Abstract
The use of extracorporeal circulation for cardiac surgery and extracorporeal life support poses tremendous challenges to the hemostatic equilibrium given its diametric tendency to trigger hyper‐ and hypocoagulopathy. The necessity of anticoagulant therapy to counteract the hemostatic activation by the extracorporeal circuitry compounded by unfavorable patient and surgical factors significantly increase the risk of catastrophic bleeding in patients who require extracorporeal circulation. Preoperative measures, such as stratification of high‐risk bleeding patients, and optimization of the modifiable variables, including anemia and thrombocytopenia, provide a crude estimation of the likelihood and may modify the risk of catastrophic bleeding. The anticipation for catastrophic bleeding subsequently prompts the appropriate preparation for potential resuscitation and massive transfusion. Equally important is intraoperative prevention with the prophylactic application of tranexamic acid, an antifibrinolytic agent that has promising benefits in reduction of blood loss and transfusion. In the event of uncontrolled catastrophic bleeding despite preemptive strategies, all effort must be centered on regaining hemostasis through surgical control and damage control resuscitation to protect against worsening coagulopathy and end organ failure. When control of bleeding is reinstated, management should shift focus from systemic therapy to targeted hemostatic therapy aimed at the potential culprits of coagulopathy as identified by point of care hemostatic testing. This review article outlines the strategies to appropriately intervene using prediction, prevention, preparation, protection, and promotion of hemostasis in managing catastrophic bleeding in extracorporeal circulation.
Learning Objectives
Approach catastrophic bleeding in patients requiring extracorporeal circulation for cardiac surgery or extracorporeal life support
Understand the role of prediction rules and point of care hemostatic testing for identification of high‐risk patients
Prepare for resuscitation and massive transfusion in the anticipation of exsanguinating patients
Manage uncontrolled and controlled bleeding during early and later stages of resuscitation
Introduction
Patients requiring extracorporeal circulation for cardiac surgery or extracorporeal life support are at increased risk for catastrophic bleeding due to a combination of preexisting risk factors, surgical complexity, the deleterious effects of extracorporeal circulation on the hemostatic system, and the necessity for anticoagulant therapy to prevent thrombosis in the extracorporeal circulation.1-3 (Catastrophic bleeding in this review refers to acute, large‐volume bleeding rather than a bleed that may be considered catastrophic solely due to its location [eg, brain hemorrhage]). Because the consequences of catastrophic bleeding can be grave if not optimally managed,4,5 it is incumbent on clinicians to be able to identify high‐risk patients, use appropriate preventive strategies, and proficiently resuscitate an exsanguinating patient. In this narrative review, we detail our approach to the management of catastrophic bleeding in the setting of extracorporeal circulation. We have classified our approach into five categories: (1) prediction and (2) prevention of catastrophic bleeding, (3) preparation for catastrophic bleeding, (4) protection against the harmful effects of catastrophic bleeding during the early phase of resuscitation with bleeding that may be uncontrolled, and (5) promotion of hemostasis during the later stages of resuscitation when bleeding is under control (Table 1).
