Abstract
Approximately 10% to 30% of patients with classical Hodgkin lymphoma (cHL) develop relapsed or refractory (R/R) disease. Of those patients, 50% to 60% show long-term progression-free survival after standard salvage chemotherapy followed by high-dose chemotherapy (HDCT) and autologous stem cell transplant (ASCT). In the past decade, novel therapies have been developed, such as the CD30-directed antibody–drug conjugate brentuximab vedotin and immune checkpoint inhibitors, which have greatly extended the treatment possibilities for patients with R/R cHL. Several phase 1/2 clinical trials have shown promising results of these new drugs as monotherapy or in combination with chemotherapy, but unfortunately, very few randomized phase 3 trials have been performed in this setting, making it difficult to give evidence-based recommendations for optimal treatment sequencing. Two important goals for the improvement in the treatment of R/R cHL can be identified: (1) increasing long-term progression-free and overall survival by optimizing risk-adapted treatment and (2) decreasing toxicity in patients with a low risk of relapse of disease by evaluating the need for HDCT/ASCT in these patients. In this review, we discuss treatment options for patients with R/R cHL in different settings: patients with a first relapse, primary refractory disease, and in patients who are ineligible or unfit for ASCT. Results of clinical trials investigating novel therapies or strategies published over the past 5 years are summarized.
Learning Objectives
Describe current and emerging therapies for patients with R/R Hodgkin lymphoma
Understand the importance for patients with R/R Hodgkin lymphoma to achieve a CMR before HDCT/ASCT
CLINICAL CASE
A 30-year-old woman presented with a persistent painless lump in the neck without B-symptoms. A biopsy of a right supraclavicular node was performed, which showed a classical Hodgkin lymphoma (cHL). An 18 F-fluorodeoxyglucose positron emission tomography (PET)–computed tomography (CT) scan revealed lymphadenopathy bilaterally in the supraclavicular and infraclavicular region, retrosternally, and in the mediastinum; hence, cHL stage IIA unfavorable was diagnosed.
After oocyte preservation, treatment with adriamycin, bleomycin, vinblastine, and dacarbazine was initiated with the intention to administer a total of 6 cycles in case of a complete metabolic response (CMR) after 2 cycles. However, the interim PET-scan showed only a partial metabolic response (PR) (Deauville score 4), and the treatment was intensified to escalated bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone. After 2 cycles, a CMR was reached and the patient received consolidative involved node radiotherapy (30 Gy).
Unfortunately, 1 year later, the patient presented with night sweats and severe itching. Imaging revealed extensive lymphadenopathy above and below the diaphragm, and a biopsy confirmed the relapse. Salvage chemotherapy with dexamethasone, high-dose cytarabine, and cisplatin (DHAP) was initiated, which resulted in a CMR after 2 cycles, and stem cells were mobilized and collected after a third cycle of DHAP with the intention to proceed to high-dose chemotherapy (HDCT) followed by autologous stem cell transplant (ASCT) rescue.
Introduction
Approximately 10% to 30% of patients with cHL will relapse or are primary refractory (R/R) to first-line treatment. Standard salvage chemotherapy and consolidation with HDCT/ASCT leads to long-term progression-free survival (PFS) in about 50% to 60% of patients. Until recently, patients who relapsed after ASCT or were ineligible for ASCT had limited treatment options, and 50% of those patients eventually died of the disease.1 In the past decades, several novel therapeutic options for patients with R/R cHL have become available, including brentuximab vedotin (BV) and immune checkpoint inhibitors (CPIs), leading to high CMR rates pre-ASCT, especially when combined with chemotherapy.2 Achieving a CMR prior to ASCT appears to be the most important prognostic factor for PFS.3-10 Therefore, a risk- and PET-adapted treatment approach could probably lead to higher cure rates.11 On the other hand, the burden of late toxicities related to HDCT, such as secondary malignancies and infertility, is considerable, especially as the disease typically affects patients early in life. For this reason, decreasing toxicity is one of the main goals in the treatment of R/R cHL.12
In this educational session, we discuss the results of studies that incorporated novel therapies and response-adapted treatment and how this could be implemented in standard practice to improve outcomes for patients with R/R cHL.
