Abstract
Hematologic malignances are more common and often higher risk in older patients. Allogeneic hematopoietic cell transplantation (alloHCT) best enables long-term disease control for patients with poor risk or relapsed/refractory hematologic malignancies such as acute myeloid leukemia, myelodysplastic syndromes, or myelofibrosis. Rates of alloHCT among older patients, while still relatively low compared with younger patients, have risen sharply over the past decade. Accumulating evidence supports alloHCT for patients ≥60 years of age relative to non-HCT therapies based on improved overall and disease-free survival. However, a significant proportion of older adults have limitations characterized by geriatric assessment. A systematic process to evaluate and optimize older patients may improve decision making, transplant outcomes, and alloHCT access. We present case-based studies to illustrate a stepwise and rational approach to proper older patient evaluation, pretransplant optimization, and posttransplant care with attention to important geriatric issues and quality of life.
Learning Objectives
Describe access barriers to allogeneic hematopoietic cell transplantation for older adults
Understand the role of GA, management, and optimization strategies for an older adult throughout the alloHCT process
Introduction
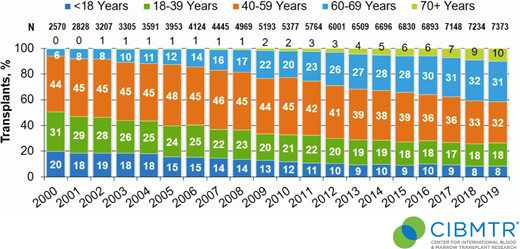
Allogeneic hematopoietic cell transplantation (alloHCT) remains the best-established curative option for many patients with advanced hematologic malignancies, particularly myeloid neoplasms.1 In recent years, we have witnessed significant advances in reducing transplant-related mortality, manipulating graft-versus-leukemia effect to prevent/treat relapse, and developing alloHCT as a platform for novel cellular therapies.2,3 Older age may have been the most formidable and important barrier, representing the next frontier.4 The demographics of blood cancer, especially myeloid malignancies, with a median age of onset in the late 60s to early 70s and frequently higher risk underscore the need.5 The era of alloHCT is upon us; the Center for International Blood and Marrow Transplantation Research (CIBMTR) reports that patients aged ≥60 years comprised more than 40% of adult alloHCT volume in the United States (Figure 1).6 In this review, we discuss unique challenges facing older patients in alloHCT and strategies to improve their outcomes.
Trends in alloHCT in the United States by increasing recipient age (N = total number of alloHCTs during each calendar year; Transplant, % reflects the percentage of alloHCT in each age group by calendar year). Data generously provided by the Center for International Blood and Marrow Transplant Research.
Trends in alloHCT in the United States by increasing recipient age (N = total number of alloHCTs during each calendar year; Transplant, % reflects the percentage of alloHCT in each age group by calendar year). Data generously provided by the Center for International Blood and Marrow Transplant Research.
CASE 1
Mr. RM is a 73-year-old man with coronary artery disease, hypertension, diabetes, and moderate obesity who resides in a rural town with his wife and children in an active lifestyle. One year ago, he initiated hypomethylating agent therapy through his local oncologist for newly diagnosed high-risk, transfusion-requiring myelodysplastic syndrome (MDS) with excess blasts. The MDS evolved to acute myeloid leukemia (AML) 1 year later, prompting induction with liposomal daunorubicin and cytarabine. His treatment course was complicated by neutropenic fever and bacteremia. A follow-up bone marrow biopsy demonstrated complete remission. Should Mr. RM be referred for consolidation alloHCT?
