Abstract
Although much less common than deep vein thrombosis of the lower extremities or lungs, clots in unusual locations, including the splanchnic, cerebral, retinal, upper-extremity, and renal locations, present with significant morbidity and mortality. In the last 2 decades, treatment of clots in these unusual locations is primarily managed medically, with interventional and surgical approaches reserved for more severe or refractory cases. The hematologist is well positioned to provide consultation to organ-specific specialties (ie, neurosurgery, hepatology, ophthalmology), especially because acquired and congenital hypercoagulability plays a major role, and anticoagulation is often the primary treatment. Historically, treatment has been based on expert opinion, but systematic reviews and meta-analyses have recently been published. Various societies have produced guidelines for the treatment of clots in unusual locations; however, randomized clinical trial data remain scarce. In the last few years, increasing data have emerged concerning the efficacy of the direct oral anticoagulants in treating clots in unusual locations. Cases have recently been described highlighting atypical thrombosis associated with COVID-19 infection as well as with the ChAdOx1 nCoV-19 (AstraZeneca) vaccine and Johnson and Johnson's Janssen Ad26.COV2.S vaccine. This article reviews clots in unusual locations with an emphasis on the splanchnic (mesenteric, portal, splenic, hepatic) and cerebral circulation. Through a case-based approach, key questions are posed, and data are presented to help guide diagnosis and treatment.
Learning Objectives
Diagnose and treat clots in the splanchnic and cerebral veins despite (1) heterogeneous clinical presentations, (2) limited high-level evidence, and (3) concomitant bleeding and clotting risks
Work within a team consisting of organ-specialized physicians as well as hematologists
Introduction
Although different definitions exist, clots in unusual sites typically refer to any location outside of the lower extremities and pulmonary arteries.1,2 Unlike the treatment of deep vein thrombosis (DVT) and pulmonary embolism, thrombosis at unusual sites is much less common, and treatment is typically guided by expert opinion based on observational studies or small randomized trials.1,3 Despite the rarity of these conditions, they are detected with increasing frequency due to modern radiographic imaging, leading to earlier diagnosis and allowing the earlier institution of effective therapy.1
Often, these unique thromboses are provoked by a pathologic condition in the organ supplied by the specific venous segments (eg, splenic vein thrombosis in a patient with pancreatitis; portal vein thrombosis (PVT) in a patient with cirrhosis).2 Frequently, these events are associated with acquired and/or congenital hypercoagulable disorders.2,4 Different thrombotic manifestations can result from the various acquired and inherited conditions (eg, myeloproliferative diseases in splanchnic vein thromboses [SVT] and estrogens in cerebral vein thrombosis [CVT]) possibly due to complex interactions with the local vessel wall, altering hemostatic balance and predisposing to thrombosis.4 Familiarity with patients with hypercoagulable disorders allows the hematologist to maintain a high index of suspicion of these rare conditions and remain an integral part of diagnosis and treatment.
During the prior year, clots in unusual locations such as the splanchnic, cerebral, and retinal circulation have been described in patients infected with COVID-19 (Table 1).5,6 In addition, a subset of vaccine-related splanchnic vein and cerebral vein clots (termed vaccine-induced thrombocytopenia with thrombosis or thrombotic thrombocytopenic syndrome) that resemble autoimmune heparin-induced thrombocytopenia with thrombosis has been described by Greinacher.7 In this case series, 9 patients developed CVT, and 3 patients developed SVT after vaccination with the ChAdOx1 nCoV-19 (AstraZeneca) vaccine.7 Thrombosis in the cerebral and splanchnic circulation has also been described after Johnson and Johnson's Janssen Ad26.COV2.S vaccine.8 At the time of this writing, it is recommended that heparin anticoagulation in these patients be avoided until an antiheparin platelet factor 4 antibody or functional assay returns negative.7 In addition, the International Society on Thrombosis and Haemostasis (ISTH) recently published guidelines suggesting the use of intravenous immunoglobulin in addition to nonheparin anticoagulation in confirmed cases.9
COVID-19 vaccination and clots in unusual locations: key points
| • The majority of thrombotic events associated with COVID-19 are DVT and pulmonary embolism |
