Abstract
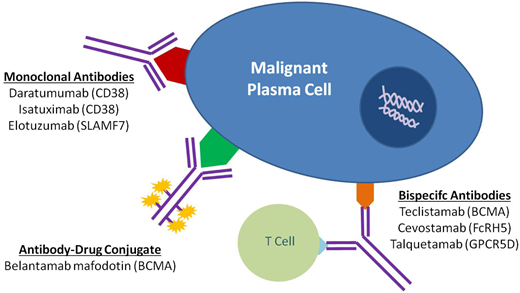
The therapeutic landscape in multiple myeloma (MM) has changed dramatically over the last 2 decades. With the introduction of novel immunotherapies, patients with MM can expect deeper responses, longer remissions, and improved overall survival. Since its approval by the US Food and Drug Administration in 2015, the monoclonal antibody specific for CD38, daratumumab, has been incorporated into both frontline and relapsed treatment regimens. Its role as a maintenance therapy is currently being explored. Subsequently, a variety of novel antibody therapeutics have evolved from the success of daratumumab, using similar concepts to target the malignant plasma cell clone. Noteworthy naked monoclonal antibodies include isatuximab, another agent directed against CD38, and elotuzumab, an agent directed against SLAM family member 7. Antibody-drug conjugates, complex molecules composed of an antibody tethered to a cytotoxic drug, target malignant cells and deliver a lethal payload. The first to market is belantamab mafodotin, which targets B-cell maturation antigen (BCMA) on malignant plasma cells and delivers a potent microtubule inhibitor, monomethyl auristatin F. Additionally, bispecific T-cell antibodies are in development that engage the immune system directly by simultaneously binding CD3 on T cells and a target epitope—such as BCMA, G-protein coupled receptor family C group 5 member D (GPRC5d), and Fc receptor homologue 5 (FcRH5)—on malignant cells. Currently, teclistamab, an anti-BCMA bispecific, is closest to approval for commercial use. In this review, we explore the evolving landscape of antibodies in the treatment of MM, including their role in frontline and relapse settings.
Learning Objectives
Review the use of MoAbs in NDMM (transplant eligible and ineligible) and RRMM
Understand the evolving role of ADCs in RRMM
Explore the current landscape and future directions of BsAbs in RRMM
CLINICAL CASE
A 54-year-old woman is diagnosed with immunoglobulin G kappa (IgGκ) multiple myeloma (MM). She undergoes induction therapy with triplet combination, followed by autologous stem cell transplant (ASCT) and lenalidomide maintenance. She experiences several relapses and is cycled through various lines of therapy. She has now had 4 lines of therapy and is considered triple-class refractory (TCR), having become refractory to a proteosome inhibitor, an immunomodulatory drug, and an anti-CD38 monoclonal antibody (MoAb). Her treating physician offers to send her to an academic center for a clinical trial, but she opts to stay close to home and is started on belantamab mafodotin.
Introduction
Despite decades of scientific advancement, MM remains incurable in the vast majority of patients.1 Currently, the primary goal of treatment is to increase survival and maintain a reasonable quality of life. This is accomplished through therapeutic suppression of the malignant plasma cell (PC) clone, which subsequently mitigates disease-related complications.1
Since their approval, MoAbs have been widely incorporated into both frontline and relapsed settings. Still, patients who become TCR remain challenging to treat and require therapies with novel mechanisms of action. In the recent LocoMMotion study, TCR MM patients who prospectively received widely available standard of care (SOC) therapies demonstrated an overall response rate (ORR) of 25%, a median duration of response (DOR) of 4.5 months, and overall survival (OS) of 11.1 months.2 To address this area of unmet need, the focus has shifted toward new approaches, such as chimeric antigen receptor (CAR) T-cell therapies, antibody-drug conjugates (ADCs), and bispecific antibodies (BsAbs).3 Although CAR T-cell therapy has shown remarkable overall efficacy of greater than 70% across various early-phase studies—leading to the approvals of idecabtagene vicleucel and ciltacabtagene autoleucel—delays in drug administration due to manufacturing time and grade 3/4 adverse events (AEs) from cytokine release syndrome (CRS), neurotoxicity, and cytopenias remain major concerns.3 The desire for safe “off-the-shelf” immunotherapies has strengthened interest in ADCs and BsAbs, which themselves have demonstrated promising efficacy in relapsed/refractory MM (RRMM).3
In this review we present the state of development of each class of antibody-directed therapy in MM, with an emphasis on the rapidly advancing ADC and BsAb therapeutic armamentarium.