AlloHCT vs chemotherapy in older patients
Older patients, especially those in their 70s, face the unique challenge of finite life expectancy that may be further constrained by medical comorbidities.7 AlloHCT for older patients with AML poses the dual dangers of complications including death after alloHCT without relapse (nonrelapse mortality) and disease relapse. As such, it is imperative that physicians and patients weigh the benefits and risks of alloHCT vs nontransplant approaches, ideally early in the treatment course. Several population-based studies have shown that invariably, older patients with intermediate- or poor-risk AML (which comprise most newly diagnosed AMLs in older patients) rarely survive for more than 5 years without an alloHCT.8,9 In a study comparing patients with AML aged ≥60 years treated with consolidation chemotherapy alone in first complete remission in several national cooperative trials vs similarly aged patients undergoing alloHCT in first complete remission from the contemporary CIBMTR transplant registry,10 survival was worse for alloHCT in the first 9 months posttransplant relative to consolidation on trials. However, after 5 years, alloHCT significantly benefited patient overall survival (OS) at 28.6% vs 13.8% in the chemo-consolidation cohort (hazard ratio, 0.53; P < .0001). Table 1 highlights similar findings from several registry studies comparing alloHCT with nontransplant chemo-consolidation trials for AML.11-13 In addition, 3 prospective, donor vs no-donor studies for patients with AML were published in abstract form, which also supports alloHCT in this population (Table 1). The most recently reported Blood and Marrow Transplantation Clinical Trial Network (BMT CTN) 1102 prospectively studied biologically assigned, newly diagnosed high-risk patients with MDS aged 60 to 75 years to alloHCT with a matched donor vs hypomethylating therapy without alloHCT in the absence of a matched donor; the presence of a matched donor conferred a 3-year OS advantage of 47.9% vs 26.6%.14
Multicenter studies comparing alloHCT to non-HCT consolidation strategies in older patients with AML or MDS
| Study . | N . | Age range . | Study design disease . | Comparison groups . | AlloHCT donor source/conditioning . | Survival outcomes . | Comments . |
|---|---|---|---|---|---|---|---|
| Farag et al. (2011)11 | AlloHCT: 94 Non-HCT: 96 | 60-70 | Retrospective, multicenter analysis AML in CR1 | AlloHCT: CIBMTR Registry Non-HCT: CT in CALGB | Matched: 77% Alternative: 23% RIC/NMA: 100% | 3-year OS: AlloHCT: 37% Non-HCT: 25% 3-year LFS: AlloHCT: 32% Non-HCT: 15% | OS benefit in all cytogenetic risk groups. |
| Kurosawa et al. (2011)12 | AlloHCT: 152 Non-HCT: 884 | 50-70 | Retrospective, multicenter analysis AML in CR1 | AlloHCT: Japanese Registry Non-HCT: Japanese Registry | Matched: 76% Alternative: 24% (15% cord) MA: 38% RIC/NMA: 62% | 3-year OS: AlloHCT: 62% Non-HCT: 51% 3-year RFS: AlloHCT: 56% Non-HCT: 29% | 183 patients in the CT group eventually received HCT. OS benefits in intermediate-risk group. |
| Versluis et al. (2015)13 | AlloHCT: 97 Non-HCT: 177 None: 366 | ≥60 | Retrospective analysis of multicenter trials AML in CR1 | AlloHCT: HOVON-SAKK trials Non-HCT: HOVON-SAKK trials | Matched: 92% Alternative: 8% RIC/NMA: 100% | 5-year OS: AlloHCT: 35% Non-HCT: 26% 5-year RFS: AlloHCT: 32% Non-HCT: 20% | Benefits are seen in both intermediate-risk and adverse risk groups. |
| Ustun et al. (2019)10 | AlloHCT: 431 Non-HCT: 211 | 60-77 | Retrospective, multicenter analysis AML in CR1 | AlloHCT: CIBMTR Registry Non-HCT: CT in cooperative group trials (CALGB, SWOG, ECOG) | Matched: 65% Alternative: 35% (24% cord) MA: 29% RIC/NMA: 71% | 5-year OS: AlloHCT: 28.6% Non-HCT: 13.8% 5-year DFS: AlloHCT: 23.7% Non-HCT: 11.1% | OS/DFS is worse in the first 9 months for alloHCT. OS/DFS benefits more for poor-risk patients. |
