrisentan | Endothelin receptor antagonist | 1, RPC, NCT02712346 | Safety and tolerability | Recruiting |
| Name . | Mechanism . | Trial phase, type, NTC # . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| Glutamine | NADH producer | 3, RDBPC, NCT01179217 | N of crises | FDA approved | Age >5 y; reduction in VOCs77 |
| Omega-3 fatty acids | Reduces endothelial activation; improved sRBC membrane and NO production | 2, RDBPC, ISRCTN80844630 | Annualized rate of VOC hospitalizations | Completed | Age 2-24 y; improved rates of VOC, anemia, and number of transfusions23 |
| 1/2, open label single arm, NCT02947100 | Safety in dose escalation, reduction in thermal sensitivity | Recruiting | Age 8-25 y | ||
| 3, RPC triple blind, NCT02525107 | Frequency and severity of VOCs, duration of hospitalization | Not yet recruiting | Age 13-70 y | ||
| 2, RDBPC, NCT02973360 | Change in baseline blood cells omega-3 fatty acid index | Completed | All SCD genotypes; age 5-17 y; advanced lipid technologies | ||
| Well tolerated with reduction in diary-reported home-managed SCD pain78 | |||||
| 3, RPC triple blind, NCT02604368 | Annualized VOC rate | Not yet recruiting | All SCD genotypes; using advanced lipid technologies for improved bioavailability | ||
| N-acetylcysteine | Reduces oxidative stress and increases glutathione | 3, RDBPC, NCT01849016 | Rate of pain d per y (diary) | Completed | All SCD genotypes; trends toward lower rates of pain, VOCs, did not reach significance; study limited by poor adherence28 |
| 1/2, single arm open label, NCT01800526 | VWF activity | Enrolling by invitation | |||
| NKTT120 | 1, open label single arm, NCT01783691 | Safety | Completed | Well tolerated; depleted iNKTs for 2-5 mo33 | |
| Arginine* | NO substrate | 2, RDBPC, NCT01796678 | Length of stay | Completed | All SCD genotypes; single center; trend toward decreased length of stay |
| Reduction in total parenteral opioid use by 54% (P = .02)37 | |||||
| 2, RDBPC, NCT02536170 | Total parenteral opioid use | Recruiting | All SCD genotypes; age 3-21 y; multicenter | ||
| 1/2, open label randomized crossover design, NCT02447874 | PK and change in NO metabolites | Enrollingby invitation | Age 7-21 y | ||
| 2, randomized open label, NCT00004412 | Leg ulcer healing | Completed | All SCD genotypes; improved leg ulcer healing79 | ||
| Citrulline* | Precursor to arginine | 1, open label, NCT02314689/NCT02697240 | AEs and citrulline level | Completed | All SCD genotypes; age 6-50 y; IV formulation; well tolerated40 |
| Riociguat | Stimulates soluble guanylate cyclase | 2, RDCPC, NCT02633397 | Treatment-related SAEs | Recruiting | All SCD genotypes |
| IW-1701 | Stimulates soluble guanylate cyclase | 2, RDBPC, NCT03285178 | Treatment-emergent AEs | Recruiting | All SCD genotypes; age 16-70 y |
| IMR-687 | PDE9 inhibitor | 2a, RDBPC, NCT03401112 | Treatment-related AEs | Recruiting | All SCD genotypes |
| Simvastatin | Upregulates NOS, reduces endothelial dysfunction | 1/2, open label single arm, NCT00508027 | Change in baseline labs | Completed | All SCD genotypes; increased NO metabolites, decreased CRP and IL-645 |
| 1/2, open label single arm, NCT01702246 | Change in frequency of vasoocclusive pain events | Completed | Decreased rates of pain and analgesic use; improved inflammatory markers46 | ||
| Atorvastatin | Upregulates NOS | 2, RDBPC crossover, NCT01732718 | Change in endothelial function on USG imaging | Completed | |
| Montelukast | Cysteinyl leukotriene receptor antagonist | 2, RDBPC, NCT01960413 | Improvement in soluble VCAM | Completed | Age 16-70 y |
| Zileuton | 5-lipoxygenase inhibitor | 1, open label single arm, NCT01136941 | AEs | Completed | All SCD genotypes; age >12 y; well tolerated49 |
| Mometasone | Inhaled corticosteroid | 2, RDBPC, NCT02061202 | Feasibility | Completed | Age ≥15 y; decreased soluble VCAM and daily pain50 |
| Ambrisentan | Endothelin receptor antagonist | 1, RPC, NCT02712346 | Safety and tolerability | Recruiting |
Unless otherwise specified, study included only adult patients with HbSS or HbSβ0.
AE, adverse event; FDA, US Food and Drug Administration; RDBPC, randomized double blind placebo controlled; SAE, serious AE; USG, ultrasound guided.
Study drug administered during acute VOC.
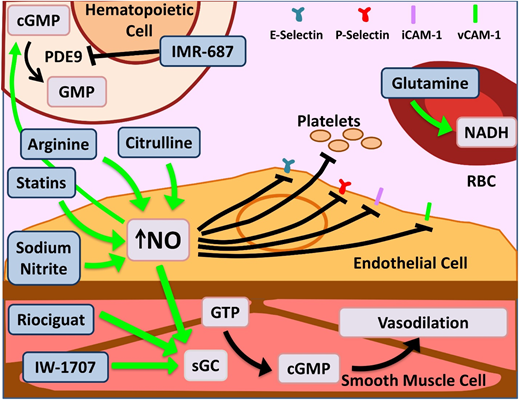
Novel agents targeting inflammation and NO bioavailability. Hemolysis in SCD and the resultant cell-free Hb leads to NO scavenging. Increased bioavailability of NO and its downstream target cyclic guanosine monophosphate (cGMP) lead to salutary effects in the endothelium, smooth muscles, leukocytes, platelets, and increased γ-globin. Therapeutic agents enhancing NO bioavailability by various mechanisms are depicted here. Of note, PDE9 has restricted tissue expression in the hematopoietic cells and brain with the potential for reduced off-target effects as opposed to PDE5, which is more widely expressed. Glutamine increases NADH within RBCs, thus reducing effects of oxidative stress. Dietary glutamine also serves as a precursor for the de novo production of arginine through the citrulline-arginine pathway, contributing to increased NO production. PDE, phosphodiesterase; sGC, soluble guanylate cyclase. Adapted from Morrone et al48 with permission.