Monoclonal antibodies
In the last decade, three MoAbs were approved for the treatment of MM. Daratumumab (anti-CD38) and elotuzumab (anti-SLAM family member 7 [SLAMF7]) were approved for commercial use in 2015 while isatuximab (anti-CD38) was approved in 2020.
Daratumumab is a humanized IgG1κ MoAb that binds to CD38 expressed on malignant PCs.4 It effectively eliminates CD38- expressing PCs through several mechanisms: antibody-dependent T-cellular cytotoxicity, antibody-dependent T-cellular phagocytosis, complement-dependent cytotoxicity, induction of apoptosis via Fcγ receptor–mediated cross-linking, and by various immunomodulatory effects.4 Several randomized controlled trials have observed the clinical benefits of adding daratumamab to immunomodulator drugs and proteosome inhibitors in RRMM, establishing its use as an SOC agent in this setting (Table 1).5–8
Pivotal randomized phase 3 trials of monoclonal antibody treatment for MM
| Clinical trial . | Patient population . | Arm (n = number of patients) . | Overall response (%) . | Median progression-free survival (months) . | Hazard ratio (P value) . |
|---|---|---|---|---|---|
| ALCYONE12 | Transplant-ineligible NDMM | D-VMP (n = 350) | 91 | 36.4 | 0.42 (P < .001) |
| VMP (n = 356) | 74 | 19.3 | |||
| MAIA11 | Transplant-ineligible NDMM | D-Rd (n = 368) | 92.9 | NR | 0.53 (P < .001) |
| Rd (n = 369) | 81.6 | 34.4 | |||
| ELOQUENT 124 | Transplant-ineligible NDMM | E-Rd (n = 374) | 83 | 31.4 | 0.93 (P = .44) |
| Rd (n = 374) | 79 | 29.5 | |||
| CASSIOPEIA9 | Transplant-eligible NDMM | D-VTd (n = 543) | 93 | NR | 0.47 (P < .001) |
| VTd (n = 542) | 90 | NR | |||
| GRIFFIN10,a | Transplant-eligible NDMM | D-RVd (n = 99) | 99 | NR | Not available |
| RVd (n = 97) | 92 | NR | |||
| GMMG-HD623 | Transplant-eligible NDMM | E-RVd (n = 555) | B1 81.5%; B2 80.7% (≥VGPR) | B1 66.2%; B2 67.2% (3 y PFS) | Not available (P = .86; no significant difference) |
| RVd (n = 559) | A1 78.9%; A2 78.2% (≥VGPR) | A1 68.8%; A2 68.5% (3 y PFS) | |||
| GMMG-HD719 | Transplant-eligible NDMM | I-RVd (n = 329) | MRD 50.1% | NR | Not available (OR = 1.82; P < .001) |
| RVd (n = 331) | MRD 35.6% | NR | |||
| POLLUX6 | RRMM, at least 1 prior line | D-Rd (n = 286) | 93 | 44.5 | 0.44 (P < .001) |
| Rd (n = 283) | 76 | 17.5 | |||
| ELOQUENT 220 | RRMM, 1-3 prior lines | E-Rd (n = 321) | 79 | 19.4 | 0.71 (P < .001) |
| Rd (n = 325) | 66 | 14.9 | |||
| CANDOR8 | RRMM, 1-3 prior lines | D-Kd (n = 312) | 84 | NR | 0.62 (P = .003) |
| Kd (n = 154) | 75 | 15.8 | |||
| IKEMA18 | RRMM, 1-3 prior lines | I-Kd (n = 179) | 87 | NR | 0.53 (P < .001) |
| Kd (n = 123) | 83 | 19.2 | |||
| CASTOR5 | RRMM, at least 1 prior line | D-Vd (n = 251) | 83 | 16.7 | 0.31 (P < .001) |