| *Niederwieser et al. (2016) ASCO Abstract e18501 | AlloHCT: 150 (donor) Non-HCT: 205 (no donor) | 50-75 | Prospective, intent-to-treat, donor vs no-donor trial AML in CR1 | AlloHCT: Donor group Non-HCT: No donor consolidation group | Matched: 79% Mismatched: 21% RIC/NMA: 100% | 9-year LFS: Donor: 25% No donor: 14% 9-year RI: Donor: 42% No donor: 78% | Benefits seen across both intermediate- and high-risk groups. |
| *Brune et al. (2018) ASH Abstract 205 | AlloHCT: 77 (donor) Non-HCT: 68 (no donor) | 50-70 | Prospective, intent-to-treat, donor vs no-donor trial AML in CR1 | AlloHCT: Donor group Non-HCT: No donor consolidation group | Matched: 100% RIC/NMA: 100% | 3-year OS: Donor: 45% No donor: 48% 3-year RFS: Donor: 40% No donor: 35% | 7 patients in control group crossed over. Extremely high relapse rate in both arms (49% vs 60%) |
| *Foran et al. (2018) EHA Abstract S857 (ECOG E2906) | AlloHCT: 135 (donor) Non-HCT: 225 (no donor) | ≥60 | Prospective, intent-to-treat, donor vs no-donor trial AML in CR1 | AlloHCT: Donor group Non-HCT: No donor consolidation group | Matched: 100% RIC/NMA: 100% | Median OS: Donor: 22.1 months No donor: 13.4 months (P = .013) | 44 patients in the donor group and 25 patients in the no-donor group crossed over. |
| Nakamura et al. (2021) (BMT CTN 1102)14 | AlloHCT: 260 (donor) Non-HCT: 124 (no donor) | 50-75 | Prospective, intent-to-treat, donor vs no-donor trial MDS IPSS Intermediate-2/high risk | AlloHCT: Donor group Non-HCT: No donor consolidation group | Matched: 100% RIC/NMA: 100% | 3-year OS: Donor: 47.9% No donor: 26.6% 3-year OS (as treated analysis): AlloHCT: 47.4% Non-HCT: 16% | Similar benefits in 3-year LFS. No decrease in QOL. |
| Study . | N . | Age range . | Study design disease . | Comparison groups . | AlloHCT donor source/conditioning . | Survival outcomes . | Comments . |
|---|---|---|---|---|---|---|---|
| Farag et al. (2011)11 | AlloHCT: 94 Non-HCT: 96 | 60-70 | Retrospective, multicenter analysis AML in CR1 | AlloHCT: CIBMTR Registry Non-HCT: CT in CALGB | Matched: 77% Alternative: 23% RIC/NMA: 100% | 3-year OS: AlloHCT: 37% Non-HCT: 25% 3-year LFS: AlloHCT: 32% Non-HCT: 15% | OS benefit in all cytogenetic risk groups. |
| Kurosawa et al. (2011)12 | AlloHCT: 152 Non-HCT: 884 | 50-70 | Retrospective, multicenter analysis AML in CR1 | AlloHCT: Japanese Registry Non-HCT: Japanese Registry | Matched: 76% Alternative: 24% (15% cord) MA: 38% RIC/NMA: 62% | 3-year OS: AlloHCT: 62% Non-HCT: 51% 3-year RFS: AlloHCT: 56% Non-HCT: 29% | 183 patients in the CT group eventually received HCT. OS benefits in intermediate-risk group. |
| Versluis et al. (2015)13 | AlloHCT: 97 Non-HCT: 177 None: 366 | ≥60 | Retrospective analysis of multicenter trials AML in CR1 | AlloHCT: HOVON-SAKK trials Non-HCT: HOVON-SAKK trials | Matched: 92% Alternative: 8% RIC/NMA: 100% | 5-year OS: AlloHCT: 35% Non-HCT: 26% 5-year RFS: AlloHCT: 32% Non-HCT: 20% | Benefits are seen in both intermediate-risk and adverse risk groups. |
| Ustun et al. (2019)10 | AlloHCT: 431 Non-HCT: 211 | 60-77 | Retrospective, multicenter analysis AML in CR1 | AlloHCT: CIBMTR Registry Non-HCT: CT in cooperative group trials (CALGB, SWOG, ECOG) | Matched: 65% Alternative: 35% (24% cord) MA: 29% RIC/NMA: 71% | 5-year OS: AlloHCT: 28.6% Non-HCT: 13.8% 5-year DFS: AlloHCT: 23.7% Non-HCT: 11.1% | OS/DFS is worse in the first 9 months for alloHCT. OS/DFS benefits more for poor-risk patients. |
| *Niederwieser et al. (2016) ASCO Abstract e18501 | AlloHCT: 150 (donor) Non-HCT: 205 (no donor) | 50-75 | Prospective, intent-to-treat, donor vs no-donor trial AML in CR1 | AlloHCT: Donor group Non-HCT: No donor consolidation group | Matched: 79% Mismatched: 21% RIC/NMA: 100% | 9-year LFS: Donor: 25% No donor: 14% 9-year RI: Donor: 42% No donor: 78% | Benefits seen across both intermediate- and high-risk groups. |