Novel agents targeting inflammation and NO bioavailability. Hemolysis in SCD and the resultant cell-free Hb leads to NO scavenging. Increased bioavailability of NO and its downstream target cyclic guanosine monophosphate (cGMP) lead to salutary effects in the endothelium, smooth muscles, leukocytes, platelets, and increased γ-globin. Therapeutic agents enhancing NO bioavailability by various mechanisms are depicted here. Of note, PDE9 has restricted tissue expression in the hematopoietic cells and brain with the potential for reduced off-target effects as opposed to PDE5, which is more widely expressed. Glutamine increases NADH within RBCs, thus reducing effects of oxidative stress. Dietary glutamine also serves as a precursor for the de novo production of arginine through the citrulline-arginine pathway, contributing to increased NO production. PDE, phosphodiesterase; sGC, soluble guanylate cyclase. Adapted from Morrone et al48 with permission.
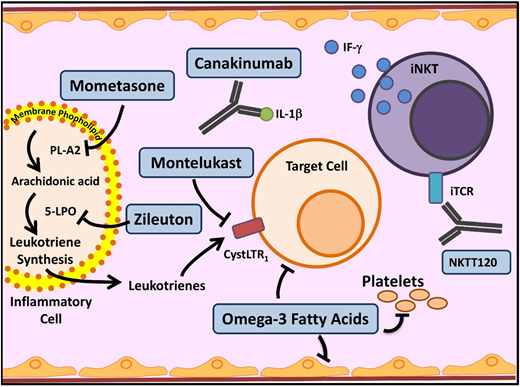
Novel agents targeting inflammation in SCD. Multiple cell types, molecules, and pathways contribute to the chronic inflammatory state in SCD. In SCD, as in asthma, leukocyte membrane phospholipids are hydrolyzed into arachidonic acid (AA), which is metabolized via the 5-lipoxygenase (5-LPO) pathway, leading to formation of inflammatory leukotrienes. Inhibition of this pathway by several agents currently used in asthma (mometasone, zileuton, and monteleukast) is being investigated in SCD. Activated leukocytes also produce various proinflammatory cytokines including IL-1β. Canakinumab in a monoclonal antibody that targets IL-1β. Invariant natural killer T cells (iNKTs) exhibit an activated phenotype and amplify the inflammatory response to hypoxia/reperfusion injury in SCD by producing IFN-ϒ. NKTT120 is a humanized monoclonal antibody that specifically depletes iNKTs. Platelets contribute to inflammation. Omega-3 fatty acids, in addition to favorably altering sRBC fatty acid membrane composition, have a myriad of anti-inflammatory effects and target included leukocytes, platelets, and endothelial cells. iTCR, invariant T-cell receptor; PLA2, secretory phospholipase A2. Adapted from Morrone et al48 with permission.
Novel agents targeting inflammation in SCD. Multiple cell types, molecules, and pathways contribute to the chronic inflammatory state in SCD. In SCD, as in asthma, leukocyte membrane phospholipids are hydrolyzed into arachidonic acid (AA), which is metabolized via the 5-lipoxygenase (5-LPO) pathway, leading to formation of inflammatory leukotrienes. Inhibition of this pathway by several agents currently used in asthma (mometasone, zileuton, and monteleukast) is being investigated in SCD. Activated leukocytes also produce various proinflammatory cytokines including IL-1β. Canakinumab in a monoclonal antibody that targets IL-1β. Invariant natural killer T cells (iNKTs) exhibit an activated phenotype and amplify the inflammatory response to hypoxia/reperfusion injury in SCD by producing IFN-ϒ. NKTT120 is a humanized monoclonal antibody that specifically depletes iNKTs. Platelets contribute to inflammation. Omega-3 fatty acids, in addition to favorably altering sRBC fatty acid membrane composition, have a myriad of anti-inflammatory effects and target included leukocytes, platelets, and endothelial cells. iTCR, invariant T-cell receptor; PLA2, secretory phospholipase A2. Adapted from Morrone et al48 with permission.
Antioxidants
Glutamine is an oral precursor for the synthesis of crucial metabolic redox cofactors.20 It increases NADH, the reduced form of nicotinamide adenine dinucleotides, within sRBCs (Figure 2), thus combating oxidative stress. Glutamine was granted US Food and Drug Administration approval in July 2017 for use in SCD patients age >5 years to prevent VOCs. The phase 3 trial demonstrated a 25% reduction in number of pain crises (3.0 vs 4.0; P < .005), 30% lower hospitalization rates (2.0 vs 3.0; P < .005), and reduced number of episodes of acute chest syndrome (ACS; ∼8% vs 23% P = .003) over 48 weeks.21 Glutamine is administered orally twice per day, can be taken along with hydroxyurea (HU) without adverse effects, and does not need additional monitoring. While dissenters focus on the modest incremental effect, it is remarkable to see multiple positive outcomes that persist in the large hydroxyurea subgroup. The noncompletion rate in this study, as in the crizanluzimab study, was ∼30%, highlighting the challenge of sustained adherence to any preventive therapy. This highlights the importance of simultaneous development of multiple types of novel therapies: preventive, abortive, and curative.
Omega-3 fatty acids promote favorable changes in the sRBC lipid membrane and NO production, dampening endothelial inflammation and coagulation activation (Figure 3).22 Single-center studies have shown reduction in the rate and severity of VOCs in both adult and pediatric patients when treated with omega-3 fatty acid supplementation.23-25 There are multiple ongoing clinical trials, including multicenter studies, examining novel formulations as well as novel delivery platforms for improved bioavailability targeting prevention of pain episodes.