| • An increasing number of reports of splanchnic and CVT have been reported in association with COVID-19 infection |
| • Although the incidence of postvaccination atypical thrombosis remains quite low, 9 cases of CVT and 3 cases of SVT have been reported after vaccination with the ChAdOx1 nCoV-19 (AstraZeneca) vaccine as well as 6 cases of CVT and 2 of concomitant SVT after Johnson & Johnson's Janssen Ad26.COV2.S vaccine |
| ○ The literature surrounding the incidence, risk, and pathophysiology continues to evolve even as this article was written |
| ○ These cases have been associated with thrombocytopenia resembling autoimmune heparin-induced thrombocytopenia |
| ○ The ISTH has developed treatment guidelines that include nonheparin anticoagulants as well as IVIG in confirmed cases |
| • Most patients had no obvious underlying risk factors or known thrombophilia |
| • Most patients were treated with anticoagulant therapy |
| • Mortality was high |
| • COVID-19 testing should be considered as well as ascertainment of vaccine status, product, and timing in patients who present with unexplained splanchnic or CVT |
| • The majority of thrombotic events associated with COVID-19 are DVT and pulmonary embolism |
| • An increasing number of reports of splanchnic and CVT have been reported in association with COVID-19 infection |
| • Although the incidence of postvaccination atypical thrombosis remains quite low, 9 cases of CVT and 3 cases of SVT have been reported after vaccination with the ChAdOx1 nCoV-19 (AstraZeneca) vaccine as well as 6 cases of CVT and 2 of concomitant SVT after Johnson & Johnson's Janssen Ad26.COV2.S vaccine |
| ○ The literature surrounding the incidence, risk, and pathophysiology continues to evolve even as this article was written |
| ○ These cases have been associated with thrombocytopenia resembling autoimmune heparin-induced thrombocytopenia |
| ○ The ISTH has developed treatment guidelines that include nonheparin anticoagulants as well as IVIG in confirmed cases |
| • Most patients had no obvious underlying risk factors or known thrombophilia |
| • Most patients were treated with anticoagulant therapy |
| • Mortality was high |
| • COVID-19 testing should be considered as well as ascertainment of vaccine status, product, and timing in patients who present with unexplained splanchnic or CVT |
IVIG, intravenous immunoglobulin.
The treatment of clots in unusual locations typically consists of anticoagulation and less commonly fibrinolytic agents, with interventional and surgical techniques playing a secondary role and often reserved for progressive organ dysfunction or infarction (eg, mesenteric vein thrombosis).1,10,11 Data on the efficacy of the direct oral anticoagulants (DOACs) compared to warfarin will be covered in greater detail in the evidence-based minireview Should Warfarin or a DOAC Be Used in Patients Presenting With Thrombosis in the Splanchnic or Cerebral Veins?
In the remainder of this article, we focus on a case-based review of thrombosis in the splanchnic and cerebral veins. Further comprehensive review of these topics as well as clots in other unusual locations can be found in several excellent reviews.1-4
SVT
SVT refers to venous thromboembolism of the portal vein (PV), mesenteric, splenic, or hepatic veins. Hepatic vein thrombosis (HVT) is typically referred to as Budd-Chiari syndrome (BCS) and includes venous outflow obstruction anywhere from the level of the hepatic venules proximally to the junction of the inferior vena cava and right atrium.12 Although the incidence of SVT is at least 25 times less than lower-extremity DVT, the presentation is often much more dramatic.1-4
Thrombosis in the splanchnic circulation is often due to a local risk factor such as liver cirrhosis, solid tumor, or pancreatitis, typically in combination with transient risk factors such as surgery or local inflammation.13 Myeloproliferative neoplasms are the most common systemic risk factor, occurring in 40% of HVT and 31% of nonmalignant, noncirrhotic PVT.14 Next-generation sequencing has also been recently used and has identified a novel JAK-2-exon 12 mutation in one-third of triple-negative patients with idiopathic or exclusively noncirrhotic SVT.15 Inherited thrombophilic disorders are recognized risk factors reported in multiple systemic reviews, but the incidence and penetrance differ between the various mutations and the various sites in the splanchnic circulation.3 SVT is considered unprovoked in 15% to 27% of cases and diagnosed incidentally in up to one-third of patients.3
CLINICAL CASE 1
A 45-year-old man with alcoholic cirrhosis noted worsening abdominal distention. Ultrasound revealed a cirrhotic liver with new thrombosis of the PV as well as new ascites. The platelet count was stable at 49 000/µL, and prothrombin time (PT) was 19 seconds.
