Abstract
Most patients with aggressive non-Hodgkin lymphoma will be cured with initial chemoimmunotherapy; however, most patients with relapsed disease will not be cured and will die as a result of their disease. In these cases, continued treatment with conventional chemotherapy is typically not of benefit and can contribute to significant toxicities and decreased quality of life for patients. Fortunately, a number of therapies are currently available or under investigation for this group of patients, ranging from oral tyrosine kinase inhibitors targeting multiple pathways within the malignant cells to adoptive cellular therapies that harness the patient’s immune system to fight disease. Additionally, many agents that are modestly effective as monotherapies can be safely combined with additional novel and conventional therapies to improve response rates and duration. Chimeric antigen receptor T cells are among the most promising group of therapies and provide the potential for cure for patients with relapsed/refractory lymphoma. In this chapter, we will review the currently available novel treatments as well as those still under investigation and discuss the most appropriate approach to patients with relapsed/refractory aggressive lymphoma. We will highlight the challenges associated with these therapies, as well as potential toxicities, and the need for additional clinical trials evaluating combinations and newer treatments.
Learning Objectives
Understand the breadth of novel therapy options for patients with relapsed/refractory aggressive lymphomas
Recognize the successes and limitations of currently available cellular therapies in relapsed/refractory aggressive lymphomas
Introduction
In this year’s edition of the American Society of Hematology (ASH) Education Program, Rosenthal and Rimsza1 present the case of a 74-year-old woman with advanced-stage non-Hodgkin lymphoma (NHL) classified as activated B-cell subtype by gene expression profiling, with a rearrangement of CMYC but not BCL2 or BCL6. The patient had high-risk molecular features and did not respond adequately to R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or second-line therapy with R-GDP (rituximab plus gemcitabine, cisplatin, and dexamethasone). She was not felt to be a candidate for autologous stem-cell transplantation (ASCT) and instead received therapy with lenalidomide plus rituximab and ibrutinib. She is currently enrolled in a clinical trial of a novel Bruton tyrosine kinase (BTK) inhibitor. This case demonstrates the current challenges in the management of patients with relapsed/refractory aggressive NHL, especially those who do not respond adequately to second-line therapy and are not candidates for potentially curative ASCT approaches. In this chapter, I will highlight some of the novel therapies available or under investigation in this setting, and I will propose potential approaches to patients with relapsed/refractory disease.
With the incorporation of rituximab into the current management for patients with untreated diffuse large B-cell lymphoma (DLBCL), most patients with DLBCL will be cured after 1 course of chemoimmunotherapy, although outcomes can be quite variable based on cell of origin, presence of specific genetic translocations, and more conventional clinical criteria identified in the International Prognostic Index.2-5 In addition, outcomes for patients who relapse after receipt of a rituximab-containing induction regimen are particularly poor, even with ASCT. In the CORAL study, which compared R-ICE (rituximab plus ifosfamide, carboplatin, and etoposide) with R-DHAP (rituximab plus dexamethasone, cisplatin, and cytarabine) for relapsed DLBCL before ASCT, for example, the 3-year event-free survival for enrolled patients who had received prior rituximab was only 21%, and even fewer rituximab-treated patients remained event free when limiting the analysis to those with early progression.6 This is particularly problematic because nearly half of the patients enrolled in that study relapsed within 12 months of diagnosis.
In the recently reported SCHOLAR-1 study, which combined patient-level data from 2 clinical trials (including CORAL) as well as 2 academic databases, 636 patients with refractory DLBCL (defined as poor response to chemotherapy or early relapse post-ASCT) were evaluated.7 The median overall survival was only 6.3 months, and only 20% of patients were alive at 2 years. These data indicate patients with relapsed aggressive NHL require novel therapies with different mechanisms of action than standard chemotherapy, because results for this subgroup of patients remain suboptimal.