Treatment for patients with a first relapse or primary refractory disease after first-line treatment
Conventional salvage chemotherapy results in pre-ASCT complete response (CR) rates of about 20% to 25% and overall response rates (ORRs) of 60% to 70%, based on evaluation by CT scan.13 More recent studies reporting response rates based on functional imaging using PET or gallium showed CMR rates of 50% to 60% after ifosfamide, carboplatin, and etoposide (ICE) or etoposide, methylprednisolone, high-dose cytarabine, and cisplatin (ESHAP). Even higher CMR rates were reported for the bendamustine, gemcitabine, and vinorelbine regimen (73%) and a sequential ICE–gemcitabine, vinorelbine, and liposomal doxorubicin (GVD) approach (78%) in which patients with no CR on ICE received additional chemotherapy with GVD before proceeding to ASCT.11,14-16 PFS ranges between 50% and 60% with an overall survival (OS) of 70% to 80% at 5 years.11,13-16 Overall, there seem to be no significant differences with regard to outcome between the most commonly used regimens (ie, ICE/DHAP/ESHAP) (Table 1). However, randomized controlled trials (RCTs) comparing different salvage chemotherapy regimens or a PET-adapted approach are lacking.
Overview of first-salvage chemotherapy regimens since 2010
| Study . | N . | Intervention . | Refractory, n (%) . | CR pre-ASCT . | ORR pre-ASCT . | PFS . | OS . |
|---|---|---|---|---|---|---|---|
| Josting et al (2010)13 (RCT) | 279 | DHAP | 0 (0) | CT: 24% | CT: 71% | 3 years: 69% (no significant difference between arms) | 3 years: 85% (no significant difference between arms) |
| Moskowitz et al (2010)14 | 105 | ICE | 48 (46) | PET/gallium: 61% CT: 33% | CT: 59% | 4 years: 56% | 4 years: 72% |
| Moskowitz et al (2012)11 | 97 | ICE + GVD (PET-adapted sequential) | 41 (42) | PET: 60% after ICE 78% after GVD | — | 51 months: 70% | 51 months: 80% |
| Labrador et al (2014)15 (retrospective) | 82 | ESHAP | 41 (50) | PET/gallium: 50% | PET/gallium: 67% | Median PFS: 56 months | 5 years: 73% |
| Santoro et al (2016)16 | 58 | BeGEV | 27 (46) | PET: 73% | PET: 83% | 5 years: 59% | 5 years: 78% |
| Study . | N . | Intervention . | Refractory, n (%) . | CR pre-ASCT . | ORR pre-ASCT . | PFS . | OS . |
|---|---|---|---|---|---|---|---|
| Josting et al (2010)13 (RCT) | 279 | DHAP | 0 (0) | CT: 24% | CT: 71% | 3 years: 69% (no significant difference between arms) | 3 years: 85% (no significant difference between arms) |
| Moskowitz et al (2010)14 | 105 | ICE | 48 (46) | PET/gallium: 61% CT: 33% | CT: 59% | 4 years: 56% | 4 years: 72% |
| Moskowitz et al (2012)11 | 97 | ICE + GVD (PET-adapted sequential) | 41 (42) | PET: 60% after ICE 78% after GVD | — | 51 months: 70% | 51 months: 80% |
| Labrador et al (2014)15 (retrospective) | 82 | ESHAP | 41 (50) | PET/gallium: 50% | PET/gallium: 67% | Median PFS: 56 months | 5 years: 73% |
| Santoro et al (2016)16 | 58 | BeGEV | 27 (46) | PET: 73% | PET: 83% | 5 years: 59% | 5 years: 78% |
BeGEV, bendamustine, gemcitabine, and vinorelbine.