N-acetylcysteine (NAC) is a ROS scavenging antioxidant. Two single-center pilot studies provided initial evidence for biomarker efficacy (reduction in the percentage of dense cells and SCD-related oxidative stress as well as reduction in pain events).26,27 A more recent phase 3 multicenter randomized placebo-controlled double-blind trial of the effect of NAC on frequency of pain using pain diaries did not demonstrate any improvement in primary or secondary end points.28 The authors reported poor adherence to study drug, which was attributed to gastrointestinal adverse effects. A registered single-arm phase 1/2 study is evaluating the tolerability and biomarker efficacy (von Willebrand factor activity and laboratory measures of red cell hemolysis and oxidation) of IV NAC.
Adenosine and iNKTs
In SCD, recurrent and ongoing episodes of vasoocclusion within the microvasculature lead to ischemia-reperfusion injury and downstream inflammation. In SCD mouse models, iNKTs were activated, increased in number, and were hypersensitive to the ischemia-reperfusion injury and played a key role in amplifying the inflammatory response. iNKT inhibition in mice demonstrated reduced levels of interferon γ and CXCR3 chemokines.29 Adenosine 2A receptors are overexpressed on iNKTs in SCD mice. Regadenoson, a selective adenosine 2A receptor agonist, dampens iNKT activation and decreases inflammation in SCD mice.30 In a phase 1 clinical trial, during acute VOCs, low-dose regadenoson decreased iNKT activation by almost 50%.31 The phase 2 trial, however, did not demonstrate a reduction in iNKT activation to the prespecified level. Additionally, no difference was seen in clinical outcomes. The authors speculated that this may have been due to a low dose of regadenoson.32 NKTT120 is an iNKT cell monoclonal antibody (Figure 3) that produces rapid and sustained iNKT cell depletion within 6 hours (earliest time point measured in the study). The highest doses in the phase 1 trial yielded undetectable levels of iNKT cells 2 to 5 months after infusion.33 The success of NKTT120 in successfully depleting iNKTs will provide the opportunity in future trials to definitively address whether this pathway is clinically important in preventing SCD-associated complications.
Enhanced NO bioavailability
L-arginine is the obligate substrate for the production of NO (Figure 2). Arginine depletion in SCD results from high levels of arginase released from hemolyzed sRBCs.34 Dysregulated arginine metabolism is an important contributor to endothelial dysfunction,34,35 and supplementation of L-arginine in SCD-transgenic mice leads to reduced inflammation.36 A phase 2 single-center randomized double-blind placebo control trial of children age 3 to 19 years using IV arginine supplementation during acute VOCs resulted in a statistically significant reduction in opioid use by 54% as well as a reduction in pain scores.37 A phase 2 multicenter study for use of arginine during acute VOCs in children is currently recruiting (registered at www.clinicaltrials.gov as #NCT02536170).
L-citrulline, a precursor of arginine, has been shown to be a significant generator of intracellular arginine and NO (Figure 2)38 and performs better than arginine in enhancing NO bioavailability and improving end-organ microcirculatory flow in murine models.39 A phase 1 single-center study of IV citrulline found it is well tolerated and safe.40 The study drug was administered to SCD patients in steady state in step 1 and then during acute VOCs in step 2. Elevation in citrulline and arginine levels was robust and well tolerated.
Riociguat and IW-1701 are activators of soluble guanylate cyclase, an enzyme that catalyzes the conversion of GTP to cGMP, leading to smooth-muscle relaxation, improved vessel function (Figure 2),41 and decreased leukocyte adhesion.42 Studies for both agents are currently recruiting for phase 2 trials. Because their targeted mechanisms, like sildenafil, result from increased cGMP, an off-target increase in pain events, as noted in the WALK-PHHAST study, remains a possibility with this approach.
IMR-687 is a selective inhibitor of phosphodiesterase-9 (PDE9; Figure 2), leading to an increase in cellular cGMP levels, which results in decreased cellular adhesion and a downstream increase in HbF.43 Because PDE9 has restricted tissue expression in hematopoietic cells and brain, as opposed to PDE5, which is more widely distributed, there is a possibility of reduced off-target effects.
Statins, 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors, prevent endothelial damage via upregulation of endothelial nitric oxide synthase in murine models.44 In a phase 1/2 pilot study in pediatrics, simvastatin increased NO metabolites by 50% overall and decreased levels of C-reactive protein and interleukin-6 (IL-6).45 In the follow-up open-label single-arm trial, once-daily simvastatin over 3 months produced a decrease from baseline in high-sensitivity C-reactive protein (P = .003), soluble E-selectin (P = .01), soluble ICAM-1 (P = .02), soluble ICAM-3 (P = .02), and soluble VEGF (P = .01) and an 83% reduction in pain frequency in both children and adults (P = .0003). Simvastatin had no effect on pain intensity or levels of NO metabolites, soluble P-selectin, or soluble VCAM-1. Patients in this trial who were simultaneously receiving HU showed maximal improvement, suggesting a synergistic effect.46 Although the phase 1 trial evaluating atorvastatin was unable to demonstrate a significant change in NO synthase response, it did show limited effect on vascular function.47 A phase 2 trial was recently completed, and results are pending.
