Abstract
Transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO) are the leading causes of transfusion-related morbidity and mortality. These adverse events are characterized by acute pulmonary edema within 6 hours of a blood transfusion and have historically been difficult to study due to underrecognition and nonspecific diagnostic criteria. However, in the past decade, in vivo models and clinical studies utilizing active surveillance have advanced our understanding of their epidemiology and pathogenesis. With the adoption of mitigation strategies and patient blood management, the incidence of TRALI and TACO has decreased. Continued research to prevent and treat these severe cardiopulmonary events is focused on both the blood component and the transfusion recipient.
Learning Objectives
Review the definitions and pathophysiology of pulmonary transfusions incorporating recent in vivo models and clinical investigations
Highlight transfusion and recipient risk factors for transfusion-associated circulatory overload (TACO) and transfusion-related acute lung injury (TRALI) with an emphasis on patients with hematological malignancies
Describe the contemporary incidence and prevention of TACO and TRALI in relation to mitigation strategies and patient blood management
Introduction
With a growing body of knowledge regarding their pathogenesis, pulmonary transfusion reactions are drawing attention as potentially preventable medical complications. Systematic data collection has played an important role in understanding the incidence and epidemiology of transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO).1-6 These often severe hazards of transfusion have been independently associated with morbidity and mortality and account for the majority of all transfusion-related deaths since 2011.7-10 This report summarizes the current definitions, pathophysiology, and risk factors for TRALI and TACO, with additional focus on patients with hematological malignancies. In addition, we will review the impact of mitigation strategies and patient blood management (PBM) on reducing their incidence and role for further prevention.11
Definitions
A lack of consensus regarding diagnostic criteria has hindered clinical recognition and research investigation of pulmonary transfusion reactions.12,13 To promote more uniform reporting and research study, 2 consensus conferences convened more than a decade ago to standardize definitions of TRALI.6,14 In parallel, working groups representing blood transfusion societies have endeavored to improve the criteria for TACO, recognizing that current surveillance definitions lacked specificity.15,16 Accurate diagnosis of these reactions relies on interpretation of clinical, radiographic, and hemodynamic data that are labor intensive to extract and require expertise to interpret.17 In this regard, research studies of TACO and TRALI frequently use a panel of expert clinicians with knowledge in both intensive care and transfusion medicine to adjudicate cases.1,9,18
Common to all pulmonary transfusion reactions is the development of acute pulmonary edema within 6 hours of blood transfusion—characterized by bilateral pulmonary opacities on chest radiography and hypoxemia by arterial blood gas or pulse oximetry testing (PaO2/FiO2 < 300, SpO2 < 90% on room air, or other evidence of hypoxia). Surveillance definitions provide a framework for the identification and characterization of individual transfusion reactions that can be differentiated by the presence or absence of risk factors for acute respiratory distress syndrome (ARDS) and findings of circulatory overload (ie, findings of left atrial hypertension) (Table 1).17 The Canadian Consensus Criteria defines TRALI as acute pulmonary edema after transfusion in the absence of circulatory overload or alternate ARDS risk factors. In contrast, possible TRALI is defined as above, except it includes a temporal association with an ARDS risk factor. TACO is pulmonary edema primarily related to circulatory overload with criteria developed by the National Healthcare Safety Network including 3 or more of the following within 6 hours of transfusion: acute respiratory distress, radiographic pulmonary edema, elevated central venous pressure, evidence of left heart failure, elevated B-type natriuretic peptide (BNP), and a positive fluid balance.
Characteristics of pulmonary transfusion reactions
| Characteristic . | TRALI . | Possible TRALI . | TACO . |
|---|---|---|---|
| Bilateral infiltrates on chest radiograph consistent with acute pulmonary edema | Yes | Yes | Yes |
| Evidence of hypoxemia* | Yes | Yes | Yes |
| Evidence of circulatory overload† | No | Yes/no | Yes |
| Presence of major ARDS risk factor(s)‡ | No | Yes | No |
| Characteristic . | TRALI . | Possible TRALI . | TACO . |
|---|---|---|---|
| Bilateral infiltrates on chest radiograph consistent with acute pulmonary edema | Yes | Yes | Yes |
| Evidence of hypoxemia* | Yes | Yes | Yes |
| Evidence of circulatory overload† | No | Yes/no | Yes |
| Presence of major ARDS risk factor(s)‡ | No | Yes | No |
Hypoxemia defined as PaO2/FiO2 < 300, SpO2 < 90% on room air, or other evidence of hypoxia.
A systematic evaluation of echocardiography (ejection fraction, diastolic function, and ventricular filling pressures), chest radiography (cardiothoracic ratio and vascular pedicle width), laboratory findings (B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide), hemodynamic parameters (systolic blood pressure and central venous pressure), and clinical course (rapid resolution of pulmonary edema after preload reduction favoring a hydrostatic rather than permeability etiology).
Major ARDS risk factors included pneumonia, sepsis, aspiration, multiple fractures, and pancreatitis.