Historical approaches
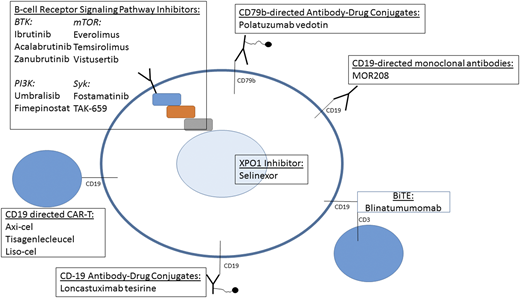
Since the publication of the PARMA study in 1995, the standard of care for eligible patients with relapsed DLBCL has included salvage chemotherapy followed by consolidation with high-dose therapy and ASCT.8 In this landmark randomized study, which was conducted before the use of rituximab, 49 patients completed ASCT, and the 5-year event-free survival for that cohort was 46% compared with 12% in the nontransplantation group (P = .001). In the current era, the posttransplantation outcomes remain similar, with 40% to 50% of patients undergoing ASCT achieving long-term remission and potential cure.6,9 Unfortunately, roughly half of patients who undergo ASCT will relapse and require additional therapy, and additional patients will not achieve adequate disease control to proceed with transplantation or will otherwise not be medically appropriate candidates. In a large multicenter study of 331 patients with primary treatment failure, defined as primary progression while receiving frontline therapy, residual disease at the conclusion of induction, or relapse within 6 months, only 40% of all patients ultimately completed ASCT, and <40% of those patients who did complete ASCT remained progression free at 2 years.10 Patients who relapse after ASCT or who are not eligible for ASCT should be considered for novel therapies and clinical trials, given the poor survival associated with historically conventional treatment; selected novel approaches are highlighted in Figure 1.
Selected therapies that are available or under investigation for management of aggressive lymphoma. axi-cel, axicabtagene ciloleucel; BiTE, bispecific T-cell engager; CAR-T, chimeric antigen receptor T cells; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol-3 kinase; syk, spleen tyrosine kinase.
Selected therapies that are available or under investigation for management of aggressive lymphoma. axi-cel, axicabtagene ciloleucel; BiTE, bispecific T-cell engager; CAR-T, chimeric antigen receptor T cells; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol-3 kinase; syk, spleen tyrosine kinase.
Oral targeted therapies
The tyrosine kinase inhibitors targeting the B-cell receptor signaling pathway have revolutionized management of many B-cell malignancies, most notably chronic lymphocytic leukemia, follicular lymphoma, and mantle cell lymphoma. In aggressive B-cell NHL, the results have been more modest and frequently limited to specific patient subsets. Agents targeting BTK, PI3K, spleen tyrosine kinase, and others have been evaluated as monotherapies and in combination with other treatments in aggressive NHL, and these results are summarized in Table 1.
Selected recently evaluated targeted therapies for relapsed/refractory aggressive B-cell NHL
| Class/agent . | Study (reference) . | Phase/design . | N . | ORR, % . | PFS . |
|---|---|---|---|---|---|
| BTK inhibitors | |||||
| Ibrutinib | 11 | 1/2/monotherapy | 80 | 37 (ABC) | 2 mo (ABC) |
| 5 (GCB) | 1.3 mo (GCB) | ||||
| Ibrutinib | 46 | 1/ with buparlisib | 10 | 14 | — |
| BGB-3111 | 47 | 1b/monotherapy | 23 | 61 (DLBCL + MCL) | — |
| PI3K inhibitors | |||||
| Buparlisib | 12,48 | 2/monotherapy | 26 | 12 | 1.8 mo |
| Umbralisib | 49 | 1/with ublituximab and ibrutinib | 6 | 17 | — |
| CUDC-907 | 13 | 1/monotherapy | 25 | 47 | 5.7 mo |
| 1/with rituximab | 12 | 18 | 1.3 mo | ||
| Syk inhibitors | |||||
| Fostamatinib | 15 | 2/monotherapy | 68 | 3 | 7.3 wk |
| TAK-659 | 50 | 1/monotherapy | 69 | 27 | 50 d |
| mTOR inhibitors | |||||
| Vistusertib | 51 | 2/monotherapy and with rituximab | 36 | 5.6 | 1.9 mo |
| Temsirolimus | 16 | 2/monotherapy | 28 | 2.6 mo | |
| Everolimus | 17 | 2/monothereapy | 77 | 30 | 3 mo |
| Other agents | |||||
| Apilimod dimesylate | 19 | 1/monotherapy | 11 | 27 | — |
| Venetoclax | 52 | 1/monotherapy | 34 | 18 | 1 mo |
| Mocetinostat | 20 | 2/monotherapy | 41 | 19 | — |
| Selinexor | 53 | 1/monotherapy | 79 | 31 | — |