BV and checkpoint inhibitors
cHL is characterized by the presence of a minority of bi- or multinucleated Hodgkin and Reed-Sternberg (HRS) cells that universally express CD30 in an inflammatory tumor microenvironment. BV is an anti-CD30 monoclonal antibody conjugated to the microtubule-disrupting agent monomethyl auristatin-E.17 PD-L1 and PD-L2 are upregulated by HRS cells in about 90% of patients and induce T-cell exhaustion, which contributes to immune escape of HRS cells.18 CPIs are monoclonal antibodies that block the interaction between inhibitory ligands such as PD-L1 and PD-L2 on the tumor cells and PD-1 receptors on immune effector cells.
Several studies have investigated the use of BV in combination with chemotherapy as first salvage regimen and showed high CMR rates prior to ASCT of up to 83%, with 2-year PFS rates ranging from 63% to 81% (Table 2).3-10 In 5 studies, BV was combined with chemotherapy in 2 to 6 cycles followed by ASCT in patients with PR or CMR, whereas in 2 studies, patients were treated initially with 4 to 6 administrations of BV monotherapy; patients with a CMR could proceed directly to ASCT, whereas patients with a PR received additional salvage chemotherapy without BV.4,5 This PET-adapted approach is interesting because approximately 30% to 50% of patients could proceed to ASCT after BV monotherapy only, thereby avoiding toxicity from salvage chemotherapy in these patients. Moreover, a trial investigating the combination of BV and the CPI nivolumab as pre-ASCT salvage regimen showed that 67 of 91 patients could proceed directly to ASCT after BV-nivolumab, without salvage chemotherapy. The study revealed low toxicity of this regimen compared with salvage chemotherapy.10
Overview of first-salvage regimens containing BV or CPI
| Study . | N . | Intervention . | Schedule . | Refractory, n (%) . | CMR pre-ASCT, n (%) . | 2-year PFS . | 2-year OS . |
|---|---|---|---|---|---|---|---|
| Moskowitz et al (2017)4 | 65 | BV + sequential ICE | BV 1.2 mg/kg d1, 8, 15 of 28-d cycles, 2 cycles. ICE salvage in case of Deauville >3. | 34 (52) | 54 (83) | 82% | 97% |
| Herrera et al (2018)5 | 57 | BV + sequential ICE/GVD | BV 1.8 mg/kg every 21 d, 4 cycles. Last 2 cycles BV escalation to 2.4 mg/kg in n = 8 patients with PR/SD. Salvage chemotherapy at discretion of treating physician. | 35 (61) | 37 (65) | 67% | 93% |
| Cole et al (2018)6 | 45 | BV + gemcitabine | BV 1.8 mg/kg on d1 and d8 every 21 days, 4 cycles. In combination with gemcitabine. | 29 (64) | 28 (67) | — | 1 year: 95% |
| LaCasce et al (2018)9 | 55 | BV + bendamustine | BV 1.8 mg/kg every 21 d, 2-6 cycles. In combination with bendamustine. Post-ASCT BV monotherapy maintenance up to 16 cycles. | 28 (51) | 39 (74) | 63% | 94% |
| Garcia-Sanz et al (2019)8 | 66 | BV + ESHAP | BV 1.8 mg/kg every 21 days, 4 cycles. In combination with 3 cycles of ESHAP. | 40 (61) | 46 (70) | 71% | 90% |
| Broccoli et al (2019)7 | 40 | BV + bendamustine | BV 1.8 mg/kg every 21 days, 4-6 cycles. In combination with bendamustine. | 20 (50) | 30 (79) | 68% | 97% |
| Abuelgasim et al (2019)19 | 28 | BV + IGEV | BV 1.8 mg/kg every 21 days, 2-4 cycles. In combination with IGEV. 64% received BV consolidation after ASCT. | 12 (43) including n = 14 with >1 line of therapy. | 70% | 73.5% (100% for patients with first relapse) | 87.1% (100% for patients with first relapse) |
