Abstract
Postpartum hemorrhage (PPH) is the leading cause of global maternal mortality and accounts for approximately one-quarter of all maternal deaths worldwide. Prevention of excess maternal deaths requires a coordinated approach to prevention, early recognition, and intervention by a multidisciplinary team. Although some women have risk factors for PPH that can be identified during pregnancy or during labor or birth, most women with severe PPH do not have any risk factors. Therefore, all pregnant women must be considered to be at risk of PPH. Common causes include uterine atony, retained placenta, trauma to the genital tract or uterus, and coagulopathy. The pivotal role of fibrinogen and hyperfibrinolysis in the evolution and as a treatment target for PPH is increasingly recognized. Coagulopathy can be an early feature in PPH that may be unrecognized, as it can be present before massive transfusion has occurred. Identification of coagulopathy by viscoelastic point-of-care testing or conventional laboratory assays can be helpful in guiding management of PPH and preventing severe maternal outcomes.
Learning Objectives
Recognize the importance of risk assessment of pregnant women to identify PPH risk factors in the antenatal period and during labor and birth
Recognize the importance of coagulation tests in women with PPH, to enable early identification and treatment of coagulopathy and hyperfibrinolysis
Clinical case
The patient was a 29-year-old Black woman in her first pregnancy. Her body mass index was 33 kg/m2. She had no other medical history of note, and antenatal care had been uncomplicated, apart from iron deficiency treated with oral iron supplements from 28 weeks’ gestation. At the 38-week scan, the fetus was well developed (estimated weight, 4100 g).
The patient had spontaneous onset of labor at 39+5 weeks’ gestation. Her admission observations were normal: afebrile, pulse 88 per minute, blood pressure (BP) 110/68 mm Hg, and respiratory rate 14 per minute. A complete blood count on admission showed hemoglobin (Hb) 10.4 × 109/L, platelets 152, white blood cell count 7.8 × 109/L. She made slow progress in the first stage of labor, requiring augmentation with IV oxytocin. At 11 hours, an epidural was placed after the IV oxytocin was started. The first stage of labor was complete at 17 hours, and initial effective pushing occurred in the second stage, with the head on the perineum at 65 minutes with no further advancement. A successful vacuum extraction (ventouse) was performed after episiotomy by the senior resident, with birth of the infant after 80 minutes in the second stage. Active management of the third stage of labor appeared complete, with controlled cord traction, intramuscular oxytocin, and delivery of the placenta. Immediate postpartum blood loss was estimated at 1200 mL. The pediatric team was called to review the infant, who had a 3990-g birth weight and some initial floppiness but responded rapidly to basic resuscitation.
Ongoing vaginal blood loss continued in the postpartum period. The patient’s uterus remained atonic but responded well to “rubbing up,” and IV oxytocin infusion was started. The team agreed to move her to an operating room (OR) for examination under anesthesia to determine whether there were any retained products of conception or any genital tract trauma that would explain the ongoing blood loss. Maternal observations were pulse, 106 per minute; BP, 98/60 mm Hg; and respiratory rate, 18 per minute.
On arrival in the OR, the observations were pulse, 114 per minute; BP, 94/60 mm Hg; and respiratory rate, 20 per minute. Vaginal blood loss continued, with estimated blood loss of 400 mL on new drapes and swabs in the OR.
Blood taken in the OR and tested on the blood gas analyzer showed Hb of 8.2 g/dL. Two units of packed red cells were ordered from the blood bank. The uterus remained atonic, and there were some abrasions of the vaginal wall and bleeding from the episiotomy; no other cause was identified. Ongoing vaginal blood loss was noted, with loss estimated at 1600 mL. Uterine atony persisted and further uterotonics were given, after which the maternal observations were pulse, 118 per minute; BP, 90/58 mm Hg; and respiratory rate, 22 per minute.
A senior obstetrician and anesthesiologist were called for support.