| Class/agent . | Study (reference) . | Phase/design . | N . | ORR, % . | PFS . |
|---|---|---|---|---|---|
| BTK inhibitors | |||||
| Ibrutinib | 11 | 1/2/monotherapy | 80 | 37 (ABC) | 2 mo (ABC) |
| 5 (GCB) | 1.3 mo (GCB) | ||||
| Ibrutinib | 46 | 1/ with buparlisib | 10 | 14 | — |
| BGB-3111 | 47 | 1b/monotherapy | 23 | 61 (DLBCL + MCL) | — |
| PI3K inhibitors | |||||
| Buparlisib | 12,48 | 2/monotherapy | 26 | 12 | 1.8 mo |
| Umbralisib | 49 | 1/with ublituximab and ibrutinib | 6 | 17 | — |
| CUDC-907 | 13 | 1/monotherapy | 25 | 47 | 5.7 mo |
| 1/with rituximab | 12 | 18 | 1.3 mo | ||
| Syk inhibitors | |||||
| Fostamatinib | 15 | 2/monotherapy | 68 | 3 | 7.3 wk |
| TAK-659 | 50 | 1/monotherapy | 69 | 27 | 50 d |
| mTOR inhibitors | |||||
| Vistusertib | 51 | 2/monotherapy and with rituximab | 36 | 5.6 | 1.9 mo |
| Temsirolimus | 16 | 2/monotherapy | 28 | 2.6 mo | |
| Everolimus | 17 | 2/monothereapy | 77 | 30 | 3 mo |
| Other agents | |||||
| Apilimod dimesylate | 19 | 1/monotherapy | 11 | 27 | — |
| Venetoclax | 52 | 1/monotherapy | 34 | 18 | 1 mo |
| Mocetinostat | 20 | 2/monotherapy | 41 | 19 | — |
| Selinexor | 53 | 1/monotherapy | 79 | 31 | — |
ABC, activated B cell; GBC, germinal center B cell–like; MCL, mantle cell lymphoma; mTOR, mammalian target of rapamycin; ORR, overall response rate; PFS, progression-free survival; syk, spleen tyrosine kinase.
The BTK inhibitor ibrutinib was evaluated in a phase 1b/2 study of 80 patients with relapsed/refractory DLBCL, and the response rate across all patients was 25%.11 However, only 1 patient with GCB DLBCL responded, whereas 37% of patients with ABC subtype achieved at least a partial response. Among the patients with ABC subtype, the median duration of response was just under 5 months, but 4 patients remained in remission for >1 year. On the basis of these results, novel BTK inhibitors are being tested in patients with non-GCB DLBCL, including acalabrutinib (ACP-196; registered at www.clinicaltrials.gov as #NCT02112526) and BGB-3111 (#NCT03145064). Ibrutinib is not currently US Food and Drug Administration (FDA) approved for patients with DLBCL, but its off-label use in patients with relapsed/refractory non-GCB DLBCL is a common consideration in patients ineligible for more aggressive treatments. PI3K inhibitors as monotherapies have been infrequently investigated in patients with aggressive NHL. Buparlisib is a panselective PI3K inhibitor and was evaluated in a phase 2 study open to many lymphoma subtypes, in which 26 patients with DLBCL were enrolled.12 Only 11.5% of patients responded, and the median duration of response was 2.2 months in DLBCL patients. An additional compound, CUDC-907 (fimepinostat), is a combination PI3K/histone deacetylase inhibitor that showed particular promise in patients with myc-altered aggressive NHL in a phase 1 study in which 30% of patients responded to monotherapy (including 64% of myc-altered patients).13 In a follow-up phase 2 study that enrolled primarily patients with CMYC alterations, the most recently reported ORR was 19% in a cohort of patients with coexpression of myc and bcl2, including 1 patient with double-hit NHL.14 The spleen tyrosine kinase inhibitor fostamatinib had an ORR of only 3% in a phase 2 study of 68 patients with relapsed/refractory DLBCL.15 Additional agents targeting the B-cell receptor signaling pathway include the mammalian target of rapamycin inhibitors. As a single agent in relapsed/refractory DLBCL, temsirolimus had an ORR of 28% and complete response (CR) rate of 13%, although the median PFS was only 2.6 months.16 The single-agent activity of everolimus is comparable; the ORR in a phase 2 study of 77 patients with relapsed/refractory aggressive lymphoma was 30% and the duration of response was <6 months.17 None of the agents targeting this pathway are currently FDA-approved in the aggressive NHL subtypes, and their primary benefit will likely be in combination with chemotherapy and/or other novel therapies.