Outline for management of catastrophic bleeding in patients on extracorporeal circulation
| Recommended actions . |
|---|
| Predict |
| Identification of high‐risk patients based on patient and procedural characteristics |
| Prediction rules |
| Prevent |
| Tranexamic acid |
| Prepare |
| Intravenous access |
| Large‐bore peripheral and central |
| Rapid infusers |
| Temperature control |
| Fluid and body warmers; warm room |
| Hemodynamic support |
| Infusion pumps |
| Vasopressors (eg, phenylephrine, norepinephrine, vasopressin) |
| Notify |
| Blood bank |
| Extra help |
| To check and infuse blood; send bloodwork; resuscitate, etc |
| Protect |
| Protect against end organ injury and severe hemostatic derangement |
| Reverse anticoagulation |
| Maintain minimal acceptable perfusion pressure |
| Vasopressors |
| Support hemostasis with allogeneic blood transfusions |
| Initiate massive bleeding/transfusion protocol |
| Ratio-based (1:1:1) transfusion guided by coagulation assays |
| Maintain acid/base and electrolyte (calcium) balance |
| Frequent arterial blood gas measurement |
| Maintain normothermia |
| Promote |
| Promote hemostasis using targeted hemostatic therapy |
| Guided by standard and POC coagulation assays |
| Combination of allogeneic and factor concentrates |
| Recommended actions . |
|---|
| Predict |
| Identification of high‐risk patients based on patient and procedural characteristics |
| Prediction rules |
| Prevent |
| Tranexamic acid |
| Prepare |
| Intravenous access |
| Large‐bore peripheral and central |
| Rapid infusers |
| Temperature control |
| Fluid and body warmers; warm room |
| Hemodynamic support |
| Infusion pumps |
| Vasopressors (eg, phenylephrine, norepinephrine, vasopressin) |
| Notify |
| Blood bank |
| Extra help |
| To check and infuse blood; send bloodwork; resuscitate, etc |
| Protect |
| Protect against end organ injury and severe hemostatic derangement |
| Reverse anticoagulation |
| Maintain minimal acceptable perfusion pressure |
| Vasopressors |
| Support hemostasis with allogeneic blood transfusions |
| Initiate massive bleeding/transfusion protocol |
| Ratio-based (1:1:1) transfusion guided by coagulation assays |
| Maintain acid/base and electrolyte (calcium) balance |
| Frequent arterial blood gas measurement |
| Maintain normothermia |
| Promote |
| Promote hemostasis using targeted hemostatic therapy |
| Guided by standard and POC coagulation assays |
| Combination of allogeneic and factor concentrates |
Prediction
In patients requiring extracorporeal circulation, risk stratification for catastrophic bleeding allows clinicians to institute preemptive measures to minimize or prevent such bleeding and prepare for management of such bleeding in case it occurs. Although it is unlikely to fully predict catastrophic bleeding in all situations, several clinical prediction rules are capable of stratifying the risk for transfusions or major bleeding in patients requiring extracorporeal circulation (Table 2).6,7 Such prediction rules have highlighted the importance of patient characteristics, such as body habitus, age, hemoglobin, platelet count, and renal function, as risk factors for bleeding. In addition, surgical complexity and duration of extracorporeal circulation have also been identified as important risk factors. Although the prediction rules are generally user friendly and accurate enough (c index > 0.7) (Table 2) to be applied clinically for risk stratification for transfusions or major bleeding, they leave a lot of uncertainty about which patients will have catastrophic bleeding. This is not surprising given that they cannot account for patient‐specific factors, such as response to anticoagulants and their reversal agents; anatomical issues that may pose technical issues; and complications related to the surgery or those that may occur during initiation, maintenance, or termination of extracorporeal circulation.8 Furthermore, existing prediction rules were primarily designed for risk stratification of cardiac surgical patients requiring short‐term extracorporeal circulation. They fail to take into consideration the risk factors that are specific to patients on long‐term extracorporeal life support, including the prolonged need for anticoagulation and the high potential for mechanical complications.2 Thus, despite being well discriminating and well calibrated, the existing prediction rules for major bleeding, which are predominantly based on nonmodifiable clinical variables, have limited application in clinical practice.9 Although they may serve to highlight the need to optimize some modifiable risk factors (eg, preoperative anemia or thrombocytopenia) and heighten vigilance for high‐risk patients, any corresponding benefits in outcomes have not been illustrated, and they are, therefore, not routinely used in clinical practice.9