Leukotrienes
Leukotrienes are a group of proinflammatory lipid molecules produced by leukocytes (Figure 3). They lead to vasoconstriction and upregulation of adhesive endothelial molecules and recruit inflammatory cells into tissues.48 Secretory phospholipase A2, which releases the leukotriene precursor arachidonic acid, and the leukotriene LTE4 (Figure 3) are elevated in SCD patients during ACS and pain crises, respectively.48 Monteleukast, a cysteinyl leukotriene receptor antagonist, is being evaluated in a phase 2 trial, which recently completed enrollment; results are pending. Zileuton inhibits an essential leukotriene synthetic enzyme, 5-lipoxygenase, and demonstrated tolerability in the phase 1 trial.49 Mometasone, an inhaled corticosteroid, has antileukotriene effect through its inhibition of arachidonic acid. The phase 2 trial established feasibility in SCD patients without a diagnosis of asthma and demonstrated reduction in pain scores and soluble VCAM levels.50
Other anti-inflammatory agents
Endothelin-1 (ET-1) is a endothelial cell–derived vasoconstrictive peptide of endothelial cells, which is increased in SCD patients in response to endothelial injury.51 An ET-1 polymorphism has been linked with risk of VOCs in a single study.52 ET-1 is mechanistically linked to chronic kidney injury, pulmonary hypertension, vasculopathy, and chronic pain. Murine studies have indicated that the use of endothelin receptor antagonists can protect from VOC-mediated mortality, as well as providing renal and pulmonary protection.52 A phase 1 trial using ambrisentan, an endothelin receptor antagonist, is recruiting patients age 18 to 65 years.
Fetal Hb induction
Fetal Hb induction is the earliest therapeutic strategy that was used in SCD. Hydroxyurea, a potent HbF inducer, was US Food and Drug Administration approved for use in adults with SCD in 1998. It has multiple mechanisms of action and profound benefit in responders. The question of whether the response is durable over the lifespan has not been put to rest, and approximately one third of adults will have a poor response, demonstrating the need for additional agents. Novel HbF-inducing agents in development are summarized in Table 3 and Figure 4.
Novel agents inducing HbF
| Name . | Mechanism . | Trial phase, type, NTC# . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| Decitabine + THU | DNA methyltransferase1 inhibitor | 1, single blinded, NCT01685515 | AEs grade ≥3 | Completed | All SCD genotypes |
| Well tolerated; HbF 4%-9% increase and doubling of F cells in higher-dose cohorts; increase in platelet counts noted54 | |||||
| Metformin | FOXO3 inducer | 1, open label, NCT02981329 | Change in HbF % or Hb | Recruiting | Age 12-40 y |
| INCB059872 | LSD1 inhibitor | 1, open label, NCT03132324 | Safety and tolerability, HbF levels | Recruiting |
| Name . | Mechanism . | Trial phase, type, NTC# . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| Decitabine + THU | DNA methyltransferase1 inhibitor | 1, single blinded, NCT01685515 | AEs grade ≥3 | Completed | All SCD genotypes |
| Well tolerated; HbF 4%-9% increase and doubling of F cells in higher-dose cohorts; increase in platelet counts noted54 | |||||
| Metformin | FOXO3 inducer | 1, open label, NCT02981329 | Change in HbF % or Hb | Recruiting | Age 12-40 y |
| INCB059872 | LSD1 inhibitor | 1, open label, NCT03132324 | Safety and tolerability, HbF levels | Recruiting |
Unless otherwise specified, study included only patients with HbSS or HbSβ0.
AE, adverse event.
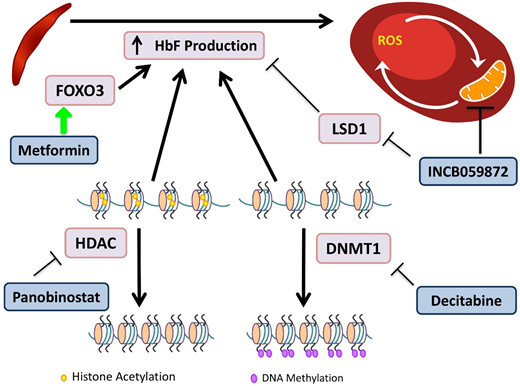
Novel agents targeting HbF induction. HbF has the ability to interfere with HbS polymerization. DNA methyltransferases (DMNTs) modify DNA by methylation of its cytidine residues, resulting in transcriptional silencing. Decitabine inactivates DNMT1, allowing for decreased methylation of the γ-globin promoter and increased gene expression. Histone deacetylators (HDACs) remove acetyl groups, primarily from histone lysine residues, leading to a more closed chromatin configuration, which represses gene expression by reducing access to interacting regulatory proteins. Panobinostat inhibits HDACs, maintaining an open acetylated chromatin configuration of the γ-globin promoter, allowing for gene expression. INCB059872 inhibits LSD1, an inhibitor of HbF production, as well as prevents ROS accumulation in RBCs. Metformin induces FOXO3, a transcription factor that upregulates HbF production, although the precise mechanism of the effect of metformin on HbF induction is still being elucidated.
Novel agents targeting HbF induction. HbF has the ability to interfere with HbS polymerization. DNA methyltransferases (DMNTs) modify DNA by methylation of its cytidine residues, resulting in transcriptional silencing. Decitabine inactivates DNMT1, allowing for decreased methylation of the γ-globin promoter and increased gene expression. Histone deacetylators (HDACs) remove acetyl groups, primarily from histone lysine residues, leading to a more closed chromatin configuration, which represses gene expression by reducing access to interacting regulatory proteins. Panobinostat inhibits HDACs, maintaining an open acetylated chromatin configuration of the γ-globin promoter, allowing for gene expression. INCB059872 inhibits LSD1, an inhibitor of HbF production, as well as prevents ROS accumulation in RBCs. Metformin induces FOXO3, a transcription factor that upregulates HbF production, although the precise mechanism of the effect of metformin on HbF induction is still being elucidated.
Decitabine plus tetrahydrouridine (THU) is an HbF-inducing regimen due to the effect of decitabine's inhibition of DNA methyltransferase, which normally directs epigenetic silencing of the γ-globin gene. Decitabine alone has limited intestinal absorption, but the use of THU before decitabine improves bioavailability.53 A randomized phase 1 trial demonstrated a tolerable regimen, which led to an increase in HbF of 4% to 9%, doubled fetal Hb-enriched RBCs, and increased patients’ total Hb levels by 1.2 to 1.9 g/dL.54 The trial showed promising results, and the drug was well tolerated. The regimen requires THU administration orally 60 minutes before decitabine twice per week; and may be cumbersome for some patients to adhere to.