Clinical presentation
Signs and symptoms of TACO and TRALI begin within 6 hours of completion of blood transfusion and include tachycardia, tachypnea, and hypoxia. These clinical findings can be blunted, particularly in postoperative or intensive care patients who may not exhibit signs of respiratory distress due to sedation or preexisting ventilatory support. Cardiovascular risk factors predominate in TACO; patients tend to be older and frequently have a history of congestive heart failure and/or coronary artery disease (Table 2).2,3 Renal impairment as reflected by a history of chronic kidney disease is also common in TACO, whereas acute kidney disease and liver failure are prevalent in both TRALI and possible TRALI.2,5,19 Surgery (both emergency or elective) is frequently associated with TACO and TRALI, with >1/2 of cases of each occurring perioperatively.2,5,20 TRALI and possible TRALI have also been associated with chronic alcohol abuse, tobacco use, shock before transfusion, and a positive fluid balance relative to transfused controls.1,21,22 Although hypertension can occur with TACO, shock is common in TACO, TRALI, and possible TRALI, with∼1/2 of cases requiring concurrent vasopressor support in one series.20
Characteristics of cases and transfused controls from the TRALI Specialized Clinical Center for Outcomes Research
| Patient characteristic . | TRALI, n = 89 . | Possible TRALI, n = 130 . | TACO, n = 83 . | Control, n = 164 . |
|---|---|---|---|---|
| Units transfused* | 4 (2-13) | 3 (2-5) | 4 (2-10) | 3 (2-10) |
| Blood component type | ||||
| Red blood cells | 67 (75%) | 83 (64%) | 66 (80%) | 127 (77%) |
| Plasma | 59 (66%) | 70 (54%) | 46 (55%) | 81 (49%) |
| Platelets | 37 (42%) | 52 (40%) | 43 (52%) | 56 (34% |
| Comorbid characteristics | ||||
| History of congestive heart failure | 7 (8%) | 8 (6%) | 29 (35%) | 13 (8%) |
| Coronary artery disease | 15 (17%) | 31 (24%) | 34 (41%) | 30 (18%) |
| Acute renal failure | 23 (26%) | 34 (26%) | 15 (18%) | 17 (10%) |
| Chronic renal failure | 5 (6%) | 11 (12%) | 15 (18%) | 14 (9%) |
| Severe liver disease | 21 (24%) | 20 (15%) | 9 (11%) | 15 (9%) |
| Chronic alcohol abuse | 12 (13%) | 17 (13%) | 5 (6%) | 6 (4%) |
| History of tobacco use | 18 (20%) | 27 (21%) | 11 (13%) | 18 (11%) |
| Hematologic malignancy | 6 (7%) | 25 (19%) | 6 (7%) | 30 (18%) |
| Recent surgery | 54 (61%) | 49 (38%) | 62 (75%) | 68 (41%) |
| Clinical characteristics | ||||
| Shock before transfusion | 39 (44%) | 59 (45%) | 32 (39%) | 30 (18%) |
| Ventilation after transfusion | 42 (47%) | 68 (52%) | 56 (67%) | 39 (24%) |
| Fluid balance, L | 3.7 (1.6-7.3) | 4.5 (1.7-7.7) | 3.7 (1.6-6) | 2.0 (0.4-4.5) |
| BNP after transfusion, pg/mL† | 252 (133-593) | 693 (361-1370) | 1907 (1108-2339) | 373 (291-589) |
| Patient characteristic . | TRALI, n = 89 . | Possible TRALI, n = 130 . | TACO, n = 83 . | Control, n = 164 . |
|---|---|---|---|---|
| Units transfused* | 4 (2-13) | 3 (2-5) | 4 (2-10) | 3 (2-10) |
| Blood component type | ||||
| Red blood cells | 67 (75%) | 83 (64%) | 66 (80%) | 127 (77%) |
| Plasma | 59 (66%) | 70 (54%) | 46 (55%) | 81 (49%) |
| Platelets | 37 (42%) | 52 (40%) | 43 (52%) | 56 (34% |
| Comorbid characteristics | ||||
| History of congestive heart failure | 7 (8%) | 8 (6%) | 29 (35%) | 13 (8%) |
| Coronary artery disease | 15 (17%) | 31 (24%) | 34 (41%) | 30 (18%) |
| Acute renal failure | 23 (26%) | 34 (26%) | 15 (18%) | 17 (10%) |
| Chronic renal failure | 5 (6%) | 11 (12%) | 15 (18%) | 14 (9%) |
| Severe liver disease | 21 (24%) | 20 (15%) | 9 (11%) | 15 (9%) |
| Chronic alcohol abuse | 12 (13%) | 17 (13%) | 5 (6%) | 6 (4%) |
| History of tobacco use | 18 (20%) | 27 (21%) | 11 (13%) | 18 (11%) |
| Hematologic malignancy | 6 (7%) | 25 (19%) | 6 (7%) | 30 (18%) |
| Recent surgery | 54 (61%) | 49 (38%) | 62 (75%) | 68 (41%) |
| Clinical characteristics | ||||
| Shock before transfusion | 39 (44%) | 59 (45%) | 32 (39%) | 30 (18%) |
| Ventilation after transfusion | 42 (47%) | 68 (52%) | 56 (67%) | 39 (24%) |
| Fluid balance, L | 3.7 (1.6-7.3) | 4.5 (1.7-7.7) | 3.7 (1.6-6) | 2.0 (0.4-4.5) |
| BNP after transfusion, pg/mL† | 252 (133-593) | 693 (361-1370) | 1907 (1108-2339) | 373 (291-589) |
Subjects are from the TRALI Specialized Clinical Center for Outcomes Research study (from 2006 to 2009).1 Data are reported as median values (interquartile ranges) or numbers (percentages).