Characteristics of some prediction rules that stratify patients who are undergoing cardiopulmonary bypass surgery for risk of major bleeding9
| Characteristics . | Biancari et al7 . | Klein et al47 . | Ranucci et al48 . | Alghamdi et al49 . | Goudie et al50 . | Karkouti et al51 . |
|---|---|---|---|---|---|---|
| Outcome predicted | >4 units of RBC | ≥1 unit RBC | ≥1 unit RBC | ≥1 unit RBC | ≥1 unit RBC;≥4 units RBC | ≥5 units RBC |
| Sample size | N = 3744 | N = 20 036 | N = 8989 | N = 11 113 | N = 33 960 for any transfusion; N = 27 353 for ≥4 units RBC | N = 10 667 |
| Origin of dataset | 16 cardiac surgery centers in 6 European countries | 10 cardiac surgery centers in the United Kingdom | 1 Italian institution | 1 Canadian institution | 3 United Kingdom and 1 Italian institutions | 1 Canadian institution |
| c Statistic (95% CI where provided) | 0.72 (0.69‐0.76) | 0.76 (0.75‐0.77) | 0.73 (0.72‐0.74) | 0.79 (0.78‐0.80) | 0.78 (0.77‐0.78); 0.81 (0.80‐0.82) | 0.88 |
| Hosmer and Lemeshow P value | .033 | .28 | .372 | .70 | .001; .32 | .20 |
| Variables scored | Sex, preoperative anemia, eGFR, antiplatelet drugs discontinued <5 d, critical preoperative state, acute coronary syndrome, use of low molecular weight heparin/fondaparinus/unfractionated heparin | Age, body surface area, sex, hemoglobin, type of surgery, creatinine, log Euroscore | Age, weight, sex, hemoglobin, type of surgery | Age, weight, sex, hemoglobin, type of surgery, creatinine, elective status, previous cardiac surgery | Age, weight, sex, creatinine, preoperative intra-aortic balloon pump, various comorbidities, previous cardiac surgery, various surgical procedure characteristics, elective status, presence of cardiogenic shock | Age, body surface area, hemoglobin, platelet count, preoperative shock, complex procedure, high blood loss, surgeon, redo surgery, elective status, duration of circulatory arrest and CPB, nadir CPB hemoglobin |
| Characteristics . | Biancari et al7 . | Klein et al47 . | Ranucci et al48 . | Alghamdi et al49 . | Goudie et al50 . | Karkouti et al51 . |
|---|---|---|---|---|---|---|
| Outcome predicted | >4 units of RBC | ≥1 unit RBC | ≥1 unit RBC | ≥1 unit RBC | ≥1 unit RBC;≥4 units RBC | ≥5 units RBC |
| Sample size | N = 3744 | N = 20 036 | N = 8989 | N = 11 113 | N = 33 960 for any transfusion; N = 27 353 for ≥4 units RBC | N = 10 667 |
| Origin of dataset | 16 cardiac surgery centers in 6 European countries | 10 cardiac surgery centers in the United Kingdom | 1 Italian institution | 1 Canadian institution | 3 United Kingdom and 1 Italian institutions | 1 Canadian institution |
| c Statistic (95% CI where provided) | 0.72 (0.69‐0.76) | 0.76 (0.75‐0.77) | 0.73 (0.72‐0.74) | 0.79 (0.78‐0.80) | 0.78 (0.77‐0.78); 0.81 (0.80‐0.82) | 0.88 |
| Hosmer and Lemeshow P value | .033 | .28 | .372 | .70 | .001; .32 | .20 |
| Variables scored | Sex, preoperative anemia, eGFR, antiplatelet drugs discontinued <5 d, critical preoperative state, acute coronary syndrome, use of low molecular weight heparin/fondaparinus/unfractionated heparin | Age, body surface area, sex, hemoglobin, type of surgery, creatinine, log Euroscore | Age, weight, sex, hemoglobin, type of surgery | Age, weight, sex, hemoglobin, type of surgery, creatinine, elective status, previous cardiac surgery | Age, weight, sex, creatinine, preoperative intra-aortic balloon pump, various comorbidities, previous cardiac surgery, various surgical procedure characteristics, elective status, presence of cardiogenic shock | Age, body surface area, hemoglobin, platelet count, preoperative shock, complex procedure, high blood loss, surgeon, redo surgery, elective status, duration of circulatory arrest and CPB, nadir CPB hemoglobin |
The c statistic is a measure of model discrimination, and it is also referred to as the area under the receiver operating characteristic curve. A nonsignificant P value for the Hosmer and Lemeshow test indicates adequate model calibration. RBC, red blood cell; eGFR, estimated glomerular filtration rate; CPB, cardiopulmonary bypass.