| Kersten et al (2021)3 | 67 | BV + DHAP | BV 1.8 mg/kg every 21 days, 3 cycles. In combination with DHAP. | 30 (45) | 53 (82) | 78% | 96% |
| Advani et al (2021)10 | 91 | BV + nivolumab | BV 1.8 mg/kg and nivolumab 3.0 mg/kg every 21 days, 4 cycles. | 38 (42) | 61 (67) | 78% | 93% |
| Moskowitz et al (2021)20 | 39 | Pembrolizumab + GVD | Pembrolizumab 200 mg every 21 days, 4 cycles. In combination with GVD. | 16 (41) | 36 (95) | 1 year: 100% | 1 year: 100% |
| Study . | N . | Intervention . | Schedule . | Refractory, n (%) . | CMR pre-ASCT, n (%) . | 2-year PFS . | 2-year OS . |
|---|---|---|---|---|---|---|---|
| Moskowitz et al (2017)4 | 65 | BV + sequential ICE | BV 1.2 mg/kg d1, 8, 15 of 28-d cycles, 2 cycles. ICE salvage in case of Deauville >3. | 34 (52) | 54 (83) | 82% | 97% |
| Herrera et al (2018)5 | 57 | BV + sequential ICE/GVD | BV 1.8 mg/kg every 21 d, 4 cycles. Last 2 cycles BV escalation to 2.4 mg/kg in n = 8 patients with PR/SD. Salvage chemotherapy at discretion of treating physician. | 35 (61) | 37 (65) | 67% | 93% |
| Cole et al (2018)6 | 45 | BV + gemcitabine | BV 1.8 mg/kg on d1 and d8 every 21 days, 4 cycles. In combination with gemcitabine. | 29 (64) | 28 (67) | — | 1 year: 95% |
| LaCasce et al (2018)9 | 55 | BV + bendamustine | BV 1.8 mg/kg every 21 d, 2-6 cycles. In combination with bendamustine. Post-ASCT BV monotherapy maintenance up to 16 cycles. | 28 (51) | 39 (74) | 63% | 94% |
| Garcia-Sanz et al (2019)8 | 66 | BV + ESHAP | BV 1.8 mg/kg every 21 days, 4 cycles. In combination with 3 cycles of ESHAP. | 40 (61) | 46 (70) | 71% | 90% |
| Broccoli et al (2019)7 | 40 | BV + bendamustine | BV 1.8 mg/kg every 21 days, 4-6 cycles. In combination with bendamustine. | 20 (50) | 30 (79) | 68% | 97% |
| Abuelgasim et al (2019)19 | 28 | BV + IGEV | BV 1.8 mg/kg every 21 days, 2-4 cycles. In combination with IGEV. 64% received BV consolidation after ASCT. | 12 (43) including n = 14 with >1 line of therapy. | 70% | 73.5% (100% for patients with first relapse) | 87.1% (100% for patients with first relapse) |
| Kersten et al (2021)3 | 67 | BV + DHAP | BV 1.8 mg/kg every 21 days, 3 cycles. In combination with DHAP. | 30 (45) | 53 (82) | 78% | 96% |
| Advani et al (2021)10 | 91 | BV + nivolumab | BV 1.8 mg/kg and nivolumab 3.0 mg/kg every 21 days, 4 cycles. | 38 (42) | 61 (67) | 78% | 93% |
| Moskowitz et al (2021)20 | 39 | Pembrolizumab + GVD | Pembrolizumab 200 mg every 21 days, 4 cycles. In combination with GVD. | 16 (41) | 36 (95) | 1 year: 100% | 1 year: 100% |
d, day; IGEV, ifosfamide, gemcitabine, vinorelbine, and prednisolone; SD, stable disease.
Thus far, in studies that incorporated a PET-adapted strategy, PFS seems to be similar for patients who proceeded to ASCT directly after having a CMR on BV, BV-nivolumab, or ICE alone as for those patients who needed additional salvage chemotherapy to achieve a CMR.4,5,10 This confirms that the most important goal is to achieve a CMR before ASCT and that patients who do not respond initially can potentially be rescued with additional salvage chemotherapy and proceed to ASCT if they subsequently reach a CMR.