Discussion
Obstetric hemorrhage is the leading cause of maternal mortality and bleeding after childbirth. Postpartum hemorrhage (PPH) accounts for two-thirds of cases of obstetric hemorrhage and for approximately one-quarter of all maternal deaths worldwide. There is no universally accepted definition of PPH, with some suggesting that blood loss volume >500 or 1000 mL represents standard or severe PPH.1 Most otherwise fit and healthy pregnant women will have minimal physiological response to this degree of blood loss, leading some clinicians to suggest more relevant clinical definitions, such as persistent PPH: ongoing active bleeding >1000 mL occurring within 24 hours after birth that continues despite the use of measures such as first-line uterotonic therapy and uterine massage.2
Maternal deaths represent only the tip of the iceberg in terms of the overall impact of major bleeding on maternal health. Women who have life-threatening hemorrhage but do not die of PPH can face long-term health complications including loss of fertility and psychological trauma. Although nearly all women with severe PPH live in countries with limited economic resources, PPH and its complications can affect women living in any resource setting. Data from the United States show that rates of severe PPH are increasing.3 Well-resourced health care settings with access to skilled practitioners, drugs, and blood banks offer the best opportunity to provide optimal care for women. In any care setting, early recognition of abnormal postpartum bleeding and mobilization of appropriate staff and resources is essential to stop the bleeding promptly and minimize morbidity and mortality.
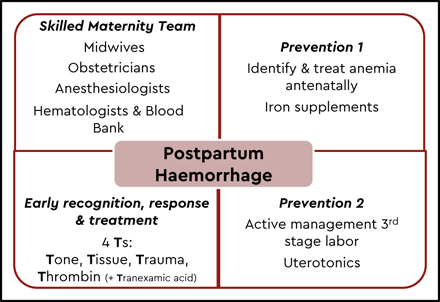
Postpartum hemorrhage should not be viewed as a diagnosis but rather a clinical manifestation of an underlying condition or conditions that require identification and treatment. The differential diagnosis is not wide and includes one or more of the following: uterine atony, retained placenta, and placental malimplantation (previa, accreta, increta, or percreta), and genital tract trauma or coagulopathy, often referred to as the “4 T’s” (tone, tissue, trauma, and thrombin). Some women enter pregnancy with risk factors for PPH or develop these risk factors during the course of pregnancy or labor and birth (Table 1). Women with risk factors identified antenatally should be managed in the appropriate setting with access to skilled staff and a blood bank and with precautionary steps taken during labor and childbirth to minimize the risk of PPH and respond early if it occurs (Figure 1). However, it is critical that all staff caring for women in labor and childbirth be aware that most women who have severe PPH have no identifiable antenatal risk factors and that a high level of awareness be maintained. Risk factors should be reassessed frequently during labor and birth.
Risk factors for PPH
| Uterine atony | Placental problems |
| Previous PPH | Retained placenta |
| Labor >12 h | Placental abruption |
| Induction of labor | Placenta previa |
| Prolonged third stage of labor | Placenta accreta |
| Baby >4 kg | Coagulopathy |
| Multiple pregnancies | Amniotic fluid embolism |
| Increased body mass index | Acute fatty liver of pregnancy |
| Infection | Maternal sepsis |
| Genital tract trauma | Massive transfusion |
| Instrumental delivery | Bleeding tendency |
| CS | Inherited |
| Uterine rupture | Acquired (receiving anticoagulant therapy) |
| Uterine atony | Placental problems |
| Previous PPH | Retained placenta |
| Labor >12 h | Placental abruption |
| Induction of labor | Placenta previa |
| Prolonged third stage of labor | Placenta accreta |
| Baby >4 kg | Coagulopathy |
| Multiple pregnancies | Amniotic fluid embolism |
| Increased body mass index | Acute fatty liver of pregnancy |
| Infection | Maternal sepsis |
| Genital tract trauma | Massive transfusion |
| Instrumental delivery | Bleeding tendency |
| CS | Inherited |
| Uterine rupture | Acquired (receiving anticoagulant therapy) |
Setting the scene for the “PPH perfect storm”
Most women with PPH respond to initial measures of uterine massage and therapeutic uterotonics. However, if bleeding continues despite these interventions, the situation can rapidly escalate with more severe blood loss, maternal morbidity, and even mortality. Failure to recognize and respond to an evolving situation of severe PPH is frequently described in reviews of adverse outcomes caused by hemorrhage. This delay in response can be explained by several factors that create the “perfect storm” where the clinical team fails to recognize the severity of the blood loss and to take the appropriate steps.