| *Brune et al. (2018) ASH Abstract 205 | AlloHCT: 77 (donor) Non-HCT: 68 (no donor) | 50-70 | Prospective, intent-to-treat, donor vs no-donor trial AML in CR1 | AlloHCT: Donor group Non-HCT: No donor consolidation group | Matched: 100% RIC/NMA: 100% | 3-year OS: Donor: 45% No donor: 48% 3-year RFS: Donor: 40% No donor: 35% | 7 patients in control group crossed over. Extremely high relapse rate in both arms (49% vs 60%) |
| *Foran et al. (2018) EHA Abstract S857 (ECOG E2906) | AlloHCT: 135 (donor) Non-HCT: 225 (no donor) | ≥60 | Prospective, intent-to-treat, donor vs no-donor trial AML in CR1 | AlloHCT: Donor group Non-HCT: No donor consolidation group | Matched: 100% RIC/NMA: 100% | Median OS: Donor: 22.1 months No donor: 13.4 months (P = .013) | 44 patients in the donor group and 25 patients in the no-donor group crossed over. |
| Nakamura et al. (2021) (BMT CTN 1102)14 | AlloHCT: 260 (donor) Non-HCT: 124 (no donor) | 50-75 | Prospective, intent-to-treat, donor vs no-donor trial MDS IPSS Intermediate-2/high risk | AlloHCT: Donor group Non-HCT: No donor consolidation group | Matched: 100% RIC/NMA: 100% | 3-year OS: Donor: 47.9% No donor: 26.6% 3-year OS (as treated analysis): AlloHCT: 47.4% Non-HCT: 16% | Similar benefits in 3-year LFS. No decrease in QOL. |
Meeting abstract only. ASCO, American Society of Clinical Oncology; ASH, American Society of Hematology; CALGB, Cancer and Leukemia Group B; CR1, first complete remission; CT, chemotherapy consolidation; DFS, disease-free survival; ECOG, Eastern Cooperative Oncology Group; EHA, European Hematology Association; HOVON-SAKK, Dutch-Belgian Hemato-Oncology Cooperative Group and the Swiss Group for Clinical Cancer Research; IPSS, International Prognostic Scoring System; LFS, leukemia-free survival; MA, myeloablative conditioning; RFS, relapse free survival; RI, relapse incidence; RIC/NMA, reduced-intensity/nonmyeloablative conditioning; SWOG, Southwest Oncology Group.
AlloHCT outcomes in older patients
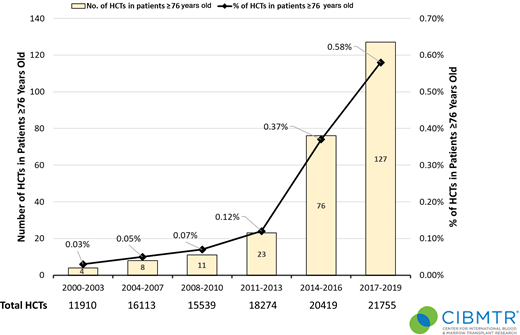
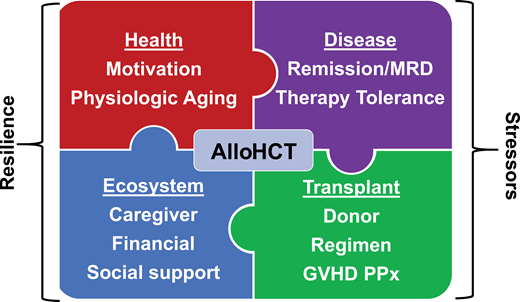
Associated with many advances in transplantation, the number and proportion of total alloHCT continue to rise in patients aged ≥60 years with hematologic malignancies (Figures 1 and 2), further stimulated by wider donor availability, including haploidentical, for most patients. Rashidi et al15 performed a meta-analysis of 13 studies of patients with AML 60 years and older who underwent alloHCT. The 2-year relapse-free survival and OS were 44% and 45%, respectively, suggesting that alloHCT is a viable option for these patients. Similar findings were demonstrated for patients with a variety of hematologic malignancies.16-18 Even among patients older than 70 years, a recent CIBMTR analysis showed acceptable if not promising 2-year progression-free survival and OS of 32% and 39%, respectively, in heterogeneous diseases, donor sources, and regimens.19 These data reinforce that chronologic age alone, at least up to 75 years, should not exclude an older patient from alloHCT candidacy. Rather, we propose the patient's “physiologic” age should be evaluated, along with a comprehensive assessment of the patient's goals of care, quality of life (QOL), and the ecosystem, including caregivers, social support system, financial resources, and living situation (Figure 3).4,20 Although beyond the scope of this review, even among reduced-intensity regimens, a range of transplant intensities exist that must be individualized based on patient health and disease risk.19-22 Furthermore, graft-versus-host disease (GVHD) remains a major cause of morbidity and functional impairment in this population, prompting consideration of lower GVHD platforms (Figure 3).23,24
Trends in alloHCT in the United States for patients 76 years or older (N = total number of transplants). Data generously provided by the Center for International Blood and Marrow Transplant Research.