Number of blood components transfused in the 6 hours before development of pulmonary edema for cases.
This is a subset of the total BNP after transfusion (n = 98), pg/mL.
BNP testing has had a major impact on the diagnosis and management of patients with pulmonary edema—including those with TACO and TRALI.23,24 BNP levels classically are elevated in patients with congestive heart failure or circulatory overload. However, elevated BNP levels have also been reported in clinical trials of ARDS, in which almost 1/3 of cases had pulmonary capillary wedge pressure measurements consistent with concomitant hydrostatic edema.25,26 In addition to volume overload, BNP is thought to be elevated through other mechanisms related to cardiac stress, such as catecholamine release or hypoxia.25 Initial studies in transfusion found elevated BNP levels in patients with TACO compared with transfused controls.27,28 Subsequent studies found higher BNP levels in cases of TACO and possible TRALI relative to cases of TRALI.29-31 Indeed, BNP levels were similar in TACO and possible TRALI, suggesting practical utility in their differentiation from TRALI.
Echocardiography can also provide critical information regarding the pathogenesis of pulmonary edema after transfusion and has supplanted invasive hemodynamic monitoring in this regard.3,26,32 Echocardiography offers a noninvasive structural and functional cardiac assessment and often reveals findings that had been unrecognized clinically.8,33 Evidence of elevated cardiac filling pressures or systolic and/or diastolic dysfunction suggest circulatory overload and a diagnosis of TACO in the setting of pulmonary edema.3 In contrast, the absence of echocardiographic abnormalities is central to the diagnosis of TRALI, and elevations in BNP are less frequent. In the setting of risk factors for ARDS, cases of possible TRALI may have findings of cardiac dysfunction and elevated filling pressures, supporting both a permeability and hydrostatic basis for pulmonary edema.20
Transfusion-specific risk factors for TRALI and TACO include the total number of blood products administered, regardless of component type.1 Plasma from a previously pregnant blood donor is a well-established risk factor for TRALI due to pregnancy-related alloimmunization (ie, development of antileukocyte antibodies).1,34-37 One study assessed the prevalence of human leukocyte antigen (HLA) antibodies in female donors using flow cytometry and found a dose-response increase in HLA antibodies with the number of prior pregnancies.38,39 Although blood components with a high volume of plasma (eg, plasma, apheresis platelet concentrates, and whole blood) have been shown to confer the highest risk, TRALI has been reported after all products—including cryoprecipitate, granulocytes, intravenous immunoglobulin preparations, and stem cell products.40-42 For TACO, the independent association with plasma transfusion is seemingly related to the large volume of multiple blood products required to reverse coagulopathy in patients with impaired cardiac and renal function.3,5,9
Patients with hematologic malignancies would seem to be at increased risk of TACO and TRALI. Patients receiving chemotherapy or undergoing hematopoietic stem cell transplantation require frequent blood transfusions in addition to significant volumes of maintenance fluids, parenteral nutrition, and intravenous medications. In addition, lymphoma and leukemia are common in older individuals who often have preexisting cardiovascular disease and are more prone to chemotherapy-induced cardiac dysfunction or kidney injury, risk factors for TACO. Severe thrombocytopenia occurs commonly in patients receiving treatment of leukemia, and frequent platelet and red blood cell (RBC) transfusions are often required. Indeed, pulmonary reactions in patients with leukemia and lymphoma tend to be associated with fewer transfusions per case and more frequent platelet transfusion compared with other populations (Tables 2 and 3).1,19 However, there are no clear differences in component type or platelet and leukocyte counts compared with transfused controls with leukemia or lymphoma, although observations are limited by small sample sizes. Despite these risk factors, TACO and TRALI occur less frequently in patients with hematologic malignancy relative to transfused controls. Although TRALI can develop through direct activation of the pulmonary endothelium, the lower incidence of TRALI in leukemia and lymphoma patients is likely related to neutropenia (see below).43
Transfusion characteristics of TRALI Specialized Clinical Center for Outcomes Research subjects with leukemia or lymphoma
| Characteristic . | TRALI, n = 6 . | Possible TRALI, n = 25 . | TACO, n = 6 . | Controls, n = 30 . |
|---|---|---|---|---|
| Total cases, % | 7 (6/89) | 19 (25/130) | 7 (6/83) | 18 (30/164) |
| Units transfused | 1 (1-1) | 1 (1-2) | 1 (1-2) | 2.5 (2-3) |
| RBCs, % | 50 | 32 | 33 | 73 |
| Platelets, % | 67 | 72 | 83 | 63 |
| Plasma, % | 0 | 24 | 0 | 17 |
| Laboratory results | ||||
| WBC count | 1.7 (0.2-2.4) | 3.1 (0.2-10.5) | 3.6 (0.1-6.9) | 1.6 (0.2-4.6) |
| Platelet count | 24 (5-33) | 25 (12-45) | 43 (17-73) | 22 (11-35) |
| Characteristic . | TRALI, n = 6 . | Possible TRALI, n = 25 . | TACO, n = 6 . | Controls, n = 30 . |
|---|---|---|---|---|
| Total cases, % | 7 (6/89) | 19 (25/130) | 7 (6/83) | 18 (30/164) |
| Units transfused | 1 (1-1) | 1 (1-2) | 1 (1-2) | 2.5 (2-3) |
| RBCs, % | 50 | 32 | 33 | 73 |
| Platelets, % | 67 | 72 | 83 | 63 |
| Plasma, % | 0 | 24 | 0 | 17 |
| Laboratory results | ||||
| WBC count | 1.7 (0.2-2.4) | 3.1 (0.2-10.5) | 3.6 (0.1-6.9) | 1.6 (0.2-4.6) |
| Platelet count | 24 (5-33) | 25 (12-45) | 43 (17-73) | 22 (11-35) |
Subjects are from the TRALI Specialized Clinical Center for Outcomes Research study.1 Data are reported as median values (interquartile ranges) or numbers (percentages). WBC, white blood cell.
