Abstract
Infections are a major cause of morbidity and can result in mortality in long-term survivors after allogeneic hematopoietic cell transplantation. Chronic graft-versus-host disease and delayed immune reconstitution are recognized risk factors. Different strategies must be utilized depending on the individual patient's situation but include prolonged antimicrobial prophylaxis and vaccination. Some important infections due to pathogens preventable by vaccination are pneumococci, influenza, varicella-zoster virus, and SARS-CoV-2. Despite the fact that such recommendations have been in place for decades, implementation of these recommendations has been reported to be poor.
Learning Objectives
Learn about important pathogens causing disease in allogeneic HCT survivors
Increase awareness of the benefits and risks of vaccination of allogeneic HCT survivors
Introduction
Infections are major causes of morbidity and can result in mortality in long-term survivors (more than 1 year) after allogeneic hematopoietic stem cell transplantation (HCT). Table 1 shows some important pathogens in this population. The risk is increased with active chronic graft-versus-host disease (GVHD) due to both incomplete immune reconstitution and ongoing immunosuppressive therapy. Although the risk for nonrelapse mortality has decreased over time,1 in a retrospective analysis infections represented 20.7% of all deaths. Furthermore, infection was the second most frequent cause of death in patients still alive 1 year after HCT.2 Two pandemics during the last 12 years have illustrated the vulnerability of this population. The 2009 H1N1 influenza “swine flu” pandemic and the recent COVID-19 pandemic both caused significant mortality in the allogeneic-HCT population in long-term survivors. It is to be expected that new infections will appear from time to time and that those who have undergone allogeneic HCT might be more vulnerable than the general population. Therefore, awareness and preventive measures are indicated.
Some important pathogens in allogeneic HCT survivors
| Bacteria | Pneumococci |
| Hib | |
| Meningococci | |
| Viruses | Influenza A and B |
| SARS-CoV-2 | |
| Respiratory syncytial virus | |
| Varicella-zoster virus | |
| Hepatitis B, C, and E | |
| Fungi | Pneumocystis jirovecii |
| Endemic mycoses |
| Bacteria | Pneumococci |
| Hib | |
| Meningococci | |
| Viruses | Influenza A and B |
| SARS-CoV-2 | |
| Respiratory syncytial virus | |
| Varicella-zoster virus | |
| Hepatitis B, C, and E | |
| Fungi | Pneumocystis jirovecii |
| Endemic mycoses |
CLINICAL CASE
A 63-year-old man who had undergone an allogeneic unrelated-donor transplantation 3 years previously came to the emergency room with a high fever that had lasted 12 hours. He had a history of moderate chronic GVHD and was still on tacrolimus. His wife reported that during the last hour at home he had become lethargic. At examination he was unconscious, was febrile, and had a blood pressure of 79/50. He was admitted. C-reactive protein was 150 and the white blood cell count was 11.5. A computed-tomography scan was performed but showed no pathology. Blood cultures were taken and a lumbar puncture performed. The latter showed a cell count of 70, prominently consisting of neutrophils. A gram stain showed gram-positive cocci, and he was started on ceftriaxone.
Bacterial infections
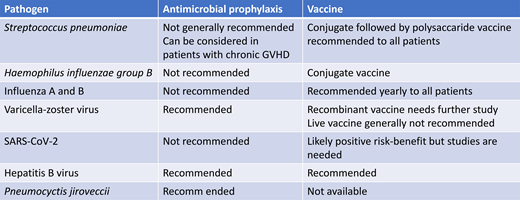
Bacterial infections are the most common type of infection causing mortality in both 1- and 5-year survivors after allogeneic HCT.2 The cause of infections occurring late after transplant is frequently not identified, especially if occurring away from transplant centers, but bacterial infections likely represent many of these “unknown” infections. The incidence of invasive pneumococcal disease (IPD) after allogeneic HCT has been reported to be 80 times that in a healthy population,3 can occur many years after allogeneic HCT, and has a high mortality. The risk is increased in patients with chronic GVHD. Vaccination is an effective way of reducing IPD in both young children and older adults. Other vaccine-preventable bacterial infections that might cause severe late infections are Haemophilus influenzae type B (Hib) and meningococci. Multiresistant bacteria can colonize allogeneic HCT patients, and it has been reported that patients becoming long-term carriers have an increased risk for late mortality.4 Some centers give antibacterial prophylaxis primarily targeting pneumococci to patients with chronic GVHD. No controlled data exist regarding this approach, and what agent to use and its efficacy is likely to depend on the antibacterial resistance situation of pneumococci in the community.