Therapies targeting additional sites are under investigation, including apilimod dimesylate (LAM-002A), an inhibitor of PIKfyve. PIKfyve is an endosomal lipid kinase that regulates endosomal membrane trafficking; it is believed to play a critical role in autophagy and may promote cancer-cell survival. Its inhibition by LAM-002A may promote tumor-cell death.18 In an ongoing phase 1 study, which included 11 patients with DLBCL, 3 of 11 patients responded, and combination regimens are currently being explored.19 The histone deacetylase inhibitor mocetinostat was also recently evaluated and had a modest single-agent ORR of 19% in relapsed/refractory DLBCL.20 Other compounds are under investigation, but unfortunately, most have had similar disappointing efficacy when administered as a single agent. As a result, most current clinical trials are evaluating such agents in combination with either additional novel agents, immunotherapies, or chemotherapy-based approaches.
Monoclonal antibodies
Although rituximab remains the most frequent CD20 monoclonal antibody used in the management of DLBCL and other CD20+ aggressive NHLs, newer antibodies have been developed that may improve upon the current efficacy of rituximab or may be effective in rituximab-refractory cases. In general, monoclonal antibodies have modest response rates and duration in patients with aggressive NHL but have been frequently used to improve outcomes when administered in combination with other agents. Obinutuzumab is a glycoengineered type 2 anti-CD20 antibody and currently has FDA approvals in follicular lymphoma and chronic lymphocytic leukemia based on randomized studies. In DLBCL, the ORR for obinutuzumab monotherapy in relapsed/refractory DLBCL was 32% in a phase 2 study that included 25 DLBCL patients.21 However, obinutuzumab did not improve upon rituximab when used in the frontline setting in combination with CHOP.22 As a result, its use in DLBCL has been limited. Additional CD20 antibodies in development include ublituximab, a type 1 chimeric glycoengineered antibody. The ORR for ublituximab monotherapy was 17% for patients with aggressive NHL in a phase 2 study across multiple lymphoma subtypes and chronic lymphocytic leukemia.23 However, when used in combination with the PI3K inhibitor umbralisib and bendamustine, the ORR for 16 patients with DLBCL was 63%, with several durable remissions.24
Monoclonal B-cell antibodies targeting CD19 are also under investigation, including MOR208, which recently received breakthrough designation from the FDA. A recently published phase 2A study of single-agent MOR-208 included 35 patients with DLBCL, with a response rate of 26%.25 However, among patients with a response, who were permitted to continue with maintenance treatment, 5 of 9 experienced a response lasting >12 months. MOR208 was subsequently combined with lenalidomide in a phase 2 study of patients with relapsed/refractory DLBCL who were not candidates for ASCT, presented at the ASH 2017 Annual Meeting.26 Of 44 evaluable patients, the ORR was 52%, and the median PFS after a median follow-up of 5.5 months was 11.3 months. The combination resulted in cytopenias as well as grade ≥3 rash in 6% of patients, but there were no severe infusion-related adverse events, and most patients were able to continue the full dose of lenalidomide.