Alternative causes of lung injury also need to be considered in patients with hematologic malignancies requiring blood transfusions. Lung injury related to sepsis or pneumonia is a common adverse event in the treatment of hematologic malignancies. Other noninfectious pulmonary complications include drug- or chemotherapy-induced pneumonitis, organizing pneumonia, diffuse alveolar hemorrhage, and idiopathic pulmonary syndromes; the latter is an infrequent albeit severe early complication of hematopoietic stem cell transplantation that may occur during a period of increased transfusion requirement. In general, pulmonary edema due to TACO and TRALI tends to be less severe and is associated with lower mortality compared with that in patients with ARDS and the above complication syndromes.2,21,22
Incidence
Historically, incidence estimates of TACO and TRALI have been based on adverse event reporting by national hemovigilance systems. The major limitation of these systems is reliance on passive reporting of transfusion reactions, which is known to underestimate true incidence given poor syndrome recognition.12,44,45 Expanded use and versatility of electronic health records (EHRs) have helped to address this limitation by incorporating algorithms that screen transfused patients for signs suggestive of pulmonary transfusion reactions. Active surveillance improves accuracy of assessment and reporting, thus offering a better approximation of the epidemiology of these reactions.1,5,13,46 As an example, the largest active surveillance study of TRALI used an electronic screening algorithm, the collection of granular clinical data, and expert panel review for outcome adjudication in a multicenter population of both medical and surgical patients. Collectively, this served to more definitively correlate the type and strength of HLA antibodies with TRALI and showed a clear decline in TRALI incidence after plasma mitigation.1
Reported incidence rates of TACO and TRALI have varied based on the studied patient population or component type. Historically, active surveillance estimates of TRALI were ∼0.1% of transfused patients but up to 5% to 8% in groups of intensive care unit populations.4,19,42 Classification of possible TRALI with ARDS risk factors and TRALI cases together accounts for some of the variability in reported incidence in hemovigilance and active surveillance studies.4,19,29,42,47 Prospective studies of TRALI in diverse, multicenter populations found lower incidence rates (0.0008% to 0.001% of transfused patients) after implementation of donor mitigation strategies.1,9 In parallel to studies of TRALI, case series have reported higher TACO incidence related to plasma transfusion and in perioperative or critically ill populations (1% to 4% of transfused patients).2,5,19,48 After declines in transfusion related to PBM, TACO has been estimated to occur in ∼1% of transfused patients.9,13
Pathophysiology
Antibody-mediated TRALI
Murine models and human studies support an antibody-mediated basis for TRALI. Donor leukocyte antibodies present in the plasma of a transfused blood component are postulated to bind to cognate (ie, corresponding) antigen in the recipient, resulting in capillary leak and lung injury. This hypothesis is supported by in vivo models, in which TRALI develops after antibodies to HLA or human neutrophil antigen (HNA) are infused into animals expressing cognate antigens.49-51 The demonstration of antibodies in blood donor plasma with specificities or cross-reactivity against cognate recipient antigens (ie, recipient HLA type or HNA) provides additional evidence for this hypothesis.1,44,52,53
However, the majority of transfused blood products that contain HLA antibodies do not cause TRALI, even when a cognate recipient antigen is present.38 In one study, TRALI occurred in only 3% of recipients who received blood products from donors that had previously been implicated in cases of TRALI.54 It was hypothesized that TRALI induction required sufficient quantity of antibody as determined by the volume of plasma transfused as well as the antibody class and strength (threshold hypothesis). In a multivariable analysis, the strength of cognate HLA class 2 antibody and the volume of HNA antibody were strong predictors of TRALI, with less evidence supporting the role of HLA class 1 antbodies.1,55 However, all classes of cognate antibody exposure have been associated with severe and fatal cases of TRALI.56
In addition to cognate antibody exposure, systemic inflammation in the transfusion recipient is thought to be critical to TRALI pathogenesis by priming neutrophils and activating pulmonary endothelial cells.57-59 The role of pretransfusion inflammation as part of a “2-event hypothesis” is supported by murine models of TRALI, in which an inflammatory response related to lipopolysaccharide or other stimuli (first event) is often required for HLA antigen/antibody interactions (second event) to induce lung injury.50,60 Elevations in inflammatory cytokines interleukin-6 (IL-6), IL-8, and C-reactive protein before transfusion have been associated with the development of TRALI; the latter was also shown to enhance TRALI when administered in a murine model.20,61-63 Systemic inflammation induces the expression of adhesion molecules on endothelial cells (CD62, ICAM-1) and neutrophils (PSGL-1), increasing interaction with peripheral granulocytes and resulting in intrapulmonary leukostasis.