Viral infections
Viral infections have been reported to be the second most common cause of infectious disease mortality in long-term survivors.2 Community-acquired respiratory virus infections are important causes of severe infection occurring years after an allogeneic HCT, especially in patients with delayed immune reconstitution. The most important of these infections until recently have been caused by influenza A and B, parainfluenza virus, and respiratory syncytial virus. However, COVID-19 emerged during the last year and has become a major cause of late mortality after HCT.5,6 Ljungman et al reported from the European Society for Blood and Marrow Transplantation registry a mortality of 28.7% in patients diagnosed with COVID-19 at 1 to 2 years after allogeneic HCT and 20.9% in patients at >2 years after the procedure.6 Sharma et al reported in a Center for International Blood and Marrow Transplant Research analysis a mortality of 15.6% in patients with COVID-19 more than 1 year after HCT.5 It is likely, however, that both these studies have a selection bias in that milder infections in patients without comorbidities have been undiagnosed.
Other important viral infections in long-term survivors are reactivated varicella-zoster virus (VZV) and chronic hepatitis virus infections. Reactivated VZV infection can be severe even during appropriate antiviral prophylaxis, especially if the patient is still receiving immunosuppression.7 Patients infected with hepatitis B virus or who have infected donors should receive prophylaxis a least during the time of ongoing immunosuppression.8 Hepatitis E virus has emerged as a potential cause of liver cirrhosis in patients on chronic immunosuppression, such as solid-organ and allogeneic HCT transplant recipients. Further studies are needed.
Fungal infections
Invasive fungal infections were reported to be the third most frequent cause of infectious disease mortality in 1-year survivors but the second in 5-year survivors after allogeneic HCT.2 Late infections occur with Pneumocystis jirovecii, especially in patients with delayed immune reconstitution, but can also occur with other fungi, particularly in the settings of relapse of the underlying disease or severe chronic GVHD. Pneumocystis prophylaxis is therefore indicated. Ibrutinib has been associated with an increased risk of invasive fungal infection, and a recent small case series of patients treated for chronic GVHD illustrates this risk.9 Ruxolitinib has also been associated with invasive fungal infections. Systemic antifungal prophylaxis should be considered in patients with severe chronic GVHD receiving intensive immunosuppression.
CLINICAL CASE (continued)
The patient was treated with ceftriaxone and improved; however, he had developed hearing loss and some additional neurological deficit. Blood and CSF cultures showed growth of Streptococcus pneumoniae. Questioning his wife provided the information that his physicians had recommended vaccination against pneumococci after the HCT, but it had never been performed.
Vaccines
Table 2 shows current recommendations for vaccination of HCT recipients. These can be broadly divided into common vaccine-preventable infections causing severe disease after allogeneic HCT and vaccines against rare but severe infections when a broad immunity in the population is desirable. Examples of the former are pneumococcal infections, influenza, and COVID-19, while examples of the latter are tetanus and diphtheria. In addition, travel vaccines are indicated for transplant recipients wanting to travel to areas where they might encounter regionally common important pathogens, such as yellow fever or Japanese encephalitis. This review discusses some examples of the 3 situations.
Recommended vaccines after allogeneic HCT
| Vaccine . | Recommendation . | Comments . |
|---|---|---|
| Nonlive vaccines | ||
| Tetanus toxoid + diphtheria toxoid | Yes | Three doses (DT) starting 6 mo after transplantation |
| Inactive influenza | Yes | Seasonal, beginning 4-6 mo after transplantation depending on season |
| Inactivated poliovirus | Yes | Three doses starting 6 (-12) mo after transplantation |
| Conjugated Hib | Yes | Three doses starting 6 (-12) mo after transplantation |
| Pneumococcal conjugate | Yes | Three doses starting 3 (-6) mo after transplantation; booster at 12 mo in patients with chronic GVHD |
| Pneumococcal polysaccharide | Yes | Booster at 12 mo in patients without GVHD no earlier than 8 weeks after conjugate |
| Acellular pertussis | Yes | Children <7 starting 6 (-12) mo after transplantation |
| Hepatitis B virus | Yes | In countries where it is recommended to the general population, starting 6 (-12) mo after transplantation |
| Papillomavirus | Yes | As in the general population, starting earliest 6-12 mo after transplantation; three doses |
| Meningococcal conjugate | Yes | As in the general population, starting 6 mo after transplantation; two doses |
| Recombinant zoster vaccine | Can be considered | Limited data after stem cell transplantation |
| Vaccines against COVID-19 | Yes | Limited data but risk/benefit favors vaccination |
| Live vaccines | ||
| MMR | Individual consideration | Children and seronegative adults, not before 24 mo after HCT; not to be given to patients with GVHD |
| Varicella | Individual consideration | Seronegative patients, not before 24 mo after BMT; not to be given in patients with GVHD |