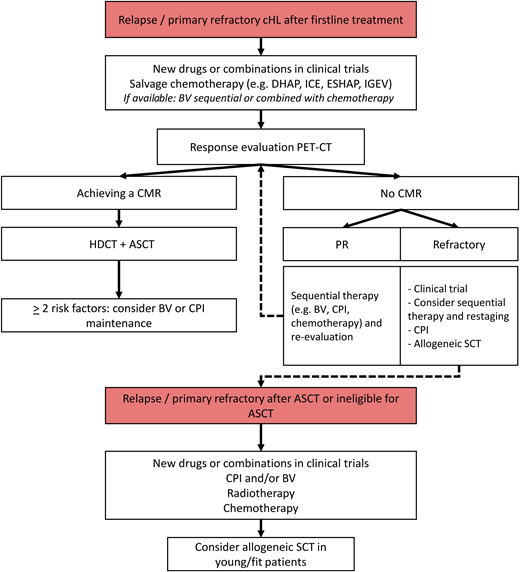
Figure 1 proposes a flowchart of PET-adapted treatment in patients with R/R cHL. Because BV and CPI are not yet available or reimbursed as first salvage treatment in many countries, salvage chemotherapy is often still the standard approach.
Flowchart of treatment for R/R cHL. IGEV, ifosfamide, gemcitabine, vinorelbine, and prednisolone; SCT, stem cell transplant.
Flowchart of treatment for R/R cHL. IGEV, ifosfamide, gemcitabine, vinorelbine, and prednisolone; SCT, stem cell transplant.
High-dose chemotherapy and ASCT
With increasing CMR rates pre-ASCT, one might question the need for consolidation with HDCT/ASCT in all patients. HDCT/ASCT is associated with immediate toxicity such as cytopenias and mucositis and long-term toxicity such as infertility and secondary malignancies.12 Superiority for HDCT/ASCT over mini-carmustine, etoposide, cytarabine, and melphalan (BEAM) (ie, reduced-dose BEAM without ASCT) in R/R cHL was shown in 2 randomized controlled trials (RCTs) in 1993 and 2002.21,22 However, in these trials, patients did not receive any salvage chemotherapy before BEAM or mini-BEAM. In addition, with the advent of effective drugs such as BV and CPIs, a subset of patients may be cured with salvage treatment alone.17
A recently published study using pembrolizumab and GVD chemotherapy followed by HDCT/ASCT showed a very high pre-ASCT CMR rate of 95%, and with a median follow-up of 1 year, no progressions have occurred.20 This study has now started a second part in which patients with a CMR after 2 cycles of pembrolizumab-GVD continue with 2 additional cycles of pembrolizumab-GVD followed by pembrolizumab consolidation instead of HDCT/ASCT. Results of this revolutionary approach could change the treatment of patients with R/R cHL significantly.
There is a high unmet need for RCTs in the pre-ASCT R/R setting. Future studies should focus on the optimal sequence of using CPIs and BV in salvage treatment and consolidation strategies to induce high CMR rates while at the same time minimizing early and late toxicity. Eventually, an RCT should be performed to establish the role of risk- and PET-adapted treatment in R/R cHL, including the role of HDCT/ASCT.
Patients with primary refractory disease
Primary refractory disease and a short interval between first-line treatment and relapse are important poor prognostic factors for response to salvage therapy and PFS.3,11,14 CMR rates to salvage therapy for refractory patients are usually lower compared with relapsed patients: 73% vs 86% after DHAP, 64% vs 84% after BV-bendamustine, and 53% vs 77% after BV-nivolumab, respectively.3,9,10 However, in patients who do achieve a CMR to salvage therapy and proceed to ASCT, post-ASCT PFS for primary refractory and relapsed patients is similar.3,11 A retrospective analysis in 78 patients who progressed on one or more salvage regimens and who were treated with regimens containing CPI showed favorable results, with 59% of patients achieving a CMR and a post-ASCT PFS of 81% at 18 months.23 This suggests that treatment with CPI may improve chemosensitivity of previously chemorefractory disease. Interestingly, pre-ASCT CMR status was not significantly prognostic for post-ASCT PFS in this cohort, suggesting a PR might be sufficient for proceeding to ASCT after CPI in this patient population.