In the setting of a systemic inflammatory process, recipient neutrophils are then activated by a sufficient quantity of cognate antibody in a transfused blood component. Neutrophil activation is associated with the release of inflammatory cytokines, reactive oxygen species, oxidases, proteases, and neutrophil extracellular traps that disrupt the alveolar-capillary barrier of the lung, resulting in an inflammatory form of pulmonary edema.58,64,65 Endothelial cell activation in areas of neutrophil aggregation in lung tissue is evidenced by high expression of adhesion molecules, such as CD31 and CD34, by immunohistochemistry.66 In some models, lung injury is dependent on neutrophil expression of Fc γ-receptors, and it is interdicted in mice through depletion of neutrophils using antigranulocyte antibodies.67 A supportive role for neutrophils in TRALI is further provided by the detection of neutrophil extracellular traps in both TRALI experimental models and human samples as well as the alleviation of lung injury by blocking neutrophil extracellular traps.64,65 In a small number of human cases, initiation of TRALI was thought to be reversed, with a leukoagglutinating antibody present in the recipient reacting to HLA antigens on the surface of transfused neutrophils, so-called “reverse TRALI.”40,68
There are supportive data on the role of other effector cells in TRALI in addition to neutrophils. Donor antileukocyte antibodies can also bind to antigens on monocytes and macrophages (HLA class 2 antibody), pulmonary endothelial cells, and platelets (HLA class 1 antibodies); these mechanisms may play a role in the development of TRALI in the setting of neutropenia (eg, patients with hematologic malignancy). For example, one lipopolysaccharide-dependent murine model found that HLA class 1 antibodies bound the vascular endothelium, resulting in complement and monocyte activation and subsequent damage to the pulmonary endothelium by reactive oxygen species.43 Other lipopolysaccharide-dependent and -independent murine models have found antibody binding of monocytes or the pulmonary endothelium, but they have also involved neutrophilic pulmonary infiltration in the development of TRALI.69,70
In parallel, platelets may play a role in TRALI pathogenesis, secreting proinflammatory mediators and interacting directly with neutrophils to promote their activation.64 Platelet migration into the alveolar spaces occurs in murine models of TRALI either alone or complexed with neutrophils. It is clear that differences in murine genotype, method of platelet depletion, and need for lipopolysaccharide explain some of the differences in proposed TRALI mechanisms as well as those between animal models and TRALI in humans. Additional investigations are needed to resolve these differences and advance our understanding of TRALI pathogenesis.
Nonantibody-mediated TRALI
In ∼20% of TRALI cases, HLA and HNA antibodies are not identified in the transfused product, despite the use of sensitive assays.71,72 These cases of “nonantibody-mediated” TRALI may be due to unidentified antibodies or exposure to other biological reactive molecules (BRMs) within the transfused blood component administered. In parallel to donor leukocyte antibodies in the 2-event hypothesis for antibody-mediated TRALI, BRMs are thought to be the necessary stimuli to trigger the cascade of lung injury in a primed transfusion recipient. Cases of TRALI associated with RBC transfusion are infrequently associated with antileukocyte antibodies; instead, inflammatory factors in the supernatant of RBCs are thought to play a role in the pathogenesis in these cases. The contribution of inflammatory mediators to development of nonimmune TRALI is partially supported by retrospective, observational data showing a decline in the incidence of TRALI after universal leukoreduction.73,74 However, in vitro evidence showing accumulation of leukocyte-derived inflammatory cytokines, soluble CD40 ligand, and nonpolar lipids (lysophosphatidylcholines) with prolonged RBC and platelet storage is not supported by case-control studies of human TRALI.1,42,75-80 Ongoing research of nonantibody-mediated TRALI is focused on the role of blood donor characteristics and component manufacturing methods.81,82
Possible TRALI
The role of transfusion in the development or exacerbation of lung injury in patients with ARDS risk factors (eg, sepsis or pneumonia) remains a topic of debate.83 Infectious risk factors for ARDS are common in patients with hematologic malignancy, further complicating distinction. Nonetheless, the high mortality associated with pulmonary edema requiring invasive mechanical ventilation in immunocompromised patients underscores the need to understand transfusion’s contribution to ARDS in this frequently transfused population.84,85
The pathogenesis and clinical course of possible TRALI are very different from those of antibody-mediated TRALI.21,22 Pretransfusion elevations in inflammatory cytokine, such as IL-8, are prominent, but antileukocyte antibodies are infrequent in possible TRALI.22,34 Research of biologic response mediators in possible TRALI has focused on the storage age of blood components. However, the age of blood has not been shown to confer differences in pulmonary, immunologic, or hemostatic function in those who receive fresh vs standard issue blood.86,87 A male-predominant plasma strategy also failed to reduce the possible TRALI incidence.1,18 Given similarities in the pathophysiology and clinical outcomes between possible TRALI and ARDS, some investigators have proposed renaming possible TRALI as “transfused ARDS.”1,83 A working group is reconsidering the nomenclature for possible TRALI given confusion regarding adverse event reporting and donor deferral as well as to allow additional characterization of its epidemiology.