Trends in alloHCT in the United States for patients 76 years or older (N = total number of transplants). Data generously provided by the Center for International Blood and Marrow Transplant Research.
Solving the puzzle of alloHCT for older patients with hematologic malignancies. MRD, measurable residual disease; PPx, prophylaxis.
Solving the puzzle of alloHCT for older patients with hematologic malignancies. MRD, measurable residual disease; PPx, prophylaxis.
Transplant access barriers for older patients
Referral bias and other barriers limit access among older patients to alloHCT. A recent systemic review of 26 studies showed that chronologic older age is the single most important barrier to refer patients for alloHCT consideration.25 Specifically, opinions differ markedly among hematologists/oncologists, transplant physicians, and transplant centers regarding the upper age limit for alloHCT, likely as a result of individual experience and expertise.26,27 Routine aging assessment could neutralize heterogeneity in opinion; however, the lack of standardized geriatric assessment (GA) tools and resources to accomplish them challenges physiologic aging evaluation.27 Other noted factors hindering access included nonwhite ethnic origin, insurance status, higher comorbidities, and lower socioeconomic status. Given recent advances in transplantation using alternative donors such as haploidentical and mismatched donors, lack of a matched donor should not be exclusionary even among older patients.28,29
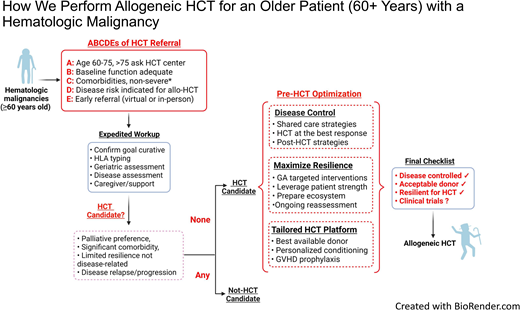
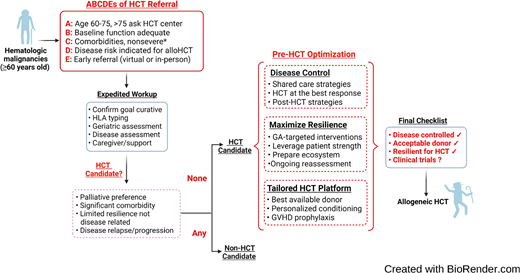
There are several potential mitigation strategies to reduce access barriers. First and foremost, disease indications for alloHCT should be clearly defined for older patients to supplement standard alloHCT guidelines,30 accounting for worse outcomes for AML, MDS, and acute lymphoblastic leukemia even in the same disease risk group. Rather than a dichotomous single decision point of “fit” or “not fit” for transplant, we recommend expedited referral for alloHCT evaluation in the appropriate disease indications for patients 60 years or older in the presence of adequate baseline functional status and without severe organ comorbidities (Figure 4). We must strive to enroll patients aged >75 years on alloHCT studies; until then, the decision must be individualized in this age group. Figure 2 quantifies the limited application of alloHCT in this cohort but also the substantial increase in utilization. Second, we should explore innovative approaches to incorporate physiologic aging evaluation by GA in the routine care of older patients, such as an embedded geriatric hematology clinic and telemedicine platform.31,32 Last, we must invest in greater educational and outreach efforts to raise awareness of the emerging, promising alloHCT outcome data, the role of GA, and clinical trial opportunities specifically designed for older patients.26
How we perform alloHCT for an older patient with a hematologic malignancy. HLA, human leukocyte antigen. *Severe comorbidities: New York Heart Association class 4 heart failure, severe renal dysfunction or end-stage renal disease on dialysis, Child class C liver cirrhosis, Gold stage 4 chronic obstructive lung disease, metastatic solid tumor, dementia, or any comorbidity significantly limiting life expectancy.
How we perform alloHCT for an older patient with a hematologic malignancy. HLA, human leukocyte antigen. *Severe comorbidities: New York Heart Association class 4 heart failure, severe renal dysfunction or end-stage renal disease on dialysis, Child class C liver cirrhosis, Gold stage 4 chronic obstructive lung disease, metastatic solid tumor, dementia, or any comorbidity significantly limiting life expectancy.