Increasingly recognized in cardiac surgery is the role of point of care (POC) hemostatic testing—primarily whole‐blood viscoelastic and platelet function assays—as an integral component of transfusion algorithms to guide appropriate treatment (see below).10,11 Although grounded from the principle that POC tests would provide snapshots of the complex coagulopathic diathesis observed in cardiac surgery, the additive utility of this POC hemostatic testing before or during surgery for prediction of major blood loss is so far unconvincing compared with the utility in transfusion management (see below).11-14 Although the tests can rule out patients who are at risk of major bleeding (ie, have high negative predictive value), they cannot accurately predict the “at‐risk” bleeding population after extracorporeal circulation (ie, have low positive predictive value).
Thus, although the concept of predicting catastrophic bleeding based on clinical risk factors and abnormal laboratory tests in patients who require extracorporeal circulation is theoretically logical, the clinical utility of this approach remains unproven. We, therefore, do not routinely rely on any existing prediction rules to definitively determine our patient’s bleeding risk. Instead, we specifically target well‐recognized modifiable risk factors—such as recent use of antiplatelet and antithrombin agents, thrombocytopenia, and anemia—to optimize patients for surgery. Importantly, we also accept and best prepare for the nonmodifiable as well as unpredictable determinants of catastrophic bleeding by instituting systemic preventative strategies and targeted therapeutic interventions.
Prevention
The administration of tranexamic acid (TXA) before the commencement of extracorporeal circulation should be standard practice in cardiac surgical patients due to its proven success in reducing blood loss and transfusion.15 As an antifibrinolytic, TXA dampens the hyperfibrinolytic state induced by extracorporeal circulation by inhibiting the conversion of plasminogen to plasmin.16 Although the hemostatic benefits of TXA are supported by class IA evidence,17,18 these benefits have yet to be translated into demonstrable mortality benefits.15 Like most of the hemostatic drugs, the use of TXA is not risk free. It is accompanied by at least a sevenfold increase in the risk of seizure, of which the exact mechanism remains unclear.15 The prothrombotic nature of TXA was once as concerning as its proconvulsive property, but this has been refuted by large‐scale, randomized, placebo‐controlled trials in cardiac surgery.15
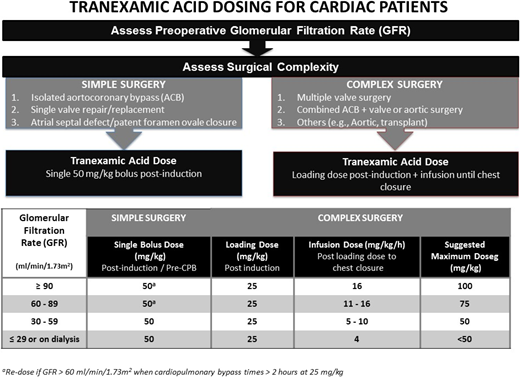
Questions remain about the appropriate dosing of TXA. Although evidence suggests that a plasma concentration of 100 mg/L maximizes the efficacy of TXA in cardiac surgery,16 there is a considerable variation in the appropriate dosing regimen to achieve this target concentration among clinicians.19 The commonly used doses range from 30 to 100mg/kg, and its administration includes an array of methods, including bolus or infusion alone or bolus followed by an infusion. In our institution, we had 2 overarching TXA dosing regimens for our low‐ and high‐risk patients who are stratified primarily based on the type of procedures involved. To the low‐risk group, which consists of aortocoronary bypass or single‐valve repair or replacement, we routinely administered a single 50-mg/kg TXA bolus after induction of anesthesia. In the high‐risk group, which consists of complex redo, multiple valves, combined aortocoronary bypass and valve, or aortic procedures, we administered a 30-mg/kg bolus followed by a 16-mg/kg per hour infusion. Although these 2 dosing regimens have been used in large clinical trials with proven hemostatic benefits, we acknowledge that they are merely empirical formulas for the general cardiac surgery population. A recent cohort study, which observed serial TXA concentration based on the above regimes in patients with chronic renal dysfunction (CKD) throughout their cardiac surgery, found that single-bolus dosing without replenishment in stages 1 and 2 CKD patients led to subtherapeutic TXA concentrations due to rapid renal clearance. In contrast, overdosing of TXA was shown in patients with stages 3 to 5 CKD, among whom 5% had seizures.20 Using the pharmacokinetic model derived in this cohort study, our institution has now adopted a modified dosing regimen for our high‐risk patients with stages 3 to 5 CKD (Figure 1).