TACO
The pathophysiology of TACO resembles that of other forms of acute cardiogenic pulmonary edema.88 In healthy individuals, pulmonary capillary pressures are counterbalanced by colloid osmotic pressure, and small volumes of transudate are removed from alveoli by the pulmonary lymphatic system. The latter is able to adapt to a range of left atrial and pulmonary capillary pressures, thus maintaining homeostasis.89 Blood transfusion can rapidly increase left atrial and pulmonary capillary pressures, resulting in transudation of fluid into the pulmonary interstitium and alveolar space (ie, TACO). Blood transfusion is thought to increase oncotic and pulmonary capillary pressures more significantly than an equivalent volume of intravenous crystalloid fluid, potentially accounting for its designation from other mechanisms of circulatory overload.90,91 However, similar to other mechanisms, TACO frequently occurs in patients with preexisting cardiac (left ventricular systolic or diastolic) dysfunction or renal impairment who are unable to compensate for an increase in circulating blood volumes.2 In these chronic conditions, pulmonary capillary wedge pressures may be persistently elevated with expansion of lymphatic capacity and result in pulmonary edema when further increased with transfusion.
A number of TACO cases occur after a single-blood component transfusion without overt signs of systemic volume overload.9,32 In these cases, TACO can be accompanied by an acute hypertensive response, where a rapid rise in systemic vascular resistance results in increased cardiac filling pressures and hydrostatic pulmonary edema. It has been hypothesized that BRMs, such as plasma free hemoglobin or nitric oxide scavengers, act on vascular smooth muscle in these cases and contribute to TACO by increasing systemic vascular resistance.92-94 Countering this hypothesis is evidence that prolonged storage of blood, in which BRMs are known to accumulate, does not result in differences in pulmonary function in transfused cardiac surgery or intensive care patients.86,95,96
The role for recipient inflammation in the pathogenesis of TACO remains an area of debate. Case series have shown that a systemic inflammatory response, including fevers, occurs in some patients with TACO. However, case-control studies have shown elevations in the inflammatory cytokine IL-8 before transfusion in patients who develop TRALI and possible TRALI but not in those who develop TACO.1,21,63 In addition, a combination of inflammatory cytokines was shown to be useful in differentiating TACO from TRALI and possible TRALI after transfusion.20 Laboratory investigations and clinical trials are needed to control for confounding factors for inflammation, such as recent surgery, which are frequently associated with TACO.
Mitigation
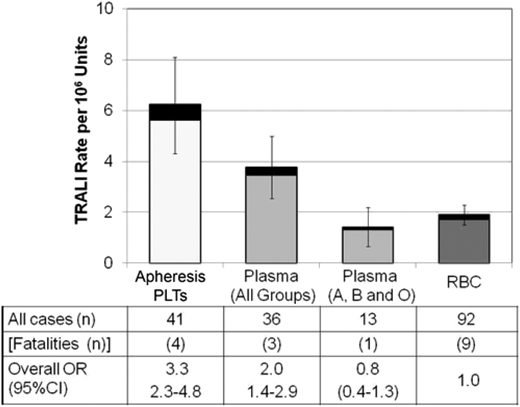
Despite underestimation of incidence, passive hemovigilance reporting mechanisms complement active surveillance studies through provision of data on trends related to mitigation strategies.55,97-100 In 2007, the American Red Cross began to distribute plasma, preferentially from male donors to limit exposure to donor leukocyte antibodies from previously pregnant female blood donors. Subsequently, the investigators observed an 80% reduction in the passively reported rate of TRALI related to plasma transfusion.97 After the adoption of male-only plasma collection, the epidemiology of TRALI shifted toward RBC and platelet blood components (Figure 1).101 Due to transfusion of a much larger number of RBCs compared with platelets and plasma, the majority of TRALI cases are associated with red cell units. However, the per component rate of TRALI reported to the American Red Cross after plasma mitigation was higher for apheresis platelets, which contain large volumes of donor plasma, than for RBCs or male donor–predominant plasma. In addition, the rate of TRALI was ∼6-fold higher with female compared with male apheresis platelet donors.
TRALI cases by blood component type after implementation of a male donor plasma mitigation strategy. TRALI cases classified in the American Red Cross hemovigilance program are expressed per million distributed components for transfusions involving only a single component type. The black stacked bars are reported fatalities. Lines show 95% confidence intervals (95% CIs) for the overall TRALI rates (fatal and nonfatal cases). OR, odds ratio; PLT, platelet. Reprinted from Eder et al104 with permission.
TRALI cases by blood component type after implementation of a male donor plasma mitigation strategy. TRALI cases classified in the American Red Cross hemovigilance program are expressed per million distributed components for transfusions involving only a single component type. The black stacked bars are reported fatalities. Lines show 95% confidence intervals (95% CIs) for the overall TRALI rates (fatal and nonfatal cases). OR, odds ratio; PLT, platelet. Reprinted from Eder et al104 with permission.