Other monoclonal antibodies evaluated in aggressive lymphomas include nivolumab and pembrolizumab, which target programmed death 1 (PD-1). In aggressive NHL, the activity of nivolumab has been evaluated in a phase 1 study across lymphoma subtypes, where 36% of 11 patients with DLBCL responded.27 A follow-up phase 2 study has been completed, but the results have not yet been reported. Pembrolizumab has not been evaluated as a single agent in DLBCL, but in primary mediastinal B-cell NHL, a disease in which PD-1 ligand (PD-L1) and/or PD-L2 overexpression is common, the ORR was 41%.28 Additional antibodies targeting the PD-1/PD-L1 axis are under investigation in NHL, including durvalumab, avelumab, and atezolizumab. In addition, the 4-1BB inhibitor utomilumab is currently being evaluated in combination with rituximab and with other PD-1/PD-L1–directed therapies.
Finally, the bispecific T-cell engagers, including blinatumomab, which targets CD19 and CD3, are effective in patients with aggressive NHL, although their use has been limited outside of clinical trials because of lack of an FDA indication. In a phase 1 study of blinatumomab in relapsed/refractory NHL (including 14 patients with DLBCL), the ORR for DLBCL patients was 55%, and the maximum tolerated dose was established at 60 µg/m2 per day.29 The median response duration was 404 days. A phase 2 study of 25 patients with DLBCL demonstrated a response rate after cycle 1 of 36%, and the median duration of response was 11.6 months.30 These agents are associated with neurologic toxicity; 9% of patients enrolled in the phase 2 study who had grade 3 encephalopathy and grade 3 aphasia. These agents are also associated with hematologic toxicity. Although not FDA approved for NHL, they do provide an intriguing option for fit patients who have progressed with available standard therapies and who may not be candidates for additional immunotherapies such as CAR-T.
ADCs
Antibody-drug conjugates (ADCs) carry a cytotoxic payload to cells targeted by the antibody portion of the compound, potentially limiting toxicity to unrelated cells and improving efficacy. Brentuximab vedotin is a CD30-directed ADC currently approved in HL, anaplastic large-cell lymphoma, and other CD30+ lymphomas. In CD30+ relapsed DLBCL, the ORR was 44%, and the median duration of response was 5.6 months (up to 16.6 months for patients achieving a CR).31 Interestingly, there has been therapeutic activity identified across a range of levels of CD30 expression, suggesting that only a small amount of detected CD30 positivity is needed for a patient to potentially respond to CD30-directed therapy. Brentuximab vedotin has also been evaluated in the frontline setting in combination with R-CHP in patients with high-intermediate or high International Prognostic Index scores.32 Although it seems to be well tolerated and effective in this setting, this drug is no longer being pursued as upfront treatment in DLBCL, although it remains a viable option for treatment of patients with relapsed/refractory CD30+ NHL, especially those who are ineligible for more aggressive treatments.
Additional agents are under development that target CD79b and CD19. The CD79b-targeted ADC polatuzumab vedotin is effective across lymphoma subtypes and was recently evaluated in a randomized study in combination with rituximab and bendamustine (vs rituximab and bendamustine alone).33 This study was recently updated at the American Society of Clinical Oncology 2018 Annual Meeting; in 80 patients with DLBCL, the ORR for the polatuzumab-containing arm was 70% vs 33% for the control arm, and the median PFS was 6.7 vs 2 months. Polatuzumab vedotin is now being evaluated in the frontline setting in combination with standard therapy (#NCT03274492). Both polatuzumab vedotin and brentuximab vedotin contain the microtubule inhibitor monomethyl auristatin E, which is associated with the development of peripheral neuropathy. As a result, monitoring for neuropathy is critical for patients receiving these therapies, especially in the relapsed setting, where prior therapies may predispose patients to developing peripheral neuropathy.