| Vd (n = 247) | 63 | 7.1 | |||
| ELOQUENT 321 | RRMM, at least 2 prior lines | E-Pd (n = 60) | 53 | 10.3 | 0.54 (P = .008) |
| Pd (n = 57) | 26 | 4.7 | |||
| APOLLO7 | RRMM, at least 1 prior line | D-Pd (n = 151) | 69 | 12.4 | 0.63 (P = .0018) |
| Pd (n = 153) | 46 | 6.9 | |||
| ICARIA-MM17 | RRMM, at least 2 prior lines | I-Pd (n = 154) | 63 | 11.5 | 0.6 (P = .001) |
| Pd (n = 153) | 32 | 6.5 |
| Clinical trial . | Patient population . | Arm (n = number of patients) . | Overall response (%) . | Median progression-free survival (months) . | Hazard ratio (P value) . |
|---|---|---|---|---|---|
| ALCYONE12 | Transplant-ineligible NDMM | D-VMP (n = 350) | 91 | 36.4 | 0.42 (P < .001) |
| VMP (n = 356) | 74 | 19.3 | |||
| MAIA11 | Transplant-ineligible NDMM | D-Rd (n = 368) | 92.9 | NR | 0.53 (P < .001) |
| Rd (n = 369) | 81.6 | 34.4 | |||
| ELOQUENT 124 | Transplant-ineligible NDMM | E-Rd (n = 374) | 83 | 31.4 | 0.93 (P = .44) |
| Rd (n = 374) | 79 | 29.5 | |||
| CASSIOPEIA9 | Transplant-eligible NDMM | D-VTd (n = 543) | 93 | NR | 0.47 (P < .001) |
| VTd (n = 542) | 90 | NR | |||
| GRIFFIN10,a | Transplant-eligible NDMM | D-RVd (n = 99) | 99 | NR | Not available |
| RVd (n = 97) | 92 | NR | |||
| GMMG-HD623 | Transplant-eligible NDMM | E-RVd (n = 555) | B1 81.5%; B2 80.7% (≥VGPR) | B1 66.2%; B2 67.2% (3 y PFS) | Not available (P = .86; no significant difference) |
| RVd (n = 559) | A1 78.9%; A2 78.2% (≥VGPR) | A1 68.8%; A2 68.5% (3 y PFS) | |||
| GMMG-HD719 | Transplant-eligible NDMM | I-RVd (n = 329) | MRD 50.1% | NR | Not available (OR = 1.82; P < .001) |
| RVd (n = 331) | MRD 35.6% | NR | |||
| POLLUX6 | RRMM, at least 1 prior line | D-Rd (n = 286) | 93 | 44.5 | 0.44 (P < .001) |
| Rd (n = 283) | 76 | 17.5 | |||
| ELOQUENT 220 | RRMM, 1-3 prior lines | E-Rd (n = 321) | 79 | 19.4 | 0.71 (P < .001) |
| Rd (n = 325) | 66 | 14.9 | |||
| CANDOR8 | RRMM, 1-3 prior lines | D-Kd (n = 312) | 84 | NR | 0.62 (P = .003) |
| Kd (n = 154) | 75 | 15.8 | |||
| IKEMA18 | RRMM, 1-3 prior lines | I-Kd (n = 179) | 87 | NR | 0.53 (P < .001) |
| Kd (n = 123) | 83 | 19.2 | |||
| CASTOR5 | RRMM, at least 1 prior line | D-Vd (n = 251) | 83 | 16.7 | 0.31 (P < .001) |
| Vd (n = 247) | 63 | 7.1 | |||
| ELOQUENT 321 | RRMM, at least 2 prior lines | E-Pd (n = 60) | 53 | 10.3 | 0.54 (P = .008) |
| Pd (n = 57) | 26 | 4.7 | |||
| APOLLO7 | RRMM, at least 1 prior line | D-Pd (n = 151) | 69 | 12.4 | 0.63 (P = .0018) |
| Pd (n = 153) | 46 | 6.9 | |||
| ICARIA-MM17 | RRMM, at least 2 prior lines | I-Pd (n = 154) | 63 | 11.5 | 0.6 (P = .001) |
| Pd (n = 153) | 32 | 6.5 |
Randomized phase 2 study
Note: This list is not exhaustive for all randomized trials including monoclonal antibodies.