CASE 1 (Continued)
Mr. RM had several telemedicine visits with the transplant physician, a clinical nurse coordinator, and a social worker, all located at an academic medical center 200 miles away. Cognizant of his comorbid conditions, necessary evaluation, and potential early loss of QOL from alloHCT, Mr. RM and his family expressed a desire to proceed. He also completed a remote, video-assisted GA, which demonstrated preserved self-reported functional status, mobility, and cognition. In parallel, the unrelated donor search proceeded, identifying a young matched unrelated donor. During chemotherapy consolidation locally, he underwent pretransplant testing also through his local oncologist. Four weeks later, he began a reduced-intensity transplant regimen inclusive of posttransplant cyclophosphamide for GVHD prophylaxis on the BMT CTN 1301 Progress 3 trial (NCT02345850) from a young well-matched unrelated donor.
GA in alloHCT
The shift from fitness alone to assessing resilience to disease-related and transplant-related stressors broadens interventional opportunities that may widen access (Figure 3). The term resiliency encompasses both the intrinsic, “physiologic” aging process and the extrinsic “ecosystem,” including caregiver, social support, finance, and resources; GA combined with standard transplant psychosocial evaluation achieves this goal.4 GA is a multidisciplinary diagnostic process that identifies medical, functional, and psychosocial limitations of an older person and place him or her on a continuous spectrum of fitness, vulnerability, and frailty and further informs a multidisciplinary care plan to maximize healthy aging, as illustrated in the following case.33
CASE 2
Mrs. LK is a 70-year-old woman who had stage II early breast cancer 2 years ago that was treated with surgery, radiation, and adjuvant chemotherapy with no evidence of disease, moderate chronic obstructive pulmonary disease, osteoarthritis, and atrial fibrillation. She sought treatment from her primary care physician for fatigue and was found to have pancytopenia with peripheral blasts. A bone marrow biopsy specimen established the diagnosis of AML harboring a monosomy 7. Due to her comorbidities, low-intensity induction commenced with azacitidine and venetoclax, which was complicated only by ongoing cytopenia. Repeat bone marrow evaluation after 1 cycle demonstrated complete remission but with positive measurable residual disease by multicolor flow cytometry and cytogenetics. She was referred for transplant consultation. The GA revealed dependence in several instrumental activities of daily living, recently depressed mood, and a screening test positive for mild cognitive impairment. She would like to pursue curative-intent alloHCT consolidation if possible. She has a highly supportive family and caregiver who concur and understand that transplant toxicity may be prohibitive, especially considering the GA-defined deficits and comorbidities. What is the appropriate next step?
GA domains affect alloHCT outcomes
Physiologic aging established through GA, coupled with anticipated stressors of the disease and treatments, begins to paint a picture of physical resilience. Serial GA may enrich understanding of resilience or “bounce back” after treatment. In the context of alloHCT, the GA should address the extrinsic ecosystem, including psychosocial support, caregiver support, and resources for alloHCT (Figure 3). Artz and colleagues conducted the initial pilot study of GA in alloHCT and found significant associations of pretransplant geriatric impairments in function and mobility with adverse survival outcomes following alloHCT.34,35 Subsequently, several groups independently validated these findings and found additional, prognostically important domains such as cognition, medication, and frailty scales. These studies are summarized in Table 2.36-44 The ongoing BMT CTN 1704 trial (Composite Health Assessment Risk Model [CHARM]) is a large national study prospectively using a standard GA and other measures prior to alloHCT among patients ≥60 years old, aiming to confirm these findings and/or identify additional risk factors (NCT03992352).