Question
What is the role of anticoagulation in PVT?
Data
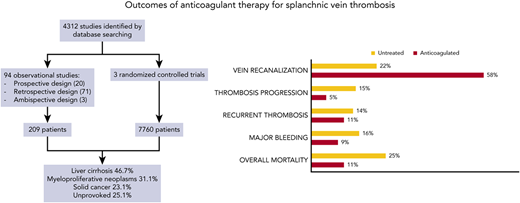
When deciding on proper treatment for a patient with PVT, it is important to consider whether the thrombosis is acute or chronic and whether the liver is cirrhotic or not.16 The goals of anticoagulation in PVT are to increase the chance of recanalization in order to improve hepatic blood flow and decrease variceal formation and subsequent bleeding.13,17 A recent meta-analysis by Valeriani and colleagues consisted of 7969 SVT patients in 97 studies (54% of whom had PVT) and documented a recanalization rate of 58% vs 22%, a progression rate of 11% vs 15%, and major bleeding in 9% vs 16% in those receiving vs not receiving anticoagulation (Figure 1).13 Compared to no treatment, anticoagulation was associated with higher recanalization (relative risk [RR], 2.29; 95% CI, 1.66-3.44), lower progression (RR, 0.24; 95% CI, 0.13-0.42), major bleeding (RR, 0.73; 95% CI, 0.58-0.92), and overall mortality (RR, 0.45; 95% CI, 0.33-0.60). The conclusion was that anticoagulation for SVT thrombosis reduced the risk of thrombus progression and improved the rate of recanalization without increasing bleeding risk.13 Two systemic reviews and meta-analyses showed the benefit of anticoagulation in cirrhotic patients with SVT.17,18 The first by Valeriani reported decreased RRs of bleeding (RR, 0.52; 95% CI, 1.42-7.17) and mortality (RR, 0.42; 95% CI, 0.24-0.73) and higher recanalization rates (RR, 3.19; 95% CI, 1.42-7.17) in the anticoagulated group.17 The second by Ghazaleh also showed that anticoagulation increased the rate of PV recanalization and decreased the rate of variceal bleeding in cirrhotic patients with nonmalignant PV thrombosis.18 Prophylactic banding should be instituted in cirrhotic patients to decrease variceal bleeding.3
Outcomes of anticoagulant therapy for splanchnic vein thrombosis, as listed on the top.13
Outcomes of anticoagulant therapy for splanchnic vein thrombosis, as listed on the top.13
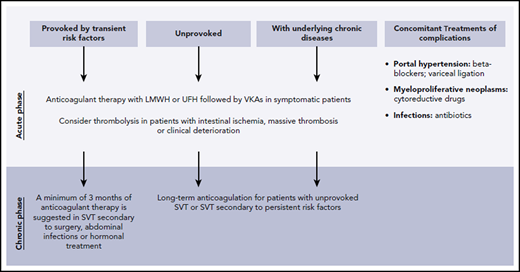
An ISTH subcommittee introduced recent guidelines recommending early anticoagulation in cirrhotic and noncirrhotic patients with acute SVT with no active bleeding or contraindications for at least 3 to 6 months.11 In patients with chronic thrombosis, an individualized care plan is recommended, while treatment similar to symptomatic acute thrombosis is recommended for incidentally detected SVT.11 The 2017 guidelines by the European Association for the Study of the Liver recommend anticoagulation for all patients with nonmalignant, noncirrhotic PVT for at least 6 months, while the 2012 American College of Chest Physicians guidelines recommend only treating symptomatic SVT and not anticoagulating those incidentally detected unless the SVT is extensive or associated with malignancy.2,19,20 However, more recent observational studies and guidelines have challenged the American College of Chest Physicians recommendations.2,19 While still controversial and based primarily on expert opinion, some experts recommend decreasing the intensity of anticoagulation to half-therapeutic doses in cirrhotic patients if the platelet counts are between 50 000 and 100 000/µL to prophylactic doses between 30 000 and 50 000/µL and withholding anticoagulation if the platelet count is below 30 000/µL.2 In patients with underlying coagulopathy, low-molecular-weight heparin (LMWH) may be preferred to warfarin, given the inability to adequately follow the effects of warfarin with PT monitoring. A recent systematic review suggested that thrombolysis is effective and safe for PVT in noncirrhotic patients who have failed anticoagulation.21 Figure 2 depicts general treatment recommendations for SVT.2
CLINICAL CASE 1 (continued)
Consistent with the ISTH guidelines referenced above, the decision was made to start anticoagulation given the new, symptomatic PV clot. We discussed the options of LWMH, warfarin, and DOACs with the patient. He opted for daily enoxaparin (Lovenox) injections. Given the platelet count of 49 000/µL and a PT of 19 seconds, the decision was made in conjunction with the patient and hepatology team to decrease the enoxaparin dose to 1 mg/kg/d. He underwent successful liver transplantation 4 months later, and anticoagulation was stopped after his postoperative recovery.