Metformin is known for its role in suppressing hepatic gluconeogenesis, but recent research has demonstrated its ability to upregulate FOXO3.55 Whole-exome sequencing of SCD patients identified FOXO3 aberrations that were associated with reduced HbF levels.55 Treatment of primary CD34+ cell-derived erythroid cultures with metformin caused increases in HbF and an additive effect with HU.56 A phase 1 clinical trial for children and adults age 12 to 40 years is currently recruiting.
Panobinostat is a nonselective pan–histone deacetylase inhibitor that induces HbF production via increased acetylation of the γ-globin promoter and inhibits cell-specific inflammation.57 A phase 1 trial for SCD is registered as active but is not yet recruiting patients.
INCB059872 is a lysine-specific demethylase 1 (LSD1) inhibitor. In SCD, there is abnormal mitochondrial retention in RBCs, with associated increased ROS levels. LSD1 inhibition increases γ-globin expression, prolongs the RBC lifespan, and decreases ROS accumulation in SCD mouse models.58 These preclinical models demonstrated that LSD1 inhibition is also able to increase HbF, with production of fewer reticulocytes and sickle cells.59 The phase 1 trial is now recruiting patients age >18 years.
Antisickling agents
The agents discussed here prevent polymerization of HbS by mechanisms other than HbF induction and are summarized in Table 4 and Figure 5.
Novel agents targeting sickling
| Name . | Mechanism . | Trial phase, type, NTC# . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| GBT440/voxelotor | Small molecule that binds to HbS α chain and increases oxygen affinity of HbS by stabilizing it in the R-state | 1/2, RDBPC, NCT02285088/ NCT03041909 | Safety, PKs, PDs | Completed | All SCD genotypes; well tolerated; improvement in Hb and hemolysis61 |
| 2a, open label, NCT02850406 | PKs, change in Hb | Recruiting | Age 12-17 y; initial partial results demonstrate improved Hb and hemolysis | ||
| 3, RDBPC, NCT03036813 | Hb level changes | Recruiting | All SCD genotypes; age 12-65 y | ||
| Sanguinate (PEGylated carboxyhemoglobin bovine)* | Bovine pegylated Hb product; dual oxygen and CO transfer results in antisickling and anti- inflammatory effects | 1b, open label, NCT01848925 | Safety compared with HU | Completed | Well tolerated.63 |
| 2, RPC single blinded, NCT02672540/NCT02411708 | Time to readiness for discharge | Completed | All SCD genotypes; interim analyses show improved RBC morphology and decreased pain scores64 | ||
| SCD 101 | Unknown | 1A, open label | Safety | Recruiting | Well tolerated; improved RBC morphology |
| 1B, RDBPC cross over, NCT02380079 | Improvement in chronic pain and fatigue81 |
| Name . | Mechanism . | Trial phase, type, NTC# . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| GBT440/voxelotor | Small molecule that binds to HbS α chain and increases oxygen affinity of HbS by stabilizing it in the R-state | 1/2, RDBPC, NCT02285088/ NCT03041909 | Safety, PKs, PDs | Completed | All SCD genotypes; well tolerated; improvement in Hb and hemolysis61 |
| 2a, open label, NCT02850406 | PKs, change in Hb | Recruiting | Age 12-17 y; initial partial results demonstrate improved Hb and hemolysis | ||
| 3, RDBPC, NCT03036813 | Hb level changes | Recruiting | All SCD genotypes; age 12-65 y | ||
| Sanguinate (PEGylated carboxyhemoglobin bovine)* | Bovine pegylated Hb product; dual oxygen and CO transfer results in antisickling and anti- inflammatory effects | 1b, open label, NCT01848925 | Safety compared with HU | Completed | Well tolerated.63 |
| 2, RPC single blinded, NCT02672540/NCT02411708 | Time to readiness for discharge | Completed | All SCD genotypes; interim analyses show improved RBC morphology and decreased pain scores64 | ||
| SCD 101 | Unknown | 1A, open label | Safety | Recruiting | Well tolerated; improved RBC morphology |
| 1B, RDBPC cross over, NCT02380079 | Improvement in chronic pain and fatigue81 |
Unless otherwise specified, study included only adult patients with HbSS or HbSβ0.
PD, pharmacodynamic; RDBPC, randomized double-blind placebo control.
Study drug administered during acute VOC.
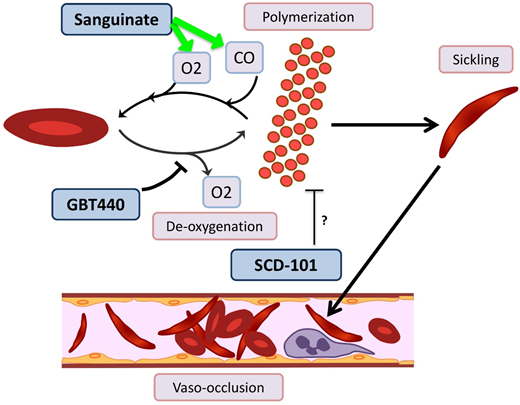
Novel agents targeting RBC sickling in SCD. GBT440 binds Hb and increases its affinity for oxygen (O2), thereby hindering polymerization. Sanguinate helps reverse polymerization and sickling by delivering carbon monoxide (CO) and oxygen to RBCs. The mechanism by which SCD-101 acts to improve sickling remains unknown.
Novel agents targeting RBC sickling in SCD. GBT440 binds Hb and increases its affinity for oxygen (O2), thereby hindering polymerization. Sanguinate helps reverse polymerization and sickling by delivering carbon monoxide (CO) and oxygen to RBCs. The mechanism by which SCD-101 acts to improve sickling remains unknown.