Two ADCs targeting CD19 (denintuzumab mafodotin and loncastuximab tesirine) have been investigated in aggressive lymphoma, and these agents are among the most promising therapies in development. Denintuzumab mafodotin includes an anti-CD19 monoclonal antibody conjugated to monomethyl auristatin F. It is active in relapsed/refractory DLBCL, and in a phase 2 study that included 53 patients with DLBCL, the ORR was 33%, and the CR rate was 22%.34 Although potentially quite effective in a refractory population, use of this therapy is associated with the development of superficial microcystic keratopathy in up to 84% of patients. This toxicity requires active management with topical steroids and is generally reversible, but it has limited the use of this therapy. A subsequent study combining denintuzumab mafodotin with R-ICE as a pretransplantation salvage approach has recently concluded enrollment, and study results are pending.
Loncastuximab tesirine similarly targets CD19 but has a different payload than denintuzumab mafodotin. In this construct, a humanized anti-CD19 antibody is conjugated to a pyrrolobenzodiazepine dimer toxin. A recently presented first-in-human study of loncastuximab tesirine in several NHL subtypes (including 35 evaluable patients with DLBCL) demonstrated excellent tolerability; no maximum tolerated dose was identified.35 In addition, there were no serious ocular toxicities, and most patients who discontinued therapy because of toxicity did so as a result of hematologic toxicities or nonhematologic laboratory abnormalities. Among the 35 evaluable patients with DLBCL, the ORR was 57%, and the CR rate was 34%. Additional evaluation of this agent is planned in an upcoming phase 2 trial in DLBCL.
Cellular therapies
The development of cellular therapies for patients with aggressive lymphoma has built upon our experience with allogeneic SCT. Although there are no prospective randomized studies supporting the use of allogeneic SCT in patients with aggressive NHL, several registry studies and other retrospective projects have identified patients who achieve long-lasting remissions with this maneuver. In a recent analysis by the Center for International Blood and Marrow Transplant Research evaluating outcomes for patients age >55 years undergoing allogeneic transplantation for NHL, the 4-year PFS was 37% for patients age 55 to 64 years and 31% for patients age ≥65 years.36 A similar 3-year PFS of 31% for patients with DLBCL who completed allogeneic transplantation after failure of autologous transplantation was described by Fenske et al37 using Center for International Blood and Marrow Transplant Research data. However, patients with refractory disease and/or active disease at the time of transplantation have a significantly poorer outcome, and allogeneic transplantation still requires a suitable donor and a patient who is able to tolerate prolonged immunosuppression. As a result, it remains a viable option for some patients but is not widely available for all patients with aggressive lymphoma.
In addition to allogeneic transplantation, there are now 2 immune effector cellular therapies approved in the United States for patients with DLBCL, and a large study in DLBCL was recently completed involving a third (Table 2). Kochenderfer et al38 published 1 of the first reports of CAR-T use in relapsed/refractory DLBCL in a series of 15 patients with NHL treated at the National Institutes of Health that included 9 patients with DLBCL. This construct (now known as axi-cel) included a CD28 costimulatory domain. This CAR-T construct was evaluated in a phase 1 multicenter study that confirmed the feasibility of a central manufacturing facility; 7 patients with DLBCL were treated, with a CR achieved in 5 of 7 patients and 3 patients maintaining the CR at 12+ months.39 In the subsequent phase 2 portion of ZUMA-1 recently published, 101 patients with relapsed DLBCL were treated (of 111 enrolled patients).40 The CR rate was 54%, and the ORR was 82%. The PFS was 41% at 15 months, and 40% of patients achieving a CR had a persistent response at data cutoff, with a median follow-up of >15 months. On the basis of these results, axi-cel is now FDA approved for the management of patients with relapsed/refractory DLBCL for whom ASCT has failed or who are not eligible for transplantation.