Studies illustrating prognostically important geriatric deficits in older patients undergoing alloHCT
| Study . | N . | Age, median (range), y . | Study design disease . | Impairment in GA domains with impact on outcomes . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Comorbidity . | Function . | Mobility . | Cognition . | Medication . | Frailty scale . | ||||
| Muffly et al. (2014)35 | 203 | 58 (54-63) | Prospective, single-center pilot study All diseases | NRM ↑ OS ↓ | NRM ↑ OS ↓ | OS ↓ | |||
| Deschler et al. (2018)36 | 106 | 66 (60-78) | Prospective, single-center trial Myeloid neoplasms | NRM ↑ PFS ↓ | OS ↓ PFS ↓ | OS ↓ | |||
| Huang et al. (2020)37 | 148 | 62 (50-76) | Prospective, single-center pilot study All diseases | OS ↓ PFS ↓ NRM ↑ Toxicities ↑ | |||||
| Pamukcuoglu et al. (2019)40 | 52 | 59 (40-73) | Prospective, single-center pilot study All diseases | OS ↓ Toxicities ↑ | |||||
| Salas et al. (2021)41 | 168 | 58 (19-77) | Prospective, single-center pilot study All diseases | NRM ↑ OS ↓ | NRM ↑ | ||||
| Olin et al. (2020)38 | 330 | 63 (50-77) | Multicenter, CIBMTR registry All diseases | NRM ↑ | NRM ↑ OS ↓ | ||||
| Polverelli et al. (2020)42 | 228 | 64 (60-76) | Two-center retrospective study | NRM ↑ OS ↓ | |||||
| Lin et al. (2020)39 | 457 | 66 (60-79) | Single-center, retrospective study | NRM ↑ | NRM ↑ OS ↓ PFS ↓ | ||||
| Bhargava et al. (2020)43 | 114 | 68 (65-75) | Single-center, retrospective study | Toxicities ↑ OS ↓ | |||||
| Sugidono et al. (2021)44 | 148 | 62 (50-76) | Single-center, retrospective study | OS ↓ | |||||
| Study . | N . | Age, median (range), y . | Study design disease . | Impairment in GA domains with impact on outcomes . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Comorbidity . | Function . | Mobility . | Cognition . | Medication . | Frailty scale . | ||||
| Muffly et al. (2014)35 | 203 | 58 (54-63) | Prospective, single-center pilot study All diseases | NRM ↑ OS ↓ | NRM ↑ OS ↓ | OS ↓ | |||
| Deschler et al. (2018)36 | 106 | 66 (60-78) | Prospective, single-center trial Myeloid neoplasms | NRM ↑ PFS ↓ | OS ↓ PFS ↓ | OS ↓ | |||
| Huang et al. (2020)37 | 148 | 62 (50-76) | Prospective, single-center pilot study All diseases | OS ↓ PFS ↓ NRM ↑ Toxicities ↑ | |||||
| Pamukcuoglu et al. (2019)40 | 52 | 59 (40-73) | Prospective, single-center pilot study All diseases | OS ↓ Toxicities ↑ | |||||
| Salas et al. (2021)41 | 168 | 58 (19-77) | Prospective, single-center pilot study All diseases | NRM ↑ OS ↓ | NRM ↑ | ||||
| Olin et al. (2020)38 | 330 | 63 (50-77) | Multicenter, CIBMTR registry All diseases | NRM ↑ | NRM ↑ OS ↓ | ||||
| Polverelli et al. (2020)42 | 228 | 64 (60-76) | Two-center retrospective study | NRM ↑ OS ↓ | |||||
| Lin et al. (2020)39 | 457 | 66 (60-79) | Single-center, retrospective study | NRM ↑ | NRM ↑ OS ↓ PFS ↓ | ||||
| Bhargava et al. (2020)43 | 114 | 68 (65-75) | Single-center, retrospective study | Toxicities ↑ OS ↓ | |||||
| Sugidono et al. (2021)44 | 148 | 62 (50-76) | Single-center, retrospective study | OS ↓ | |||||
NRM, nonrelapse mortality; PFS, progression-free survival.
CASE 2 (Continued)
The transplant team recommended short-term deferral to address GA-defined deficits while continuing chemotherapy to deepen disease response. Mrs. LK underwent rehabilitative therapy with physical and occupational therapy supplemented by home walking and strengthening supervised by her family. The resolution of transfusion-dependent anemia further boosted physical recovery. The geriatrics team managed polypharmacy by actively deprescribing nonessential medications thought to contribute to the mild cognitive deficits. Repeated GA 2 months later demonstrated improved functional status and cognition (no longer in the impaired range). Depressive symptoms resolved with more social engagement and physical independence. Based on these results and another informed discussion, the patient and transplant team elected to proceed. She subsequently underwent reduced-intensity conditioning alloHCT using her 36-year-old haploidentical son with posttransplant cyclophosphamide for prevention of GVHD. The patient had a caregiver starting the day before transplant infusion and continuing throughout. The transplant admission was complicated by an episode of delirium initially recognized by the caregiver. After excluding organic causes, she received haloperidol as needed, and occupational therapy prescribed intensive cognitive exercises. She was discharged on posttransplant day +37 to home with a walker and a home exercise regimen, avoiding a subacute rehabilitation facility. She continued “virtual” clinics visits and physical face-to-face encounters and ongoing rehabilitation.