GBT440/voxelotor is a small molecule that binds the α chain of HbS and increases its affinity for oxygen by allosteric modification to favor the R-state.60 In a phase 1/2 trial with healthy volunteers age 18 to 55 years and patients with HbSS age 18 to 60 years, GBT440 was well tolerated and led to reduction of sickle cells and an increase in Hb, and evidence of decreased hemolysis was found.61 Interim results from a phase 2a trial demonstrate improvement of transcranial Doppler velocity and clinical symptom reduction.62 A phase 3 double-blind randomized placebo-controlled multicenter trial is recruiting SCD patients age 12 to 65 years. Additionally, a pediatric phase 2a open-label single- and multiple-dose study is recruiting patients age 12 to 17 years to evaluate the PKs, safety, tolerability, and exploratory treatment effect.
Sanguinate (PEGylated carboxyhemoglobin bovine) is a dual oxygen and carbon monoxide transferring agent. A phase 1b trial demonstrated safety and tolerability, with mild self-limited adverse events.63 The phase 2 trial has completed enrollment, and interim results demonstrate the ability to return sickled RBCs to normal morphology, decrease inflammation, and decrease pain scores64 ; additional results are pending.
SCD 101 is a botanical drug with antisickling activity; however, the mechanism is unknown (although it does not bind Hb directly or alter oxygen affinity). The completed phase 1b open-label single-arm dose-escalation study65 in adults was well tolerated, with improvement of RBC morphology on smear review. Oral SCD 101 was administered over 4 weeks at 4 dosing levels. Those who received high doses reported improvement in chronic pain and fatigue. Patients who received the highest two doses reported improvement in exercise tolerance, sleep, and leg ulcers. The results of this study have been presented in abstract form only, and a peer-review publication is pending.
Activated coagulation and platelets
The hypercoagulable state in SCD is often underappreciated, and patients are at risk for significant thrombotic complications. Multiple factors of SCD pathophysiology contribute to the heightened thrombotic risk: ischemia reperfusion, hemolysis, ROS-driven externalization of sRBC phosphatidylserine and decreased NO bioavailability, inflammation, and endothelial disease. This leads to a myriad of perturbations, such as increased thrombin generation, elevated tissue factor expression, platelet activation, and increased large molecular weight von Willebrand multimers.17,18,66 Targeting these abnormalities provides a therapeutic opportunity that is even more compelling with the ease of use of direct oral anticoagulants, 2 of which are in clinical trials. The cross talk between thrombosis and inflammation also makes it likely that targeting Xa or thrombin with these newer agents would reduce vasculopathy, inflammation, and end-organ damage. Heparins, in addition to their anticoagulant effect, have additional antiadhesive properties via blockade of P-selectin. Agents targeting activated coagulation and platelets are summarized in Table 5.
Anticoagulants and antiplatelet agents in SCD
| Name . | Mechanism . | Trial phase, type, NTC# . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| Unfractionated heparin* | Inhibits P-selectin in addition to anticoagulant effect | 2, open label randomized, NCT02098993 | Time to discharge | Recruiting | HbSS, HbSC, or HbSβ0 with ACS |
| Tinzaparin* | Inhibits P-selectin in addition to anticoagulant effect | 3, randomized to 2 different dosing levels, NCT02580773 | Time to ACS resolution | Recruiting | HbSS, HbSC, or HbSβ0 with ACS |
| Rivaroxiban | Direct oral factor Xa inhibitor | 2, NCT02072668 | VCAM1 and IL-6 levels | Enrolling patients by invitation | |
| Apixiban | Direct oral factor Xa inhibitor | 3, RDBPC, NCT02179177 | Daily outpatient pain score | Completed | HbSS, HbSC, or HbSβ0 |
| Ticagrelor | P2Y12 receptor antagonist | 2 (adults), RDBPC, NCT02482298 | Proportion of pain days (e-diary) | Completed | |
| 2 (pediatrics), NCT02214121 | PKs, PDs, safety | Completed | Age 2-18 y; safe and well tolerated69 | ||
| 1 (infants), NCT03492931 | PKs | Recruiting | Infants and toddlers age 0-24 mo |
| Name . | Mechanism . | Trial phase, type, NTC# . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| Unfractionated heparin* | Inhibits P-selectin in addition to anticoagulant effect | 2, open label randomized, NCT02098993 | Time to discharge | Recruiting | HbSS, HbSC, or HbSβ0 with ACS |
| Tinzaparin* | Inhibits P-selectin in addition to anticoagulant effect | 3, randomized to 2 different dosing levels, NCT02580773 | Time to ACS resolution | Recruiting | HbSS, HbSC, or HbSβ0 with ACS |
| Rivaroxiban | Direct oral factor Xa inhibitor | 2, NCT02072668 | VCAM1 and IL-6 levels | Enrolling patients by invitation | |
| Apixiban | Direct oral factor Xa inhibitor | 3, RDBPC, NCT02179177 | Daily outpatient pain score | Completed | HbSS, HbSC, or HbSβ0 |
| Ticagrelor | P2Y12 receptor antagonist | 2 (adults), RDBPC, NCT02482298 | Proportion of pain days (e-diary) | Completed | |
| 2 (pediatrics), NCT02214121 | PKs, PDs, safety | Completed | Age 2-18 y; safe and well tolerated69 | ||
| 1 (infants), NCT03492931 | PKs | Recruiting | Infants and toddlers age 0-24 mo |
Unless otherwise specified, study included only adult patients with HbSS or HbSβ0.
PD, pharmacodynamic; RDBPC, randomized double-blind placebo control.
Study drug administered during acute VOC are marked with an asterix
Tinzaparin is a low molecular weight heparin, and a single-center randomized double-blind placebo-controlled study of tinzaparin administered during acute VOCs demonstrated reduction in the severity and length of the acute painful events.67 The role of unfractionated heparin plus tinzaparin is currently being evaluated in ACS, with the primary outcome being time to hospital discharge/length of ACS.
Rivaroxiban, a direct oral factor Xa inhibitor, has been studied in SCD mouse models and demonstrated decreased tissue inflammation and pulmonary neutrophil congestion.66 The phase 2 randomized crossover trial is enrolling patients by invitation to evaluate inflammation, coagulation and endothelial cell activation, and improved microvascular blood flow assessed by laser Doppler velocimetry. Similarly, apixaban, another oral anti-Xa inhibitor, was studied in a phase 3 trial evaluating its efficacy in decreasing patient-reported daily pain scores. The study has completed enrollment, but the results are not yet available.