Comparison of outcomes for largest CAR-T trials for patients with DLBCL
| . | JULIET42 . | ZUMA-140 . | TRANSCEND43 . |
|---|---|---|---|
| N of enrolled patients | 147 | 111 | 134 |
| N of treated patients | 99 | 101 | 114 |
| Median time from apheresis to infusion | — | 17 d | — |
| ORR, % | 53 | 82 | 75 |
| CR rate | 40 | 54 | 55 |
| Median follow-up, mo | 5.6 | 15.4 | — |
| Duration of response, mo | Not reached | 8.1 | — |
| Rate of any CRS, % | 58 | 93 | 39 |
| Rate of grade ≥3 CRS, %* | 15 | 13 | 1 |
| Rate of any neurotoxicity, % | 21 | 64 | 23 |
| Rate of grade ≥3 neurotoxicity, % | 12 | 28 | 13 |
| Frequency of tocilizumab use, % | 15 | 43 | 10 |
| Frequency of steroid use, % | 11 | 27 | 9 |
| . | JULIET42 . | ZUMA-140 . | TRANSCEND43 . |
|---|---|---|---|
| N of enrolled patients | 147 | 111 | 134 |
| N of treated patients | 99 | 101 | 114 |
| Median time from apheresis to infusion | — | 17 d | — |
| ORR, % | 53 | 82 | 75 |
| CR rate | 40 | 54 | 55 |
| Median follow-up, mo | 5.6 | 15.4 | — |
| Duration of response, mo | Not reached | 8.1 | — |
| Rate of any CRS, % | 58 | 93 | 39 |
| Rate of grade ≥3 CRS, %* | 15 | 13 | 1 |
| Rate of any neurotoxicity, % | 21 | 64 | 23 |
| Rate of grade ≥3 neurotoxicity, % | 12 | 28 | 13 |
| Frequency of tocilizumab use, % | 15 | 43 | 10 |
| Frequency of steroid use, % | 11 | 27 | 9 |
CRS, cytokine release syndrome.
Grading varied among studies.
Similar efficacy has been seen with CTL019 (tisagenlecluecel), which is manufactured with a 4-1BB costimulatory domain and was initially developed at the University of Pennsylvania. This construct was first evaluated in lymphoma in a recently published series that included 23 patients with DLBCL (of whom 14 were treated).41 Eighty-six percent of patients with DLBCL achieving a response maintained the response through the end of study follow-up, and none of the patients achieving a CR had relapsed. This therapy was also evaluated in a multicenter study most recently updated at ASH 2017.42 Among patients with follow-up through 3 months, the ORR was 53%, and the CR rate was 40%. At 6 months, the CR rate was 30%, and the median duration of response was not reached. This therapy has also been FDA approved. Additional CAR-T constructs are under development, including JCAR017 (liso-cel), another 4-1BB–based product. Among 69 treated patients with DLBCL in a recently reported phase 1 study of JCAR017, the ORR was 75%, and the CR rate was 56%, with 37% of patients in CR at 6 months.43
Ultimately, CAR-T seems to result in long-term remissions for 30% to 40% of patients with relapsed/refractory DLBCL; patients who do not achieve a CR typically experience a fairly rapid progression. There are a number of projects under way designed to recover responses in patients who progress after CAR-T and to improve responses to cellular therapy, including a currently open study of atezolizumab that is available in the National Cancer Institute Experimental Therapeutics Clinical Trials Network (#NCT02862275) for patients with residual disease and/or progression after adoptive cell transfer. In addition, significant immune-mediated toxicities complicate an appreciable proportion of treatment courses. CRS is typically characterized by fever, hypoxia, hypotension, and other markers of systemic inflammation that can mimic sepsis, including elevated ferritin, C-reactive protein, and interleukin-6. Fortunately, the anti–interleukin-6 antibody tocilizumab is effective in the management of CRS and is frequently used in patients with grade ≥2 CRS; corticosteroids are also used in severe cases. Prophylactic tocilizumab on day 2 postinfusion was evaluated in a subset of patients treated with axi-cel, and only 1 patient experienced grade ≥3 CRS.44 Further investigation is needed, but such an approach may significantly limit the toxicity of this therapy.
CAR-T can also be complicated by the development of neurologic toxicity in up to two thirds of patients treated with axi-cel; it can range from mild word-finding or calculation difficulties reflected in handwriting and other complex tasks to encephalopathy and coma. Fatal cerebral edema has also been described.44 As a result, close monitoring and assessment of neurologic status are critical during the first several weeks postinfusion.