TXA dosing schedule for cardiac surgery patients requiring cardiopulmonary bypass.
TXA dosing schedule for cardiac surgery patients requiring cardiopulmonary bypass.
TXA is undoubtedly an indispensable drug for the prevention of catastrophic bleeding during extracorporeal circulation. The dosing regimens currently used, however, require refinement to tailor to at least subgroups, if not individual patients. In the absence of a tested algorithm that accounts for the pharmacokinetics of TXA and the appropriate tool to rapidly measure TXA concentration, we can only assume that the bolus, the infusion, or the combination of both would lead to a plasma concentration of maximum effectiveness. As it stands, we know that the current use of TXA is effective, but much improvement in dosing regimen is necessary to potentiate its antifibrinolytic effect and decrease its proconvulsive risk.
Preparation
More so than in most surgical settings, careful and thorough preparation is needed to set the foundation for a successful outcome in case of catastrophic bleeding in high‐risk patients. All team members should be made aware of the patient’s established massive bleeding determinants and the patients’ associated risks to fulfill their roles in the event of catastrophic bleeding. Preoperatively, if the patient is on any anticoagulant or antiplatelet agents, specialist consultation may be required to determine the safety of their temporary discontinuation and appropriate reversal.
Before the start of surgery, the designated operating room must be set up to facilitate the management of catastrophic bleeding. It should be warm to help prevent hypothermia, and it should be equipped with warming blankets, rapid fluid infusers with warming capacity, and infusion pumps with vasopressors, such as phenylephrine, norepinephrine, and vasopressin. The anesthesiologist must establish sufficient vascular access to allow for the resuscitation of and the administration of medication and monitoring of hemodynamics in the potential exsanguinating patient. For the truly high‐risk patient, we secure two large‐bore peripheral intravenous lines, a large‐bore central venous line with pulmonary artery catheter, and two arterial lines (one for monitoring blood pressure and another for blood sampling).
The patient’s blood must be typed and crossmatched with advance notice to the blood bank about the pending high‐risk nature of the surgery for timely preparation of massive transfusion support. In patients with low hemoglobin levels (ie, hemoglobin <100 g/L), it may be prudent to start red cell transfusions early to a target hemoglobin of 100 g/L to allow adequate reserve for hemodilution resulting from the prime in the extracorporeal circulation and the anticipated bleeding. The institution should have a massive bleeding/transfusion protocol, and all team members should be aware of their roles if the protocol is instituted. Visual aids outlining indications and the process are encouraged to be easily accessible in the operating room.
Protection against end organ injury and coagulopathy
During the early phase of catastrophic bleeding, the overarching objective should be to allow time for the surgeons to gain control of the bleeding source while simultaneously resuscitating the exsanguinating patient to protect against end organ (brain, heart, kidney, and liver) injury and the development of severe coagulopathy.