Patients with a late relapse
Although most relapses after first-line treatment occur within the first 2 years, a minority of patients has a late relapse more than 5 years after first-line treatment.24 A large retrospective analysis showed that late relapses occur more frequently in patients with early-stage favorable disease compared with patients with unfavorable or advanced-stage disease. Whether these relapses represent true relapses or a new second cHL manifestation, possibly due to genetic or environmental risk factors in these patients, remains largely unknown. About half of these patients were treated with ASCT, which was associated with favorable PFS and OS compared with other salvage therapies; however, non-ASCT approaches, such as those using combination chemotherapy or occasionally radiation alone, could be considered depending on the patient's initial treatment and underlying comorbidities.24
Maintenance treatment after ASCT
For patients with a high risk of relapse after ASCT, maintenance treatment with BV can be considered. In a study investigating BV maintenance, 329 patients with unfavorable risk R/R cHL (defined as primary refractory disease, relapse <1 year, or extranodal disease) received either up to 16 cycles of BV maintenance or placebo after ASCT.25 The study showed improvement in PFS in patients receiving BV maintenance, with a 5-year PFS of 59% vs 41% for placebo. However, there was no difference in OS, probably because 87% of patients who relapsed in the placebo arm received BV at the subsequent relapse. Therefore, the use of BV maintenance after ASCT could potentially be restricted to patients with at least 2 risk factors, or alternatively, its use could be delayed until progression. With the increasing use of BV in the first-line setting, it is also important to investigate whether patients who relapse after BV in combination with chemotherapy will still show advantage of BV maintenance after ASCT.26 Alternatively, CPI could be used as maintenance treatment; a small phase 2 trial in 30 high-risk patients showed high post-ASCT PFS.27 The combination of CPI and BV maintenance in 59 high-risk patients has also shown promising results, with 5 patients with PR converting to CR during maintenance.28 Further studies should investigate the role of post-ASCT maintenance in high-risk patients with CPI and/or BV vs reserving these treatments for a subsequent relapse.
BV and CPI treatment for patients who relapse after ASCT or are ineligible for ASCT
Patients who relapse after ASCT or are ineligible for ASCT due to chemotherapy-resistant disease generally have a poor prognosis.1 In Table 3, we summarize the most important recent studies in patients with R/R cHL who have progression after at least 1 line of salvage treatment. The first breakthrough in the post-ASCT setting was the application of monotherapy with BV in heavily pretreated R/R patients, which showed an ORR of 75% and a CMR rate of 34% with a median PFS of 20.5 months in those with a CMR. The PFS rate at 5 years, however, was only 22% with an OS of 41%, highlighting the need for additional treatment options (Table 3).17
Overview of recently reported trial results incorporating BV or CPIs for patients after ≥1 line of salvage treatment
| Study . | N . | Intervention . | CMR . | ORR . | PFS . | OS . |
|---|---|---|---|---|---|---|
| Chen et al (2016)17 | 102 | BV | 34% | 75% | 5 years: 22% | 5 years: 41% |
| O'Connor et al (2018)29 | 65 | BV + bendamustine | 32% | 71% | 2 years: 50%* | 2 years: 65%* |