CLINICAL CASE 2
A 43-year-old woman who has no prior medical history presented with 2 weeks of progressive jaundice, abdominal pain, and a distended abdomen. She took no medications and did not smoke or consume alcohol. Her family history was unknown. Physical examination was significant for scleral icterus and jaundice. The abdomen was distended, the liver edge was palpated 3 cm below the costal margin, and a fluid wave was detected. There was mild ankle edema, but the rest of the examination was unremarkable. Hemoglobin was 16.7 g/dL with an MCV of 76 fL and a platelet count of 608 000/µL. Imaging confirmed occlusive thrombosis of the main hepatic vein.
Questions
How common is the JAK2 V617F mutation in BCS? Is anticoagulant therapy, local intervention, or both preferred in newly diagnosed BCS?
Data
BCS occurs due to obstruction of the hepatic venous outflow anywhere between the liver and the heart.12 An underlying prothrombotic state is identified in 88% of BCS patients.13 A myeloproliferative neoplasm (MPN) has been reported in up to 62% of cases of idiopathic BCS, with polycythemia vera being the most common subtype (18%-43%). A JAK2 V617F mutation is found in 26% to 52% of patients.2 The diagnosis of BCS is the presenting symptom of an MPN in 74% of cases, with many patients having normal blood counts at the time.2 Calreticulin mutations have been reported in 2.9% of BCS patients, in the absence of the JAK2 V617F mutation.12 The MPL mutation has been reported in only a few cases.12 Any patient who presents with idiopathic BCS should be evaluated with an MPN genetic panel consisting of at least the above mutations.12 Antiphospholipid antibody syndrome and paroxysmal nocturnal hemoglobinuria are also overrepresented in patients with BCS and should be considered if an MPN panel is negative.12
The management of BCS, compared to other SVTs, is unique as treatment generally consists of both short- and long-term anticoagulation as well as decompression of the hepatic venous outflow track (Figure 3). Lifelong anticoagulation is typically recommended for BCS, although based mostly on expert opinion with no randomized controlled trials having been reported.12,19 LMWH or unfractionated heparin is typically begun, and endoscopy is performed to evaluate for and treat varices.12 Historically, long-term anticoagulation has been with warfarin.
Endovascular intervention consists of angioplasty, stenting, and thrombolysis aimed at opening up the hepatic outflow tract and preserving hepatic function. Transvenous angioplasty is currently performed in a majority of BCS patients.12,22 Stent placement should be pursued if there is inadequate pressure reduction with angioplasty, based on superior results in a recent randomized clinical trial.12,23 If this is not effective or feasible, transjugular intrahepatic portosystemic shunt is usually performed to reduce portal pressure.3 Surgical shunting, while the historical treatment of choice, has mostly given way to endovascular intervention and anticoagulation.1,3 Using the stepwise approach, 1-year and 5-year survival have improved to 96% and 89%, respectively.11 Liver transplantation has been performed in patients with acute or progressive liver failure.12
CLINICAL CASE 2 (continued)
The patient was immediately placed on therapeutic unfractionated heparin, and angioplasty with stenting of the main hepatic vein was performed within 48 hours with excellent results. JAK-2 V617F testing subsequently returned positive, and she started therapeutic phlebotomy as well as hydroxyurea. After a discussion about oral anticoagulant options, the patient decided to be treated with apixaban 5 mg twice a day. Six months later her hemoglobin and platelet count remained normal, and there was no recurrent thrombosis on a regimen of periodic phlebotomy, hydroxyurea, and apixaban.