Potential for rapid loss of a large volume of blood
In pregnancy, the total blood volume is ∼5 to 7 L (70-80 mL/kg lean body mass). By term, the blood supply to the uterine arteries is ∼500 to 600 mL per minute, increased from its normal level of 10 to 15 mL per minute outside of pregnancy.4 After delivery of the placenta, the uterine muscles contract, effectively staunching blood flow from the uteroplacental bed. Uterine atony, retained placental tissue, and abnormal placental implantation impede the normal action of the uterus in completing this critical mechanical hemostatic process. Given the high blood flow to the uterine arteries, it is easy to appreciate how rapidly a large volume of blood can be lost in a short time.
Underestimation of the degree of blood volume loss
In many clinical settings the volume of blood loss is estimated by using visual assessment rather than objective measurement, which significantly underestimates the actual blood volume, especially at higher volumes.5 Accurate measurements of blood volume using graduated containers and gravimetric measurement of blood-soaked pads and swabs reported to the clinicians in real time can help alert them to the development of severe continued bleeding. Providing a cumulative total of blood volume loss is especially important when women are moved during the process of PPH management (for example, from the delivery room to the OR) if required for an examination while the patient is under anesthesia, to assess for retained products of conception or genital tract trauma.
Most pregnant women are healthy and physiologically robust
Healthy pregnant women show minimal physiological response to blood loss of 1000 to 1500 mL, perhaps only becoming slightly tachycardic with a minor decline in systolic BP. By the time women have significant hypotension or tachycardia or an increased respiratory rate or become distracted or agitated, they usually have lost in excess of 2000 to 2500 mL. Careful and repeated clinical assessments and documentation of vital signs, such as pulse rate, BP, temperature, and respiratory rate is essential for identifying a trend indicating physiological decompensation in response to hypovolemia. The use of maternity early warning scoring to improve early detection of clinically deteriorating patients and escalation of the clinical response is increasing.6,7 These systems assign a score to a range of clinical vital signs to form a total maternity early warning score. An increasing score suggests a deviation from a normal physiological state and indicates clinical deterioration, which should prompt an escalation in response by clinicians who have the appropriate level of skill to care for the patient.
Lack of anticipation of the presence of early coagulopathy with PPH, before a massive transfusion is needed
Dilutional coagulopathy is common in patients with hemorrhage after multiple transfusions. Urgent red cell transfusion is preferred to infusion of significant volumes of crystalloid, but in an unstable patient, volume replacement with up to 2 to 3 L of crystalloid may be necessary prevent severe hypotension.2 Coagulopathy resulting from conditions, such as amniotic fluid embolism, placental abruption, and sepsis, can be present early, before administration of IV fluids or blood products. Early identification of coagulopathy by testing basic hemostatic function, with either laboratory-based assays or point of care (POC) testing, can help identify coagulopathy early and enable directed transfusion of the appropriate blood products.
Standard thrombin-based functional clotting assays for fibrinogen, such as the Clauss fibrinogen assay, measure the time it takes for a fibrin clot to form. In most clinical settings the final result will not be available for at least 45 to 60 minutes. Viscoelastic point of care (VE-POC) tests, such as rotational thromboelastometry (ROTEM) and thromboelastography, are increasingly used to assess global hemostasis, determining the activity of the coagulation factors and the amount of fibrinogen available to form a fibrin clot, as well the resistance of the clot to fibrinolysis.8 In the setting of PPH, hyperfibrinolysis is not uncommon, especially in more severe PPH. The results of POC tests are usually available within 15 to 20 minutes, especially if the instrument is available in the OR.