Thus far, clinical trials of antiplatelet agents in SCD have been disappointing. Prasugrel, an irreversible P2Y12 antagonist, in a pediatric phase 3 trial did not demonstrate a significant reduction in VOC rates.68 Levels of platelet inhibition in the prasugrel study were modest and may have contributed to its lack of efficacy. Given the thromboinflammatory role of activated platelets in SCD, it is also possible that different end points such as thrombosis or neurovascular events may be more meaningful. Ticagrelor, a reversible P2Y12 receptor antagonist, also inhibits ADP-induced platelet aggregation, but unlike prasugrel, it does not require metabolic activation. The phase 2 trial in adults is now complete, with results posted but not yet published. In a phase 2 2-part pediatric study (age 2-17 years), ticagrelor was well tolerated, but no difference in pain ratings or analgesic use was shown, although the study was not powered to detect a difference for these outcomes.69 A phase 1 trial for children <24 months is now recruiting.
Complex mechanisms of pain in SCD
Pain is a hallmark of SCD and is associated with increased morbidity and mortality. In addition to acute nociceptive crisis pain, chronic neuropathic or mixed-pain states are frequent in adult patients and contribute to a poor quality of life. Mechanisms of chronic pain in SCD patients are poorly understood, and much of the recent work has been in murine models.70 Noxious stimuli from tissue damage ultimately contribute to hyperexcitability resulting from peripheral and central sensitization (Figure 6). Opioids, the mainstay of current treatment, contribute to central sensitization and therefore chronic pain. There is an urgent need for effective treatment of acute pain states with opioid-sparing agents and decreased reliance on opioids for chronic pain. Current strategies draw upon approaches used in other chronic pain states and targets identified in murine models of SCD pain (Table 6; Figure 6).
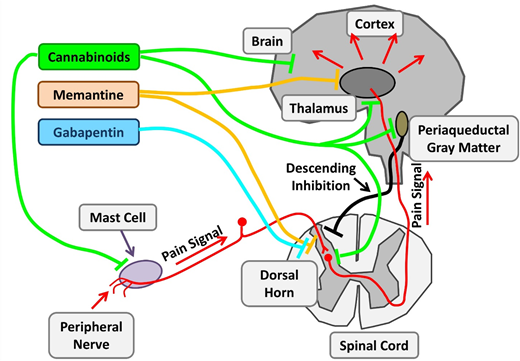
Therapeutic targets investigating chronic pain in SCD. Chronic pain in SCD is likely multifactorial and results from various mechanisms, such as mast cell activation and neurogenic inflammation, peripheral nociceptor sensitization, and central sensitization. Nonopioid agents with established benefits in non-SCD chronic pain are being investigated. Cannabinoids decrease chronic pain by their central effects as well as stabilization of mast cells and reduced neurogenic inflammation. Memantine is an NMDA receptor antagonist, and gabapentin binds to voltage-gated calcium channels in neurons. Adapted from Morrone et al48 with permission.
Therapeutic targets investigating chronic pain in SCD. Chronic pain in SCD is likely multifactorial and results from various mechanisms, such as mast cell activation and neurogenic inflammation, peripheral nociceptor sensitization, and central sensitization. Nonopioid agents with established benefits in non-SCD chronic pain are being investigated. Cannabinoids decrease chronic pain by their central effects as well as stabilization of mast cells and reduced neurogenic inflammation. Memantine is an NMDA receptor antagonist, and gabapentin binds to voltage-gated calcium channels in neurons. Adapted from Morrone et al48 with permission.
Novel opioid-sparing agents targeting SCD pain
| Name . | Mechanism . | Trial phase, type, NTC# . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| Vaporized cannabis | Activation of cannabinoid receptors | 1, RDBPC cross over, NCT01771731 | Daily pain level over 5 d | Completed | All SCD genotypes; results pending |
| Canakinumab | IL-1β mAb | 2, RDBPC, NCT02961218 | Daily pain scores | Recruiting | Age 12-20 y |
| Buprenorphine | Partial μ agonist and κ antagonist | 2, open label, NCT03492099 | Hospitalization in 72 h subsequent to full analgesia rotation to buprenorphine | Active but not yet recruiting | All SCD genotypes |
| Ketamine* | NMDA receptor antagonist | 2, open label, NCT03296345 | Treatment-associated AEs | Recruiting | All SCD genotypes; age 8-25 y |
| Randomized triple blinded, NCT03431285 | Pain scores | Recruiting | All SCD genotypes | ||
| Randomized triple blinded, NCT02573714 | Pain scores | Recruiting | All SCD genotypes; age 4-16 y | ||
| Intranasal ketamine | |||||
| 3, randomized open label, NCT02801292 | Reduction in pain scores | Not yet recruiting | All SCD genotypes; age 3-17 y | ||
| Memantine | NMDA receptor antagonist | 2, single group, NCT02615847 | AEs | Completed | Results pending |
| 2a/2b, single arm, NCT03247218 | AEs | Not yet recruiting | ≥10 y | ||
| Gabapentin* | Binding to voltage-gated calcium channels in neurons | 2, RDBPC, NCT01954927 | Percentage of patients with ≥33% pain reduction in 3 h | Recruiting | All SCD genotypes; age 1-20 y |
| Topical sodium nitrite | NO | 2, RDBPC, NCT02863068 | Tolerability | Recruiting | All SCD genotypes |
| 1, single arm open label, NCT01316796 | Safety, tolerability, dose finding | Completed | All SCD genotypes; well tolerated with positive efficacy signals |
| Name . | Mechanism . | Trial phase, type, NTC# . | Primary end points . | Status . | Results and comments . |
|---|---|---|---|---|---|
| Vaporized cannabis | Activation of cannabinoid receptors | 1, RDBPC cross over, NCT01771731 | Daily pain level over 5 d | Completed | All SCD genotypes; results pending |
| Canakinumab | IL-1β mAb | 2, RDBPC, NCT02961218 | Daily pain scores | Recruiting | Age 12-20 y |
| Buprenorphine | Partial μ agonist and κ antagonist | 2, open label, NCT03492099 | Hospitalization in 72 h subsequent to full analgesia rotation to buprenorphine | Active but not yet recruiting | All SCD genotypes |
| Ketamine* | NMDA receptor antagonist | 2, open label, NCT03296345 | Treatment-associated AEs | Recruiting | All SCD genotypes; age 8-25 y |