CLINICAL CASE 3
A 50-year-old woman with a prior history of a left popliteal DVT developed severe midabdominal pain, cramping, and fever. The pain progressed over the next few days, and she presented to her local emergency room. A complete blood count showed a mild leukocytosis with a left shift. Lactic acid was moderately elevated. A CT venogram (CTV) showed thrombosis of the superior mesenteric vein with bowel wall edema throughout the jejunum and proximal ileum.
Question
Should MVT be managed medically, surgically, or both?
MVT is a rare cause of mesenteric ischemia, representing only 5% to 20% of cases.1,4,24 With improved imaging techniques, including contrasted CTV or magnetic resonance imaging (MRI), the diagnosis can typically be made radiographically instead of surgically.1 Guidelines published by the European Association for the Study of the Liver recommend treatment with LMWH or, preferably, intravenous heparin, as early laparotomy may be required.3,4,24 A recent systematic review of 11 published studies recommended early and immediate anticoagulation.24
CLINICAL CASE 3 (continued)
Despite therapeutic anticoagulation, intravenous fluids, bowel rest, and antibiotics, she became hypotensive and lactic acid levels increased over the next 12 hours. The decision was made to proceed with exploratory laparotomy due to concern for peritonitis and necrotic bowel.
Endovascular therapy (EVT) should be limited to centers with expertise in the procedure.24 In the aforementioned systematic review, EVT was only used at 2 centers, and the bowel resection rate remained high at 65% and 78%, respectively.24-26 However, in an additional study 5 out of 8 patients who underwent EVT avoided surgical resection.27
Surgical exploration is often required when bowel ischemia is suspected. However, the distinction between reversible and irreversible bowel ischemia is difficult.24 Often a primary laparotomy is performed, but only frankly necrotic bowel should be resected.24 The abdomen is typically left open, and a second-look operation is performed at a later date.24
CLINICAL CASE 3 (continued)
During the initial surgical exploration, 5 cm of necrotic jejunum was resected. Upon transection of the bowel wall, multiple “wormlike” clots were extruded. Anticoagulation was restarted and she clinically improved and did not require additional resection. A thrombophilia workup including JAK-2 V617F testing was undertaken and returned significant for high-titer anticardiolipin and beta-2-glycoprotein antibodies. Long-term anticoagulation with warfarin was initiated.
CVT
CVT refers to thromboses of the cerebral veins or dural venous sinuses. CVT is rare, with an approximate incidence of 13.2 to 15.7 per million persons per year. CVT is more common in women than men (3:1) and in younger age groups (mean age of 40).3
Clinical manifestations of CVT include neurological symptoms ranging from headaches, papilledema, focal deficits, seizures, encephalopathy, and coma.28 As with other atypical sites of thrombosis, risk factors for CVT can be divided into local, mechanical, and systemic factors (Table 2).
Risk factors for CVT
| Local risk factors (8%-12%) . | Mechanical causes (2%-5%) . | CNS disorders (2%) . | Systemic risk factors . |
|---|---|---|---|
| Infections involving the ears, sinuses, mouth, face, neck, CNS | Trauma, lumbar puncture, jugular vein catheterization, neurosurgical interventions | Arteriovenous malformation, dural fistulae, CNS malignancies | Pregnancy/puerperium (10%-17% women), hormonal treatment (50%-53% women), thrombophilia (approx. 33%), myeloproliferative neoplasms (3%-4%), COVID-19 infection, COVID-19 vaccines (AstraZeneca, J&J) |
| Local risk factors (8%-12%) . | Mechanical causes (2%-5%) . | CNS disorders (2%) . | Systemic risk factors . |
|---|---|---|---|
| Infections involving the ears, sinuses, mouth, face, neck, CNS | Trauma, lumbar puncture, jugular vein catheterization, neurosurgical interventions | Arteriovenous malformation, dural fistulae, CNS malignancies | Pregnancy/puerperium (10%-17% women), hormonal treatment (50%-53% women), thrombophilia (approx. 33%), myeloproliferative neoplasms (3%-4%), COVID-19 infection, COVID-19 vaccines (AstraZeneca, J&J) |
Adapted from Riva et al.4
CNS, central nervous system.