| Live zoster | Not recommended |
| Vaccine . | Recommendation . | Comments . |
|---|---|---|
| Nonlive vaccines | ||
| Tetanus toxoid + diphtheria toxoid | Yes | Three doses (DT) starting 6 mo after transplantation |
| Inactive influenza | Yes | Seasonal, beginning 4-6 mo after transplantation depending on season |
| Inactivated poliovirus | Yes | Three doses starting 6 (-12) mo after transplantation |
| Conjugated Hib | Yes | Three doses starting 6 (-12) mo after transplantation |
| Pneumococcal conjugate | Yes | Three doses starting 3 (-6) mo after transplantation; booster at 12 mo in patients with chronic GVHD |
| Pneumococcal polysaccharide | Yes | Booster at 12 mo in patients without GVHD no earlier than 8 weeks after conjugate |
| Acellular pertussis | Yes | Children <7 starting 6 (-12) mo after transplantation |
| Hepatitis B virus | Yes | In countries where it is recommended to the general population, starting 6 (-12) mo after transplantation |
| Papillomavirus | Yes | As in the general population, starting earliest 6-12 mo after transplantation; three doses |
| Meningococcal conjugate | Yes | As in the general population, starting 6 mo after transplantation; two doses |
| Recombinant zoster vaccine | Can be considered | Limited data after stem cell transplantation |
| Vaccines against COVID-19 | Yes | Limited data but risk/benefit favors vaccination |
| Live vaccines | ||
| MMR | Individual consideration | Children and seronegative adults, not before 24 mo after HCT; not to be given to patients with GVHD |
| Varicella | Individual consideration | Seronegative patients, not before 24 mo after BMT; not to be given in patients with GVHD |
| Live zoster | Not recommended |
DT, diphtheria and tetanus toxoids; MMR, measles, mumps, and rubella. Adapted from Cordonnier et al.10
An important aspect of vaccinating allogeneic HCT recipients is safety. Currently available data with nonlive vaccines support a high level of safety with side effects similar to those in a normal population. Knowledge about risks with new vaccine platforms such as those used in the recently licensed COVID-19 vaccines is less and will require continued and careful surveillance. Live attenuated vaccines can cause vaccine-induced disease, which can be fatal in severely immunosuppressed individuals. Careful weighing of risk/benefit is therefore needed.
Although international recommendations regarding vaccination have existed for several years,10,11 several reports state that the implementation is poor, as illustrated by the clinical case.12-14 The reasons vary, including compliance, unclear responsibilities, and reluctance to immunize patients with GVHD or ongoing immunosuppression. Data clearly suggest, however, that patients with GVHD should be vaccinated with nonlive vaccines, although it must be recognized that the immune response might be lower.
Pneumococcal vaccines
Pneumococcal polysaccharide vaccine is a poor inducer of an immune response, especially in patients with chronic GVHD. On the other hand, studies with the pneumococcal conjugate vaccines (PCV) showed that they could induce strong and durable immune responses. The current recommendations are to start vaccination with 3 doses of PCV at 3 to 4 months after HCT followed by either a pneumococcal polysaccharide vaccine V23 dose in patients without or a fourth PCV dose in patients with chronic GVHD.10,11 The introduction of PCV in the management strategy has reduced the risk of IPD after allogeneic HCT.15 The long-term retainment of antibodies against pneumococci has been studied after varying vaccine schedules. However, the majority received at least 3 PCV doses, as currently recommended. Fifty percent of patients were protected against all 7 analyzed serotypes at a median of 9.3 years after transplantation while 70% were protected against 5 of 5 serotypes.16 A lack of protective antibodies was associated with chronic GVHD, relapse of underlying malignancy, and cord blood transplantation. This shows that seroprotection ought to be regularly assessed to define the need for booster doses. New conjugated vaccines covering additional serotypes are in development.
Hib
Immunization against Hib is recommended after HCT. However, nontypable Haemophilus influenzae strains has become more common, and there is currently no vaccine against these strains.
Influenza vaccine
Influenza A and B infections can be severe and life-threatening, and fatal infections can occur several years after HCT. Therefore, yearly influenza vaccination with the inactivated vaccine is recommended for all allogeneic HCT recipients starting at 6 months after HCT but could be initiated as early as 4 months after HCT during a community outbreak. Children 6 months to 8 years of age should be given a second dose. The live attenuated influenza vaccine should not be used in HCT recipients. Family members and hospital staff should receive influenza vaccine, thereby reducing the risk for transmission.10,11
Several studies have shown poorer immune serological responses after vaccination in allogeneic HCT recipients, both adults and children, compared to healthy controls. The time after transplantation is the most important factor for vaccine efficacy, with patients vaccinated later responding better. Two-dose vaccine schedules and the use of adjuvanted vaccines have been studied with varying results.