Concern that the current platelet supply would not be sufficient to support conversion to male-only donations led to a strategy to screen female apheresis platelet donors for leukocyte antibodies. In 2009, the National Health Service Blood and Transplant in the United Kingdom began to test female apheresis platelet donors and defer those positive for HLA class 1 and/or class 2 antibodies. Over a 1-year period, this policy identified higher-risk antibody-positive donors, and when combined with other measures, it further reduced TRALI incidence.102 More recently, one blood center reported that platelet mitigation strategies had reduced TRALI incidence related to platelet transfusion nearly 3-fold.103
In 2009, the vast majority of US blood centers surveyed had initiated some measures of screening for high-risk platelet donors in response to an AABB (formerly American Association of Blood Banks) bulletin recommending platelet and plasma mitigation strategies.11 In October 2016, the AABB issued an updated standard now requiring blood centers to distribute apheresis platelets exclusively collected from males, females who have never been pregnant, or females with negative HLA antibody test results since their most recent pregnancy. One estimate suggested that a strategy to test all previously pregnant female plateletpheresis donors for HLA antibodies could further reduce the risk of TRALI related to platelet transfusion by 60%.101 However, data on the impact of universal platelet mitigation on reduction in TRALI incidence in the United States are not yet available.
Although mitigation strategies have been successful in reducing the risk of TRALI, they do revisit the challenges of maintaining an adequate blood supply. One study found that the residual risk of TRALI from plasma transfusion was confined almost entirely to group AB female donors.104 The latter are universally compatible plasma donors, and they are in short supply but high demand. Although the majority of group AB plasma components were from male donors, 40% were from female donors unscreened for HLA antibodies and therefore, had an associated increased risk for TRALI. Recent data supporting the safety of group A plasma in trauma situations rather than ABO-compatible group AB plasma may decrease the need for collection of female plasma due to blood supply limitations.105
Additional prevention of TACO and TRALI requires identification and mitigation of modifiable risk factors. The risk related to plasma is ascribed, in part, to emergency transfusion with unscreened AB plasma in the case of TRALI and large infused volumes in individuals with impaired cardiac and renal function in the case of TACO. In the case of TACO, slowing of blood infusion rates and prophylactic volume reduction with diuretics may benefit selected patients.8,121 The use of homeostatic agents, such as factor concentrates, prothrombin complex concentrates, and antithrombolytics, may be an alternative to emergency plasma transfusion, and a factor concentrate was less frequently associated with pulmonary edema compared with plasma in a small clinical trial of patients receiving anticoagulation.122
Pooling of products and the use of additive solutions seem to be other practical ways to reduce the risk of TRALI from blood components. In the case of pooling, platelet and plasma components are prepared from multiple donors; this may dilute any leukocyte antibodies or neutralize any antibodies by residual leukocytes or soluble HLA or HNA antigens in the pool. For solvent/detergent plasma products, plasma from up to 2500 blood donors is pooled and treated with solvent 1% tri-N-butylphosphate and detergent 1% Triton X-100, resulting in lower antibody titers or antibody neutralization. Observational studies of solvent/detergent plasma suggest a significant reduction in the risk of TRALI.98,123,124 Some platelet additive solutions replace ∼2/3 of the plasma contained in the platelet component and have been associated with a decline in TRALI incidence in buffy coat–derived platelet concentrates.125
In addition to mitigation strategies, the widespread adoption of PBM has played a significant role in reducing the incidence of both TACO and TRALI. The number of units transfused is a risk factor for all pulmonary transfusion reactions, and reduction in transfusion is a core tenet of PBM. PBM strategies are derived from randomized control trial data that support the safety of restrictive transfusion practice in both medical and surgical populations.106-109 A meta-analysis of clinical trials suggested that restrictive transfusion resulted in a lower incidence of posttransfusion pulmonary edema compared with liberal transfusion.110 In a randomized pilot study of patients with leukemia, restrictive RBC transfusion was also not associated with differences in neutropenic fever or bleeding complications.111 PBM strategies have led to a substantial decline in blood transfusion in many developed countries over the past 5 years.112-120 In parallel with reduction in blood transfusion, recent active surveillance studies of TACO and TRALI found lower incidence rates of transfusion-related adverse events.5,9
Future directions
Significant progress has been made in both the understanding of TRALI and its mitigation. Future strategies for the prevention of TRALI may include immune-based strategies to block donor antibodies or the immune response to transfused antibodies. Human investigations have found increased plasma levels of the inflammatory cytokine IL-8 but lower levels of the anti-inflammatory cytokine IL-10 in both antibody- and nonantibody-mediated cases of TRALI compared with those of possible TRALI.1,20,126 Recently, it was shown that regulatory T cells and dendritic cells were protective in a murine model of antibody-mediated TRALI, and this protection was associated with increased plasma levels of IL-10.127 In addition, there was evidence of a benefit of IL-10 administration in the prevention of murine TRALI as well as treatment after its induction with HLA antibodies (Figure 2). These findings may indicate a distinct mechanism of pathogenesis in TRALI compared with other forms of lung injury as well as opportunities for prevention and treatment.