CVT is confirmed by neuroimaging. Most patients are initially evaluated with a noncontrast CT to quickly evaluate for intracranial hemorrhage as well as other pathologies. However, unenhanced CT scans lack the sensitivity to detect the majority of CVT. A contrast-enhanced CT increases sensitivity to 88% to 99%, and MRI remains the gold standard for diagnosis.29 CTVs may be useful in patients who have a contraindication to MRI.3
CLINICAL CASE 4
A 23-year-old woman presented to the emergency room with worsening headache over the past week. She had no significant past medical history. Her only medication was a combined oral contraceptive pill. A computed tomography (CT) scan of the head without contrast showed a “dense triangle sign.” While in the emergency room, she suffered a tonic-clonic seizure. MRI of the brain showed thrombosis involving the superior sagittal sinus with an associated small area of intracranial hemorrhage.
Question
What is the initial treatment of acute-phase CVT?
Many cases of CVT are accompanied by intracerebral hemorrhage, making treatment complicated given the concern for worsening hemorrhage. Two randomized trials examined the benefit of parenteral anticoagulation compared to placebo in the treatment of acute-phase CVT. In the first trial, published in 1991, patients were randomized to intravenous heparin treatment (n = 10) vs placebo (n = 10). The heparin group had improved recovery and better long-term outcomes.30 The second trial consisted of 60 patients randomized to nadroparin vs placebo. Better outcomes in the nadroparin arm were noted, although they did not reach statistical significance.31 Although sample sizes were small, these trials demonstrated that anticoagulation with heparin or LMWH is better than no treatment in acute CVT. Approximately 43% of these patients had intracranial hemorrhage at diagnosis, and none of the patients randomized to anticoagulation developed a new intracranial hemorrhage, whereas 3 patients in the placebo group experienced a new hemorrhage.30,31 In the International Study on Cerebral Vein and Dural Sinus Thrombosis, a trend toward lower rates of death or dependency in the anticoagulated group (12.7% vs 18.3%; hazard ratio, 0.73; CI, 0.44-1.21) was seen.32 A subsequent systematic review and meta-analysis showed a trend toward decreased mortality and neurological outcomes with LMWH compared to UFH.33 Bleeding events were similar in both groups.33 Based on these studies, the most recent guidelines from the European Stroke Organization (2017), the American Heart Association/American Stroke Association (2014), and the American Academy of Chest Physicians (2012) recommend either UFH or LMWH for treatment of acute CVT even in cases with associated cerebral hemorrhage.27,34,35
CLINICAL CASE 4 (continued)
The patient was started on an antiepileptic and intravenous heparin; however, she developed worsening mental status requiring mechanical intubation. Repeat imaging did not show new intracranial hemorrhage. The neurointerventional radiology team was consulted to evaluate for thrombectomy.
Question
What is the role of endovascular interventions in the treatment of CVT?
The evidence thus far suggests no added benefit from endovascular thrombolysis or thrombectomy. Data primarily come from systematic reviews that show higher fatality rates, more bleeding (mostly intracranial), and poor neurological outcomes with thrombolysis (Table 3). A randomized controlled trial (TO-ACT) examined the benefit of thrombolysis in addition to anticoagulation and was stopped prematurely for futility.36 Given the lack of high-quality evidence suggesting benefit, the consensus is to avoid up-front EVT except in select cases with high pretreatment risk of poor outcomes.