If patients are still anticoagulated at the time of bleeding, reversal agents should be administered if appropriate. Thereafter, early resuscitation during catastrophic bleeding should follow the recommendations for exsanguinating trauma patients, which are to use a balanced approach (also referred to as damage control resuscitation). The three facets of this approach are minimize crystalloid use, allow permissive hypotension (systolic blood pressure of 80‐90 mmHg), and transfuse allogeneic blood products empirically in ratios that approximate whole blood (typically a 1:1:1 ratio for plasma, platelet, and red cell units).21 Over the past decade, several observational and randomized studies have found that this approach is associated with improved outcomes in combat and civilian trauma populations.21 More recently, the Pragmatic, Randomized Optimal Platelet and Plasma Ratios trial compared the outcomes of patients randomized to a ratio of 1:1:1 versus 1:1:2, and it found that the primary outcome of mortality was similar between the groups but that the secondary outcomes of improved hemostasis and deaths due to bleeding were lower in the 1:1:1 group.22 It seems, therefore, that for the early empiric phase of resuscitation in the setting of catastrophic bleeding, fixed ratios of 1:1:1 or 1:1:2 are both appropriate. However, because data outside of the trauma setting are scant, clinicians should recognize that this has been given a weak recommendation based on low‐level evidence.23
If possible, cell salvage should be instituted to reduce the number of allogeneic red cell transfusions,17 because processed red cells via modern cell salvage machines have better survivability and oxygen-carrying capacity than stored allogeneic red cells.24,25 Hemoglobin should be measured frequently (via POC blood gases every 30 minutes) to guide the amount of red cell transfusions. Despite the proven safety of restrictive transfusion triggers in cardiac surgery,26 we aim for a hemoglobin of 100 g/L in rapidly bleeding patients to allow for reserve in oxygen delivery capacity and take advantage of the hemostatic properties of red cells.27 POC arterial blood gases should also be used to aid optimization of oxygenation, acid‐base balance, and electrolytes (calcium and potassium).
Promotion
After rapid bleeding is under some control, such that the patient is no longer exsanguinating, the intervention must shift from a systemic defensive coverage to promotion of normal hemostasis using the principle of “optimal blood management.” The principle states that, although the futile overuse of blood should be averted, it should not be delayed or underused in lifesaving situations.28 At this “promotion” stage of resuscitation, the primary objective is to rapidly identify the potential cause(s) of coagulopathy and institute targeted hemostatic therapy to correct the coagulopathy.
The coagulopathy related to extracorporeal circulation is usually multifactorial, but it is primarily caused by a combination of excessive fibrinolysis, platelet dysfunction or deficiency, and impaired thrombin generation due to coagulation factor deficiency.29,30 The most important instigator of these abnormalities is the contact of blood with the extracorporeal circulation, which activates the intrinsic and extrinsic coagulation pathways (despite the use of heparin), causing excessive clot formation and breakdown. Other contributory factors include circuit‐related hemodilution, protamine and heparin effects, hypothermia, surgical trauma, and blood loss.29,30 Unless the specific causes of coagulopathy are rapidly diagnosed and appropriate therapy is instituted, not only will ongoing bleeding necessitate additional red cell transfusions to maintain adequate hemoglobin levels, but it will also cause further loss of clotting factors, creating a vicious cycle of worsening coagulopathy and increased need for transfusions.
Until recently, clinicians predominantly relied on conventional laboratory tests that consist of prothrombin time, partial thromboplastin time, platelet count, and fibrinogen level to identify the causes of coagulopathy. These tests, applied individually or collectively, have major shortcomings: they have long turnaround times (usually >1 hour),31 are insensitive to even major reductions in coagulation factor levels, and cannot detect important coagulation defects, such as excessive fibrinolysis, platelet dysfunction, or specific coagulation factor deficiencies.29,32 These shortcomings severely hamper the management of coagulopathy, forcing clinicians to delay therapy until the results become available or resort to empiric therapy based on their clinical judgment. These strategies are inefficient and potentially harmful, because they can lead to underuse of blood products in some patients (worsening bleeding) and overuse of blood products in others,30,33 violating the principle of “optimal blood management” outlined above.28
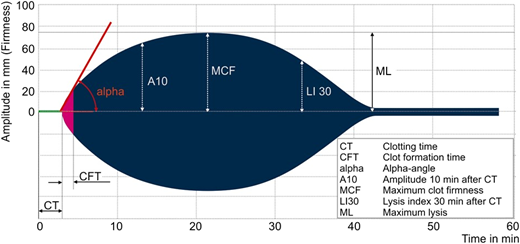
To better and more rapidly identify the specific cause(s) of coagulopathy, we use a combination of a POC viscoelastic coagulation assay (rotational thromboelastometry; Instrumentation Laboratory, Mississauga, ON, Canada) and a platelet function test (PlateletWorks; Helena Laboratories, Beaumont, TX) as part of a pragmatic algorithm that targets the three primary coagulation defects in the setting of extracorporeal circulation (Figure 2).10,34 Variables obtained from rotational thromboelastometry are illustrated in Figure 3.