| Armand et al (2018)18 | 243 | Nivolumab | 16% | 69% | 1 year: 50%* | 1 year: 92% |
| Chen et al (2019)2 | 210 | Pembrolizumab | 27.6% | 71.9% | 2 years: 31.3% | 2 years: 90.9% |
| Shi et al (2019)30 | 92 | Sintilimab | 34% | 80.4% | 6 month: 77.6% | 6 month: 100% |
| Song et al (2019)31 | 75 | Camrelizumab | 28% | 76% | 9 month: 76.6% | 9 month: 96%* |
| Armand et al (2020)32 | 31 | Pembrolizumab | 19% | 58% | 2 years: 30% | 2 years: 87% |
| Song et al (2020)33 | 70 | Tislelizumab | 63.9% | 87.1% | 9 month: 74.5% | 9 month: 98.6% |
| Diefenbach et al (2020)34 (including n = 26 first relapse) | 64 | Ipilimumab + BV vs nivolumab + BV vs ipilimumab + nivolumab + BV | 60%/65%/84% | 80%/94%/95% | 1 year: 59%/77%/87% | 1 year: 90%*/80%*/95%*/ |
| Kuruvilla et al (2021)35 | 304 | Pembrolizumab (n = 151) vs BV (n = 152) | 25% vs 24% | 66% vs 54% | 2 years: 35% vs 25% | — |
| Liu et al (2021)36 | 61 | Camrelizumab monotherapy vs camrelizumab + decitabine | 79% vs 32% for camrelizumab + decitabine vs camrelizumab | 95% vs 89% | 2 years: 67% vs 42% | 2 years: 100% |
| Study . | N . | Intervention . | CMR . | ORR . | PFS . | OS . |
|---|---|---|---|---|---|---|
| Chen et al (2016)17 | 102 | BV | 34% | 75% | 5 years: 22% | 5 years: 41% |
| O'Connor et al (2018)29 | 65 | BV + bendamustine | 32% | 71% | 2 years: 50%* | 2 years: 65%* |
| Armand et al (2018)18 | 243 | Nivolumab | 16% | 69% | 1 year: 50%* | 1 year: 92% |
| Chen et al (2019)2 | 210 | Pembrolizumab | 27.6% | 71.9% | 2 years: 31.3% | 2 years: 90.9% |
| Shi et al (2019)30 | 92 | Sintilimab | 34% | 80.4% | 6 month: 77.6% | 6 month: 100% |
| Song et al (2019)31 | 75 | Camrelizumab | 28% | 76% | 9 month: 76.6% | 9 month: 96%* |
| Armand et al (2020)32 | 31 | Pembrolizumab | 19% | 58% | 2 years: 30% | 2 years: 87% |
| Song et al (2020)33 | 70 | Tislelizumab | 63.9% | 87.1% | 9 month: 74.5% | 9 month: 98.6% |
| Diefenbach et al (2020)34 (including n = 26 first relapse) | 64 | Ipilimumab + BV vs nivolumab + BV vs ipilimumab + nivolumab + BV | 60%/65%/84% | 80%/94%/95% | 1 year: 59%/77%/87% | 1 year: 90%*/80%*/95%*/ |
| Kuruvilla et al (2021)35 | 304 | Pembrolizumab (n = 151) vs BV (n = 152) | 25% vs 24% | 66% vs 54% | 2 years: 35% vs 25% | — |
| Liu et al (2021)36 | 61 | Camrelizumab monotherapy vs camrelizumab + decitabine | 79% vs 32% for camrelizumab + decitabine vs camrelizumab | 95% vs 89% | 2 years: 67% vs 42% | 2 years: 100% |
Data have been extracted from available Kaplan-Meier curves using WebPlot Digitizer.
A study that investigated pembrolizumab monotherapy showed an ORR of 69% and a CMR rate of 22%, with a 2-year PFS of 31% and OS of 91%.2 Several different CPIs and also combinations of CPIs have been investigated in R/R cHL.30,31,33,34 In a phase 1 trial, 64 patients were randomized between ipilimumab-BV, nivolumab-BV, and triple therapy with ipilimumab-nivolumab-BV. The trial showed differences in toxicity profile and efficacy between the 3 regimens, with the highest percentage of grade 3/4 adverse events in the triplet and ipilimumab-BV group, whereas the highest ORR and CMR rates were found in the triplet and nivolumab-BV group.34
In a recently published head-to-head comparison of monotherapy with pembrolizumab vs monotherapy with BV, pembrolizumab showed a significantly higher median PFS of 13.2 months vs 8.3 months for BV.35 The incidence of adverse events was comparable between the 2 groups, with immune-mediated adverse events in the pembrolizumab arm and neuropathy in the BV arm.