Endovascular therapy in acute CVT
| Trial . | Study design . | No of patients . | Intervention . | Results . | Limitations . |
|---|---|---|---|---|---|
| Coutinho et al36 (TO-ACT trial) | Randomized, controlled | 67 | Endovascular treatment in patients Inclusion criteria: ≥1 Risk factor for clinical deterioration (coma, mental status changes, CVT in deep venous system, intracerebral hemorrhage) | Stopped prematurely for futility | Small sample size |
| Dentali et al37 | Systematic review of 15 studies | 156 | Mechanical thrombectomy/thrombolysis | Death rate 9% Major bleeding 10% with local thrombolysis 8% intracranial hemorrhage 58% fatal | |
| Siddiqui et al40 | Systematic review of 42 studies | 185 | Mechanical thrombectomy/thrombolysis (71%) 60% pretreatment intracranial hemorrhage 47% stupor, coma | 84% good outcome 12% mortality 10% new/worsened intracerebral hemorrhage 95% recanalization rate | Concern for bias as the studies were not blinded |
| Trial . | Study design . | No of patients . | Intervention . | Results . | Limitations . |
|---|---|---|---|---|---|
| Coutinho et al36 (TO-ACT trial) | Randomized, controlled | 67 | Endovascular treatment in patients Inclusion criteria: ≥1 Risk factor for clinical deterioration (coma, mental status changes, CVT in deep venous system, intracerebral hemorrhage) | Stopped prematurely for futility | Small sample size |
| Dentali et al37 | Systematic review of 15 studies | 156 | Mechanical thrombectomy/thrombolysis | Death rate 9% Major bleeding 10% with local thrombolysis 8% intracranial hemorrhage 58% fatal | |
| Siddiqui et al40 | Systematic review of 42 studies | 185 | Mechanical thrombectomy/thrombolysis (71%) 60% pretreatment intracranial hemorrhage 47% stupor, coma | 84% good outcome 12% mortality 10% new/worsened intracerebral hemorrhage 95% recanalization rate | Concern for bias as the studies were not blinded |
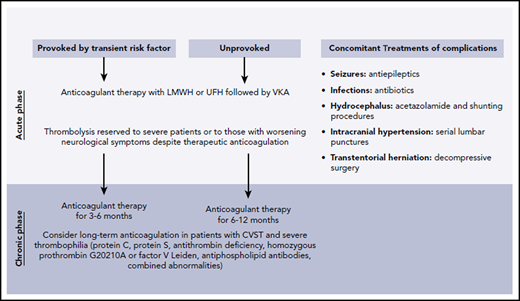
CVT can lead to other complications, including seizures, elevated intracranial pressure, brain herniation, and infections, requiring additional supportive measures (Figure 4).
CLINICAL CASE 4 (continued)
In the following days, she slowly recovered and was extubated, with a plan to discharge her to a rehabilitation facility.
Question
What is the best long-term treatment for CVT?
The Cerebral Vein Thrombosis International Study reported on 706 patients with CVT. Of these, 85% were treated with heparin in the acute phase followed by 84% with warfarin for a median duration of 12 months. At a median follow-up of 40 months, 89.1% of patients were found to have completely recovered.37 In the VENOST study, 67% of 1144 CVT patients were treated with warfarin after initial treatment with heparin. At 1-year follow-up, 93.1% of 691 patients for whom data were available showed a complete recovery.38 Most society guidelines recommend the use of warfarin, although emerging data show that DOACs may have similar efficacy and safety as compared to warfarin. This topic will be covered in greater detail in the accompanying evidence-based minireview Should Warfarin or a DOAC Be Used in Patients Presenting With Thrombosis in the Splanchnic or Cerebral Veins?
There is no definitive evidence regarding the optimal duration of anticoagulation in the treatment of CVT. Extrapolating from evidence from lower-extremity DVT, most experts recommend 3 to 12 months of anticoagulation. If CVT occurred in the setting of a transient risk factor, 3 to 6 months of anticoagulation may be adequate. If there were no provoking factors, we treat for at least 6 to 12 months. In cases in which persistent hypercoagulability and a risk for thrombosis have been identified, long-term anticoagulation should be considered. Similarly, a longer duration of anticoagulation is suggested for the treatment of recurrent CVT. The EXCOA-CVT study is an ongoing prospective study that aims to compare the outcomes of short-duration (3-6 months) vs long-term (12 months) anticoagulation treatment in CVT patients.39
Conflict-of-interest disclosure
Carol Mathew: no competing financial interests to declare.
Marc Zumberg: member: American Board of Internal Medicine Hematology Board; American Society of Hematology speaker reimbursement, law case review.
Off-label drug use
Carol Mathew: direct oral anticoagulants for splanchnic and cerebral vein thrombosis are discussed.
Marc Zumberg: direct oral anticoagulants for splanchnic and cerebral vein thrombosis are discussed.