POC-based transfusion algorithm in use at Toronto General Hospital.10 Rotational thromboelastometry (ROTEM; and its EXTEM and FIBTEM assays) and PlateletWorks are described in the text and Figure 3. ACT, activated clotting time; CPB, cardiopulmonary bypass.
Rotational thromboelastometry (ROTEM) curve and parameters. Curve and parameters apply to the INTEM (mild intrinsic coagulation activation) and EXTEM (mild extrinsic coagulation activation) assays. Our algorithm uses the EXTEM assay, which is largely heparin insensitive and can, therefore, be used on a heparinized patient. EXTEM is also insensitive to extracorporeal‐induced platelet dysfunction. Prolonged EXTEM‐CT indicates reduced coagulation factor levels, and low‐amplitude EXTEM‐A10 indicates impaired clot firmness due to low fibrinogen levels or low platelet count. To differentiate between low fibrinogen levels and low platelet count, the algorithm uses the FIBTEM assay (mild extrinsic coagulation activation in the presence of a thrombocyte inhibitor to measure only the fibrin part of the clot). A low‐amplitude FIBTEM‐A10 indicates low fibrinogen levels. A normal FIBTEM‐A10 in the presence of a low‐amplitude EXTEM‐A10 indicates low platelet count.
Rotational thromboelastometry (ROTEM) curve and parameters. Curve and parameters apply to the INTEM (mild intrinsic coagulation activation) and EXTEM (mild extrinsic coagulation activation) assays. Our algorithm uses the EXTEM assay, which is largely heparin insensitive and can, therefore, be used on a heparinized patient. EXTEM is also insensitive to extracorporeal‐induced platelet dysfunction. Prolonged EXTEM‐CT indicates reduced coagulation factor levels, and low‐amplitude EXTEM‐A10 indicates impaired clot firmness due to low fibrinogen levels or low platelet count. To differentiate between low fibrinogen levels and low platelet count, the algorithm uses the FIBTEM assay (mild extrinsic coagulation activation in the presence of a thrombocyte inhibitor to measure only the fibrin part of the clot). A low‐amplitude FIBTEM‐A10 indicates low fibrinogen levels. A normal FIBTEM‐A10 in the presence of a low‐amplitude EXTEM‐A10 indicates low platelet count.