Fibrinogen and fibrinolysis in PPH
The importance of fibrinogen and fibrinolysis in major postpartum bleeding has been brought into focus in recent years. Fibrinogen levels in pregnant women at term are increased at ∼4 to 6 g/L, compared with levels of 2 to 4 g/L in nonpregnant patients. In 2007, Charbit et al showed that fibrinogen levels were lower in women who developed severe PPH than in women with nonsevere PPH (median levels, 3.3 and 4.4 g/L, respectively).9 Importantly, this difference was evident early in the evolution of PPH and before any blood or blood products had been administered. Other studies have confirmed this finding10,11
Although these studies suggested that a low level of fibrinogen is predictive of development of severe PPH, they did not ascertain whether early fibrinogen replacement could modify the degree of blood loss. A controlled study of women with moderate PPH (blood loss >1000 mL after Cesarean section [CS], >500 mL in women who required manual removal of placenta, and >1000 mL in women who required exploration of the uterus) randomized women to 2 g of fibrinogen or placebo.12 Transfusion rates were the same in women (n = 25 of 123; 20%) given fibrinogen as in those who received placebo (n = 26 of 121; 22%). Of note, the mean fibrinogen level in each group was normal (4.5 g/L) and the median blood loss was <1500 mL, indicating that perhaps fibrinogen would not be a key factor in this population.
A second multicenter, double-blind, randomized, placebo-controlled trial of early fibrinogen replacement in women with severe PPH (>1000-1500 mL measured blood loss, with ongoing bleeding and reduced fibrinogen on ROTEM: FIBTEM A5 <15 mm, equivalent to ∼3 g/L fibrinogen).13 Of 606 women eligible for inclusion, only 57 (9.4%) were randomized, with 55 women analyzed for the primary outcome (the number of allogeneic units transfused: red blood cells and plasma products). There was no difference in blood products transfused or in any of the secondary outcomes, such as invasive procedures for control of blood loss or transfer to intensive care. Analysis of prespecified subgroups showed that, in women with fibrinogen >2 g/L (n = 22), there was no difference in blood loss or blood transfusion after administration of the study medication, whereas the median blood loss was lower in the fibrinogen arm than in the placebo arm in women with fibrinogen <2 g/L. The researchers concluded that a fibrinogen level of >2 g/L appeared sufficient for hemostasis in the setting of PPH. Too few women in the cohort had very low fibrinogen (<2 g/L) for them to determine whether early administration of fibrinogen would modify outcomes in that patient group.
Although Collins et al13 did not demonstrate an improvement in outcomes in women who were given fibrinogen concentrate, they observed that, over the course of the clinical trial, their approach of routine risk assessment for PPH, objective measurement of cumulative blood loss, and early involvement of senior staff enabled early recognition and intervention in the course of the PPH to take steps to respond to and control blood loss. The prompt recognition allowed for timely and appropriate escalation of care and involvement of senior staff at an early stage. Also, the study led to the practice of early assessment of hemostasis, Hb, and lactate by using POC tests. The clinicians used the study protocol to inform the development of a nationwide interventional program for standardized management of PPH, “OBS Cymru,” which has been effectively implemented across all obstetric units in Wales.14
Approach to transfusion of blood and plasma products in management of PPH
Using empiric fixed ratios of red blood cells, fresh frozen plasma (FFP), and platelets in women with PPH >1500 mL has been shown to reduce progression to severe PPH. Most women with PPH <2000 mL do not have low levels of fibrinogen or other clotting factors, and fibrinogen does not appear to decrease to <2 g/L until blood volumes of >4000 mL are lost, although early coagulopathy is a feature of placental abruption and amniotic fluid embolism. Empiric transfusion in the absence of hemostatic testing may lead to overtransfusion of blood and plasma products increased risk of complications, such as transfusion-associated circulatory overload and transfusion-associated lung injury. Collins et al advocate using targeted transfusion protocols with viscoelastic-POC (VE-POC) testing.15 They reported that fibrinogen levels decrease sooner than other coagulation factors, so that correction of low fibrinogen levels may be a more important therapeutic target.
The International Society on Thrombosis and Haemostasis (ISTH) recommends using either cryoprecipitate (∼15 g/1000 mL) or fibrinogen concentrate (20 g/1000 mL) to maintain fibrinogen >2 g/L when managing PPH.16 The fibrinogen concentration of FFP is much lower (2 g/1000 mL), and its use for fibrinogen replacement could lead to hemodilution.