Additional cellular therapies are under development, including ACTR087, a cellular product expressing antibody-coupled T-cell receptors, which is coadministered with rituximab in CD20+ lymphomas.45 Unlike CAR-Ts, this product requires administration in conjunction with a monoclonal antibody. In a phase 1 multicenter study of the combination of ACTR087 and rituximab, 2 of 6 evaluable patients had a CR, with 1 additional patient achieving a partial response. At the initial dose levels, there were no serious adverse events, CRS, or neurologic toxicities, although continued close monitoring is needed, with dose escalation and subsequent expansion to larger patient cohorts.
Recommendations and future directions
Despite the plethora of novel therapies currently available and those in development, many patients with recurrent aggressive lymphomas have inadequate treatment options, either because of poor activity of the available agents or because of declining performance status related to progression of the disease. Even the most promising therapies, such as CAR-T, require patients to wait for apheresis and manufacturing and require a performance status that is adequate to tolerate potential toxicities. In addition, a majority of patients who receive a CAR-T infusion will not obtain a prolonged remission. The path to future success for management of such patients likely includes combinations of novel therapies designed to target the specific molecular abnormalities that are driving the disease process in a specific patient. In addition, further modulation of the immune system through post–CAR-T therapies, such as PD-1 antibodies, may improve the CR and prolonged remission rates of patients with DLBCL who receive such therapies. Indeed, several studies are currently under way or in development to investigate such approaches.
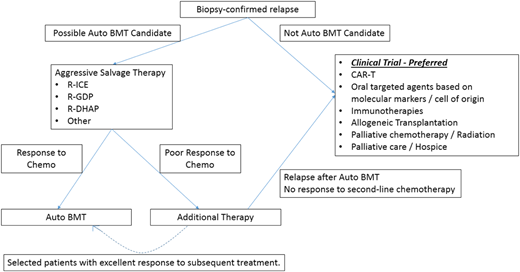
My current approach to patients with relapsed/refractory aggressive NHL is presented in Figure 2. When presented with a patient with refractory disease for whom autologous transplantation has failed or is not an option, similar to the patient case presented at the beginning of this chapter, my goal is to use conventional chemotherapy only as a bridge to an alternative therapy, such as CAR-T, because conventional chemotherapy without a more definitive end point is unlikely to result in prolonged benefit for most patients. The evaluation for CAR-T must include not only a disease assessment and medical evaluation, but also a discussion of the logistic requirements of CAR-T therapy and consideration of insurance benefits. Such patients should also be considered for post–CAR-T adjunctive therapies in a study if appropriate. At our center, potential candidates for CAR-T must meet with our financial counselor and social worker in addition to the physician team, and a patient must meet a series of criteria to proceed, including appropriate disease status (ie, planned on-label use or appropriate for study), control of comorbidities, adequate coverage for cellular therapy, and psychosocial support, to ensure he or she can adhere to the required follow-up. If CAR-T is not an option, the patient should be strongly considered for a clinical trial evaluating novel therapies in combination when feasible or considered for off-label use of novel agents such as ibrutinib or lenalidomide based on his or her molecular phenotype. Unfortunately, many such patients remain refractory to therapy despite the use of all currently available treatments, and open discussions regarding the expected prognosis and the possibility of end of life remain a key component of the care process.
My approach to patients with relapsed/refractory aggressive B-cell NHL. auto, autologous; BMT, bone marrow transplantation; chemo, chemotherapy.
My approach to patients with relapsed/refractory aggressive B-cell NHL. auto, autologous; BMT, bone marrow transplantation; chemo, chemotherapy.
Correspondence
Jonathon B. Cohen, Emory University–Winship Cancer Institute, 1365 Clifton Rd NE, Atlanta, GA 30322; e-mail: jonathon.cohen@emory.edu.
References
Competing Interests
Conflict-of-interest disclosure: J.B.C. is on the board of directors or an advisory committee for Genentech, AbbVie, Janssen, Seattle Genetics, and BioInvent and has received research grants from American Society of Hematology and the Lymphoma Research Foundation and research funding from LAM, Bristol-Myers Squibb, Novartis, Seattle Genetics, and Takeda.
Author notes
Off-label drug use: This chapter includes discussion of off-label uses of medications as well as non–FDA-approved therapies.