Despite suboptimal serological responses, there might be clinical effectiveness of vaccination since protective antibody levels against severe influenza disease are poorly defined. Kumar et al showed in a prospective cohort study that influenza vaccination during the relevant season reduced the risk of severe diseases such as pneumonia and the need for admission to an intensive care unit.17 Piñana et al showed in a similarly designed cohort study that influenza vaccination reduced the risk of lower-respiratory tract influenza and hospital admissions.18 Thus, there is a substantial clinical benefit to regular influenza vaccination of HCT recipients.
COVID-19 vaccines
COVID-19 is associated with substantial morbidity and mortality after allogeneic HCT. Thus, vaccination is indicated but must be evaluated regarding the risk of possibly inducing GVHD or other immune activation phenomena. Currently, no information is available addressing these issues in allogeneic HCT recipients. Data in patients with hematological malignancies, especially chronic lymphocytic leukemia and multiple myeloma, and after solid- organ transplantation suggest lower rates of response than in healthy controls.19-24 It is currently unclear how to interpret the results of antibody assays in severely immunocompromised individuals such as HCT patients regarding protection against COVID-19. Thus, the continued use of protective measures is recommended.
HPV vaccine
HCT patients are prone to develop papillomavirus-driven complications such as cervical dysplasia. Stratton et al showed that strong immune responses can be elicited by the quadrivalent human papillomavirus (HPV) vaccine without significant side effects.25 It would be logical to vaccinate HPV-seronegative individuals to prevent acquisition of HPV, but whether vaccination of HCT recipients already infected with HPV would result in a clinical benefit is unknown. This could be a topic for future studies.
Varicella vaccine
Primary varicella can be severe after HCT. The existing vaccine is live and attenuated and should not be used soon after HCT. A seronegative patient should, if possible, be immunized before transplantation, providing that enough time can elapse after the vaccination. Vaccination of seronegative family members is recommended. A few uncontrolled studies have reported that varicella vaccination is safe and can result in seroconversion if performed more than 2 years after HCT in patients without chronic GVHD or ongoing immunosuppression.
A high proportion of HCT patients develop herpes zoster that occasionally becomes severe. Both live and nonlive recombinant vaccines exist. Issa et al gave 1 dose of live attenuated zoster vaccine to 58 allogeneic HCT recipients and noted no significant side effects.26 However, fatal infections have been documented after autologous HCT. Due to the proven efficacy and safety of acyclovir prophylaxis, vaccination with the live zoster vaccine is not recommended during at least the first few years after HCT.
The recently licensed recombinant zoster vaccine showed high immunogenicity and decreased the rate of herpes zoster in autologous HCT recipients.27 It has also been studied in allogeneic HCT recipients but not in a controlled trial. Camargo et al demonstrated that 2 doses of recombinant zoster vaccine were less immunogenic in allogeneic HCT recipients compared to autologous recipients and that VZV reactivations occurred.28 Baumrin et al showed that a 2-dose regimen was safe and tolerable, with high rates of mild to moderate local and systemic side effects but without increasing the rates of GVHD; however, VZV reactivations were seen after vaccination.29 Additional studies are therefore needed.
Measles vaccine
The majority of allogeneic HCT patients will become seronegative to measles during extended follow-up. There are documented cases of fatal measles in BMT recipients.30,31 The loss of immunity is more rapid in patients previously vaccinated against measles compared to those having experienced natural infection. Vaccination can only be considered in a patient without chronic GVHD or ongoing immunosuppression. The reported effect of vaccination varies between different studies, with a seemingly higher response rate in adults than in children.
Travel vaccines
Vaccines are available for HCT patients living in certain areas of the world or traveling to areas where certain infections are endemic. Nonlive vaccines include those against tick-borne encephalitis32,33 and Japanese encephalitis.34 The response to hepatitis A vaccine was shown to be poor in both previously seronegative and seropositive individuals.35 Yellow fever is endemic in some areas of the world, and the only vaccine is live attenuated. In a retrospective French multicenter study, 21 patients who received yellow fever vaccination a median of 39 months after HCT demonstrated a high response rate without documented side effects.36 For patients who live in or must visit areas where yellow fever is endemic, immunization could be considered at least 2 years after HCT and if the patient is without GVHD or ongoing immunosuppression.
Conflict-of-interest disclosure
Per Ljungman: research funding, consultancy, lecturing: Pfizer.
Off-label drug use
Per Ljungman: nothing to disclose.