Deficiencies of other clotting factors tend to occur at a later stage in PPH, suggesting that there is a more limited requirement for FFP. When VE-POC testing is not available, conventional laboratory testing may be helpful and the ISTH Scientific and Standardization Committee recommends targeting using 15 mL/kg FFP to maintain activated partial thromboplastin time/prothrombin time >1.5 × normal.16
Severe thrombocytopenia is uncommon in most women with PPH, leading to a recommendation (ISTH) to limit platelet transfusions, unless platelet count is <75 × 109/L.16
Inhibition of fibrinolysis
Over the past 20 years or so, the impact of hyperfibrinolysis in major bleeding has been recognized. The CRASH-2 study demonstrated tranexamic acid, a potent inhibitor of fibrinolysis, reduction in death related to bleeding by 21% (risk ratio [RR], 0.79; 95% confidence interval [CI], 0.64-0.97) in trauma patients who received it within 3 hours and by 32% (RR, 0.68; 95% CI, 0.57-0.82) in patients who received it within 1 hour.17
The WOMAN study is a placebo-controlled trial conducted in 21 countries that assessed the impact of tranexamic acid in 20 021 women with PPH >500 mL after vaginal birth or >1000 mL after CS.18 The maternal mortality rate was 2.4% (n = 483) with 72% (n = 346) of deaths caused by hemorrhage. Administration of 1 g of tranexamic acid (with a second dose given for ongoing bleeding) resulted in an overall reduction in death related to bleeding of 19% (RR, 0.81; 95% CI, 0.65-1.00) when given within 3 hours.
As a result of this study, the World Health Organization now strongly recommends early use of IV tranexamic acid (within 3 hours of birth) in addition to standard care for women with clinically diagnosed PPH after vaginal birth or CS.19
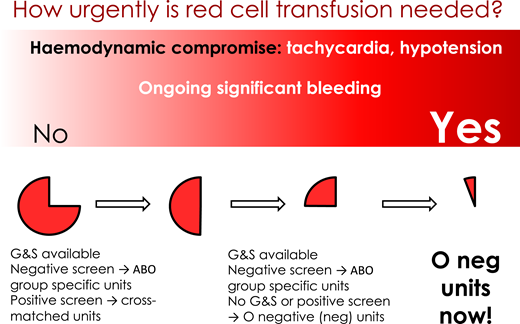
Recognition and response to major PPH also requires rapid response with transfusion of red blood cells to maximize oxygen delivery and prevent tissue hypoxia, development of acidosis, organ failure, and worsening of shock. The urgency of red cell transfusion depends on the degree of maternal clinical instability and rapidity of blood loss. Transfusion of O− red blood cells may be required in women who are clinically unstable or who are losing blood rapidly when cross-matched blood is not available.
Comments on the case
Antenatal risk factors
In the clinical case, the only identifiable antenatal risk factors for PPH were increased body mass index and fetal macrosomia. Although the patient was iron deficient, she was not anemic. However, during labor she needed augmentation with oxytocin and had prolonged first and second stages of labor, with an assisted delivery. The immediate estimated postpartum blood loss was high, confirming a PPH, and importantly, the bleeding did not stop after initial interventions of uterine massage and therapeutic uterotonics.
An opportunity for escalation of intervention and a call for backup was missed at the time the patient was transferred to the OR. The degree of tachycardia and the increase in the respiratory rate were signs that this otherwise healthy, physiologically robust woman had lost a significant amount of blood. As a rule of thumb, a pulse rate higher than the systolic BP indicates a problem.
Estimated rather than measured blood loss
It is likely given the drop in the Hb that the woman had lost >1600 mL blood. Accurate cumulative measurement of blood loss would have alerted the clinicians to the severity of blood loss and the potential for progression.
Failure to perform an early coagulation test
A test of coagulation is helpful in identifying unanticipated coagulopathy. If available, a VE-POC test (ROTEM or thromboelastography) can provide a result within 15 minutes. Even though a conventional laboratory-based fibrinogen assay may be delayed by 60 minutes, it could still provide additional help if the PPH is not being controlled by initial maneuvers.
Ongoing significant postpartum bleeding in a woman with a well-contracted uterus with no evidence of genital tract trauma or retained placenta should alert the clinicians to the possible presence of coagulopathy.
Correspondence
Claire McLintock, Auckland City Hospital, Grafton Road, Auckland 1143, New Zealand; e-mail: e-mail doctorclaire@redhealth.org.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.