Geriatric management and optimization
While GA may uncover vulnerabilities in older patients considering alloHCT, how best to optimize patients prior to alloHCT remains a work in progress. Challenges include short time available before alloHCT due to the pace of disease and delayed referral, nonmodifiable deficits such as comorbidities, and limited institutional resources. Low-intensity interventions would be ideal; however, the BMT CTN conducted a multicenter, randomized study of structured home exercise and a stress management program prior to transplantation, finding no improvement in physical and mental functioning posttransplant.45 While not limited to older patients, this accentuated the need for targeted and/or more intensive pretransplant optimization. Recently, Derman, Artz and colleagues46 conducted the first pilot study applying GA-guided interventions in a multidisciplinary team clinic (MDC) to optimize patients prior to transplant. They found that, compared to historical cohorts with similar disease and transplant characteristics, the MDC cohort experienced fewer inpatient deaths, shorter length of stay, fewer discharges to a skilled nursing facility, and improved survival. The critical components of the MDC approach likely involve more careful patient selection, targeted optimization, and multidisciplinary collaboration.46,47 In addition, early recognition, especially of ecosystem barriers, through routine evaluation best affords opportunities to optimize. Telehealth and a shared care model, for example, may alleviate distance barriers for routine pre- and posttransplant visits, at least when patients can safely reside at home.47 GA-guided management and integration of geriatric principles of care should not be limited to pretransplant care. The development of geriatric syndromes of functional decline, fall, delirium, and cognitive impairment posttransplant is not uncommon, and these syndromes are associated with impaired survival and QOL.24,48,49 In addition, discharge to a rehabilitation facility posttransplant has been shown to be a marker of poor survival.50 These issues require further prospective validation with patient-centric outcomes of function and QOL.
How we perform alloHCT in an older patient
We summarize our approach to alloHCT in an older patient with hematologic malignancy in Figure 4, working toward successful completion of a final checklist. We recommend that hematologists, patients, and institutions first consider the “ABCDE” to triage (early) referral. We believe resiliency measurement, through GA or equivalent, is essential in older candidates to supplement standard pre-alloHCT testing and the subjective “fitness” criteria. We advocate a collaborative model partnering the transplant team and the disease management team (when separate) to harmonize disease therapy with anticipated transplant timing, often dictated by donor availability. Disease treatment may occur distant from the transplant center, particularly as a range of lower-intensity treatments exist for common alloHCT indications. This shared care model ensures more uniform messaging to patients related to alloHCT plans, risks, and benefits from all physicians. Shared care promotes the parallel process of maximizing resilience through GA-targeted interventions and preparation of the supporting ecosystem during disease treatment. Alignment of these processes facilitates meeting a “final checklist” before alloHCT (Figure 4). We acknowledge that not all older patients who embark on this process will ultimately pursue alloHCT because of disease relapse, inadequate resilience, and/or changes in goals of care, underscoring the value of multiple touch points to discuss patient goals and recalibrate patient expectations about the likelihood of meeting the final checklist.
Conclusion and future directions
We recommend alloHCT as a standard of care option for older patients with high-risk hematologic malignancies best established for AML and MDS. Not only has utilization in older patients risen markedly, but outcomes in older patients also continue to improve due to incorporation of novel transplant platforms with reduced toxicities, an increased donor pool, and the better selection and care of older transplant patients. Moreover, we are beginning to appreciate the impact of aging biology on transplant outcomes and to explore mechanism-based, therapeutic interventions to target aging pathways.51 The convergence of success in disease-based therapies, education to address age misconceptions, novel interventions to bolster patient resilience, and transplant regimens promises more widespread and more successful application of alloHCT for older adults with high-risk hematologic malignancies.
Acknowledgments
R.J.L. acknowledges the support from the ASH Clinical Research Training Institute and the ASH Scholar Award. We acknowledge editorial support from Sally Mokhtari. We thank Dr. Sergio Giralt for comments on the concept. This work was also supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center and P30 CA033572 to City of Hope National Medical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Likewise, the views expressed in this article are those of the authors and do not reflect the position of the CIBMTR.
Conflict-of-interest disclosure
Richard J. Lin: no competing financial interests to declare.
Andrew S. Artz: no competing financial interests to declare.
Off-label drug use
Richard J. Lin: nothing to disclose.
Andrew S. Artz: nothing to disclose.