| Randomized triple blinded, NCT03431285 | Pain scores | Recruiting | All SCD genotypes | ||
| Randomized triple blinded, NCT02573714 | Pain scores | Recruiting | All SCD genotypes; age 4-16 y | ||
| Intranasal ketamine | |||||
| 3, randomized open label, NCT02801292 | Reduction in pain scores | Not yet recruiting | All SCD genotypes; age 3-17 y | ||
| Memantine | NMDA receptor antagonist | 2, single group, NCT02615847 | AEs | Completed | Results pending |
| 2a/2b, single arm, NCT03247218 | AEs | Not yet recruiting | ≥10 y | ||
| Gabapentin* | Binding to voltage-gated calcium channels in neurons | 2, RDBPC, NCT01954927 | Percentage of patients with ≥33% pain reduction in 3 h | Recruiting | All SCD genotypes; age 1-20 y |
| Topical sodium nitrite | NO | 2, RDBPC, NCT02863068 | Tolerability | Recruiting | All SCD genotypes |
| 1, single arm open label, NCT01316796 | Safety, tolerability, dose finding | Completed | All SCD genotypes; well tolerated with positive efficacy signals |
Unless otherwise specified, study included only adult patients with HbSS or HbSβ0.
AE, adverse event; RDBPC, randomized double-blind placebo control.
Study drug administered during acute pain event
With changing policies regulating cannabis use, there is a burgeoning interest in cannabis pharmacotherapy for pain. Cannabinoids have potent nociceptive effects as a result of their direct effect on widely distributed receptors within the central nervous system. SCD mice treated with cannabinoid receptor agonists demonstrate decreased mast cell granulation, neurogenic inflammation, and substance P levels, leading to amelioration of the chronic pain phenotype. Thus, cannabinoids have both disease-modifying and central analgesic effects. Results of the recently completed phase 2 trial of vaporized cannabis are pending.
Canakinumab is a monoclonal antibody against IL-1β. Heme activates the NLRP3 inflammasome, which leads to induction of IL-1β and consequent inflammation in various hemolytic disorders. In vitro studies using peripheral blood monocytes from SCD patients provide evidence for these pathways in SCD.71 Canakinumab is successful in treating the IL-1β–mediated chronic inflammation in cryopyrin-associated periodic syndromes, leading to the hypothesis that it may help mitigate hemolysis–IL-1β–mediated chronic pain in SCD.72 In an SCD mouse model, IL-1β inhibition led to improvement in vascular occlusion, granulocyte extravasation, and microvascular flow,73 providing further support for this hypothesis. A phase 2 trial of canakinumab in patients with SCD is currently recruiting patients.
Buprenorphine is a partial μ agonist and κ antagonist with high affinity for the μ receptors. It has a long half-life of 28 to 37 hours for sublingual administration. In chronic pain studies, it was shown to be associated with a lower risk for misuse, diminished withdrawal symptoms and cravings for opioids, as well as the reduced risk of overdose.74 A registered phase 2 trial to assess the benefits of rotating all chronic opioids to buprenorphine is active but not yet recruiting.
Ketamine is a phencyclidine derivative with analgesic and anesthetic properties. As an N-methyl-D- aspartate (NMDA) receptor antagonist, ketamine has the ability to modulate the normal development of tolerance and hyperalgesia to opioids, which develops secondary to NMDA receptor–mediated activation of the nociceptive system. Several registered clinical trials are recruiting patients to evaluate low-dose ketamine for use of pain control in acute VOCs as an opioid-sparing approach. Memantidine, another NMDA receptor antagonist, is being evaluated in phase 2 trials as a potential long-term treatment.
Gabapentin binds to the α2δ subunits of Ca2+ channels at the postsynaptic dorsal horns. It is believed to hinder the development of opioid tolerance and has been shown to have opioid-sparing effects in the postoperative setting. It is approved for use in neuropathic pain states such as diabetic neuropathy and regional pain syndromes. There have only been a few case reports describing its use in SCD.75 A current phase 2 trial is evaluating its use in acute pain crises in the ambulatory setting in pediatrics.
Conclusion
In summary, there continue to be advances in novel therapeutics in SCD, with >30 in clinical trials. Despite challenges with recruitment, funding, and appropriate choice of end points, these are truly exciting times. Awareness of the patient voice and partnership with industry continue to strengthen. Many agents have completed robust trials with definitive results. Although those with positive results move onto the next phases of study, the well-conducted studies with negative outcomes provide critical insights and are also valuable. With the availability of novel therapies, the thoughtful design of multiagent trials of agents with different mechanisms of action and nonoverlapping toxicities is crucial. The development of a robust longitudinal patient database with uniform phenotypic data will be critically important in comparing outcomes of these different regimens. Using the success of pediatric acute lymphoblastic leukemia multiagent therapy as a model, we speculate that the bar for health outcomes in SCD will continue to be set higher in the coming decades.
Acknowledgments
This work was supported in part by grant R01 FD005341 from the US Food and Drug Administration (D.M.).
Many important scientific and clinical contributions in SCD are beyond the scope of this report. We apologize to authors of important contributions that could not be cited because of space limitations.
Correspondence
Deepa Manwani, Children's Hospital at Montefiore, 3415 Bainbridge Ave, Bronx, NY 10467; e-mail: dmanwani@montefiore.org.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.