Importantly, in patients who received earlier treatment with BV or CPI, response rates seem to be similar to patients who have not received BV or CPI before. A retrospective study evaluated 18 patients with R/R cHL and 10 patients with R/R anaplastic large cell lymphoma who received treatment with BV in 2 lines and showed CMR and ORR that are comparable with patients who received BV for the first time.37 Retreatment with CPI in 78 patients with R/R cHL who relapsed after nivolumab also showed comparable efficacy.38
Role of radiotherapy in the management of R/R cHL
The role of radiotherapy in the R/R setting has not been revisited well in this era of novel treatment options. Radiotherapy can be used pre-ASCT or post-ASCT on residual lesions or in patients with extranodal or bulky disease and as part of the conditioning regimen using total lymphoid irradiation, but comparative data about efficacy of radiotherapy in these settings are scarce and outdated.39 Earlier studies have shown that patients who receive radiotherapy have a decreased risk of local recurrence, and thus for patients with limited-stage disease at relapse, radiotherapy may be an effective option.39 Using radiotherapy in patients who have a PR pre-ASCT would be an interesting strategy to increase the CMR rate, and studies investigating this approach are warranted. In addition, the synergistic effects of radiation with immunotherapy, as described in a few case reports, should be investigated more extensively and could be an option for patients who relapse after ASCT or are ineligible for ASCT.
General considerations
In conclusion, the primary goal of treatment for patients with R/R cHL is to achieve a CMR before HDCT/ASCT as this significantly correlates with a favorable outcome after ASCT. Using a sequential approach, treatment intensity and toxicity can be reduced in a subset of fast-responding patients. Patients who are ineligible for ASCT or relapse after ASCT can be treated with BV or CPIs. There is a need to develop novel therapies to increase response rates without increasing toxicity. One of the next goals for clinical trials is to investigate which patients can possibly be cured without HDCT/ASCT. Risk-stratified and PET-adapted prospective studies could help achieve this goal. Optimized risk stratification and response evaluation will guide future treatment decisions and will help to find the right treatment for the right patient.
CLINICAL CASE (continued)
Despite the initial CMR after 2 cycles of DHAP, soon after the third and last cycle of DHAP, B-symptoms and itching returned and a relapse was again confirmed by PET-CT and a biopsy.
Given the poor prognosis in this patient with chemorefractory disease, combination treatment with BV and nivolumab was started based on the encouraging results of a phase 1/2 trial.10 Already after 1 cycle of BV-nivolumab, her B-symptoms disappeared, and after 4 cycles, she reached a CMR. We decided to proceed to haploidentical ASCT, because the patient initially progressed during salvage chemotherapy. As mentioned above, though, emerging data on the role of ASCT after CPI for patients refractory to multiple prior lines of chemotherapy suggest that ASCT could be considered in this setting as well.23
Conflict-of-interest disclosure
Julia Driessen: travel support from Takeda.
Sanne H. Tonino: no relevant disclosures.
Alison J. Moskowitz: honoraria: Seattle Genetics; consulting or advisory role: Seattle Genetics, Takeda, Imbrium Therapeutics, Merck, Janpix, Kyowa Kirin International, miRagen, ADC Therapeutics, Bristol Myers Squibb; research funding: Incyte, Seattle Genetics, Merck, Bristol Myers Squibb, miRagen, ADC Therapeutics, BeiGene.
Marie José Kersten: honoraria for consulting or advisory boards: Kite/Gilead, Novartis, BMS/Celgene, Miltenyi Biotech, Research funding Takeda, Roche, Celgene, Kite/Gilead.
Off-label drug use
Julia Driessen: nothing to disclose.
Sanne H. Tonino: nothing to disclose.
Alison J. Moskowitz: nothing to disclose.
Marie José Kersten: nothing to disclose.