PlateletWorks assesses platelet function as reflected by the change in platelet count in whole blood before and after platelet activation and aggregation induced by platelet agonists (collagen activator used in our algorithm) using an electronic impedance–based cell counter. The algorithm recommends a stepwise approach for patients with moderate blood loss while allowing for a multifaceted approach to those with rapid bleeding. The first recommendation is to administer platelets in patients who have low functioning platelet counts, which are a common cause of coagulopathic bleeding in patients requiring extracorporeal circulation. The recommendation for the treatment threshold of <75 000 × 106 in functioning platelet count is derived from the anticipation of an additional 20% to 30% drop (ie, to below 50 000 × 106) in functioning platelet count after heparin is neutralized with protamine.35 The next step in the algorithm is to administer fibrinogen supplementation (either with fibrinogen concentrate or cryoprecipitate) to correct hypofibrinogenemia, defined as A10‐FIBTEM ≤ 8 mm, which corresponds to fibrinogen <1.5 g/L via the Clauss method.36,37 There is currently scant comparative data on fibrinogen concentrate and cryoprecipitate for treatment of acquired hypofibrinogenemia, but a large randomized trial is underway that should help clarify which should be the therapy of choice.38 The final step targets impaired thrombin generation by recommending the administration of plasma or prothrombin complex concentrate (PCC). Impaired thrombin generation due to the combination of cardiopulmonary bypass–induced factor deficiency and heparin‐induced release of endothelial tissue factor pathway inhibitor may potentially be an important cause of bleeding after extracorporeal circulation.39 Verification of such postulation, however, is challenging given the limited capacity to measure thrombin generation clinically. CT‐EXTEM (similar to the International Normalized Ratio) is useful in measuring the time to the initiation of thrombin generation, but it provides no information on the dynamics of thrombin generation thereafter. Because normal CT‐EXTEM, INR, or both do not preclude the possibility of impaired thrombin generation, it may be appropriate to empirically target thrombin generation when bleeding continues and coagulation assays have been normalized. Among the scarce comparative data on plasma and PCC for enhancing thrombin generation, one in vitro study showed superiority of PCC over plasma in correcting impaired thrombin generation.40 In observational studies comparing PCC and plasma, patients who received PCC had less blood loss and required fewer transfusions,41,42 but confirmatory randomized trials are needed. Activated factor VII can be an alternative to plasma or PCC to enhance thrombin generation.40 However, the cost and the associated thromboembolic complications of activated factor VII dictate that it be used only in those well‐resuscitated patients whose bleeding is intractable to the correction of any identifiable coagulation defects.17,43
This algorithm was evaluated both at our institution and in a cluster randomized study at 12 other institutions. We found that implementation of the algorithm resulted in fewer red cell transfusions (relative risk, 0.91; 95% confidence interval [95% CI], 0.85-0.98), platelet transfusions (relative risk, 0.77; 95% CI, 0.68-0.87), and major bleeding episodes (relative risk, 0.83; 95% CI, 0.72-0.94). In other studies, POC‐based algorithms have also been associated with improved clinical outcomes (such as renal failure, sepsis, and death),44 but this was not the case in our experience.10,34
It is important to note that POC coagulation assays have direct (reagents) and indirect (personnel) costs that make them more costly than conventional coagulation assays, which likely hindered the wider adoption of POC‐based transfusion algorithms into practice.45,46 Arguably, similar results in the reduction of transfusion may be achieved using algorithms that rely on conventional coagulation assays, particularly if the issue of long turnaround times for those assays can be resolved (as has been done at some centers). Thus, the overarching objective at each institution should be to develop a transfusion algorithm that allows for rapid and targeted management of coagulation defects in patients who develop coagulopathy after catastrophic bleeding.
To conclude, in this review, we outlined the approach that we use at our institution to effectively prevent and manage catastrophic bleeding in extracorporeal circulation for cardiac surgery and extracorporeal life support. Our approach (which we structured in a 5 Ps mnemonic of prediction, prevention, preparation, protection, and promotion) is a pragmatic, evidence‐based approach that highlights the importance of addressing both anticipated and real‐time bleeding during the entire perioperative or periprocedure period. We believe that the appropriate application of this integrated approach, which yielded encouraging clinical outcomes in our institution, may also lead to favorable outcomes in the same high‐risk group of patients at other institutions. On the same note, we acknowledge that there is a paucity of data in many of the areas that we addressed, leaving much uncertainty about how to best manage such patients, which necessitates clarification and objective support from high‐quality, randomized, control studies in the future.
Acknowledgments
The authors thank Drs. Angela Jerath and Marcin Wasowicz for allowing them to use the tranexamic acid dosing schedule that they developed.
Correspondence
Keyvan Karkouti, Department of Anesthesia, Toronto General Hospital, 200 Elizabeth St, 3EN, Toronto, ON M5G 2C4, Canada; e-mail: keyvan.karkouti@uhn.ca.
References
Competing Interests
Conflict of interest: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.