TRALI suppression in CD4+ T cell–depleted mice with murine IL-10 administration. (A) Decreased lung wet/dry (W/D) weight ratios of CD4+ T cell–depleted C57BL/6 mice infused with 34-1-2s/AF6-88.5.5.3 and treated prophylactically with murine IL-10 administration (45 mg/kg intravenously). (B) Lung W/D weight ratios of CD4+ T cell–depleted C57BL/6 mice infused with 34-1-2s/AF6-88.5.5.3 and treated therapeutically 15 minutes later with or without murine IL-10 administration (45 mg/kg intravenously) after onset of TRALI (at least a 2° drop in rectal temperature 10 minutes after TRALI antibody injection). Comparisons in both panels were analyzed with one-tailed unpaired t test. Each dot represents 1 mouse, and error bars represent standard deviation. *P < .05; ****P <. 0001. PBS, phosphate-buffered saline. Reprinted from Kapur et al127 with permission.
TRALI suppression in CD4+ T cell–depleted mice with murine IL-10 administration. (A) Decreased lung wet/dry (W/D) weight ratios of CD4+ T cell–depleted C57BL/6 mice infused with 34-1-2s/AF6-88.5.5.3 and treated prophylactically with murine IL-10 administration (45 mg/kg intravenously). (B) Lung W/D weight ratios of CD4+ T cell–depleted C57BL/6 mice infused with 34-1-2s/AF6-88.5.5.3 and treated therapeutically 15 minutes later with or without murine IL-10 administration (45 mg/kg intravenously) after onset of TRALI (at least a 2° drop in rectal temperature 10 minutes after TRALI antibody injection). Comparisons in both panels were analyzed with one-tailed unpaired t test. Each dot represents 1 mouse, and error bars represent standard deviation. *P < .05; ****P <. 0001. PBS, phosphate-buffered saline. Reprinted from Kapur et al127 with permission.
Prior studies have suggested a lack of benefit of the anti-inflammatory effects of systemic corticosteroids in ARDS and a lipopolysaccharide-stimulated murine model of TRALI.60,128 Differences in murine TRALI models or the timing of corticosteroid administration may explain these disparate effects. Other anti-inflammatory modalities to prevent or treat TRALI have also been proposed, including the targeting of C-reactive protein, IL-8, reactive oxidative species, neutrophil extracellular traps, or Fc receptors.129 Additional investigations are needed to confirm that these murine model findings parallel human pathophysiology before embarking on clinical studies of TRALI prevention or treatment. The recognition that any immunomodulatory therapy could increase the risk of infection is especially relevant in immunocompromised patients (eg, those with hematologic malignancies). In contrast to TRALI, few in vitro or in vivo investigations have been conducted to further understand the pathogenesis of TACO. In vivo models of TACO are needed to study the effect of individual blood components relative to other intravenous fluids on pulmonary capillary pressures, specific rates of blood administration, and the benefit of prophylactic diuretics as well as the role of systemic inflammation.
Work to improve blood collection and storage may also provide the opportunity to prevent TACO and TRALI. Modifications to blood components include the development of novel filters, apheresis collection systems, pathogen reduction, extended storage of platelets, and new methods of leukodepletion or irradiation. A novel prestorage filter absorbed HLA antibodies and lipids in addition to leukocytes and platelets, and it was associated with reduced neutrophil activation and TRALI incidence in an animal model.130 Translation of an effective filter to clinical practice could obviate the need for specific donor mitigation strategies. Continued research to develop RBC and platelet storage solutions that preserve blood product quality but also mitigate TRALI also hold promise.131-134 In addition, an ongoing randomized clinical trial is revisiting the potential benefit of washing allogeneic RBCs in reducing the incidence of TACO and TRALI.92,135
At a systems level, prevention of TACO and TRALI requires implementation science research to harness the ever-expanding role of EHRs in medical care. The application of natural language processing or machine learning methods to EHR data of transfusion recipients would allow for more widespread surveillance and sophisticated approaches to adverse event reporting for blood and blood-derived products. Automated identification of adverse events utilizing large sources of blood donor, component, and transfusion recipient data would allow assessment of the safety of blood component modifications (eg, pathogen-reduced products or extended storage of platelets) in addition to additional mitigation strategies. Expanded active surveillance of pulmonary transfusion reactions would also serve to aid in the refinement of current diagnostic criteria for TACO and TRALI, allowing better discrimination of individual reactions from other causes of pulmonary edema.
Predictive algorithms could also be embedded within the EHR to allow for real-time identification of patients at increased risk of an adverse pulmonary transfusion event. For example, clinical decision support systems incorporating hemodynamic parameters or creatinine clearance could trigger recommendations for diuretic administration or alternatives to transfusion in patients at risk for TACO. Evidence of a systemic inflammatory response or other recipient risk factors could trigger allocation of lower-risk platelet or plasma units to hospitalized patients at increased risk for TRALI. Real-time identification of at-risk individuals could also be coupled to clinical trials of immune-based therapies for TRALI. Continued collaboration by bench and clinical researchers, clinicians, epidemiologists, and blood donor centers will be required to further understand and minimize these severe complications of blood transfusion.
Correspondence
Nareg Roubinian, Blood Systems Research Institute, 270 Masonic Ave, San Francisco, CA 94118; e-mail: nroubinian@bloodsystems.org.
References
Competing Interests
Conflict-of-interest: N.R. has declared no competing financial interests.
Author notes
Off-label drug use: None disclosed.