D, daratumumab; d, dexamethasone; E, elotuzumab; I, isatuximab; K, carfilzomib; M, melphalan; OR, odds ratio; P, pomalidomide; p, prednisone; R, lenalidomide; T, thalidomide; V, bortezomib.
In transplant-eligible patients, the addition of daratumamab to bortezomib-thalidomide-dexamethasone (phase 3 CASSIOPEIA trial) improved depth of response and led to a significant progression-free survival (PFS) benefit.9 Similarly, the addition of daratumumab to lenalidomide-bortezomib-dexamethasone (RVd; phase 2 GRIFFIN trial) in transplant-eligible patients improved depth of response, including stringent complete remission and minimal residual disease (MRD) (10−5), and showed PFS benefit (HR 0.45; p = 0.324), while median OS was not reached in either group with limited follow-up.10 In both CASSIOPEIA and GRIFFIN, there was a benefit to adding daratumumab across all subgroups except those with high-risk cytogenetics or International Scoring System stage III disease.
A PFS benefit is also seen for frontline daratumamab in combination with bortezomib-melphalan-prednisone (VMP; phase 3 ALCYONE trial) or lenalidomide-dexamethasone (Rd; phase 3 MAIA trial) in transplant-ineligible patients.11,12 Further, daratumumab has been combined with carfilzomib/Rd (KRd) as an induction therapy, with or without ASCT, in the phase 2 MASTER and MANHATTAN studies. Early results show impressive MRD-negative (10−5) remissions; 80% in the MASTER trial and 71% in the MANHATTAN trial.13,14
The question of daratumumab maintenance was first addressed in the second randomization of the CASSIOPEIA trial, in which patients were further randomized after ASCT/consolidation to daratumumab for up to 2 years or active surveillance. The median PFS was not reached (NR) in the daratumumab maintenance arm and was 46.7 months in the observation arm (hazard ratio [HR], 0.53; 95% CI, 0.42–0.68; P < .0001).15 Unexpectedly, those patients who had received daratumumab during induction and consolidation derived no incremental benefit from daratumumab maintenance alone vs observation alone (HR, 1.02; 95% CI, 0.71–1.47; P = .91), suggesting that depth of response, rather than continuous therapy, may have been the determining factor.15 Ongoing trials such as GRIFFIN (NCT02874742), DRAMMATIC (NCT04071457), PERSEUS (NCT03710603), and AURIGA (NCT03901963) will help elucidate the optimal use of daratumumab or daratumamab plus lenalidomide as maintenance therapy.
Isatuximab is another IgG1κ MoAb that binds to CD38. One notable difference from daratumumab is that isatuximab can induce direct cytotoxicity via caspase-dependent apoptosis and lysosome-mediated nonapoptotic cell killing.16 Isatuximab has been successfully combined with pomalidomide- dexamethasone (Pd; phase 3 ICARIA-MM trial) and Kd (phase 3 IKEMA trial) in RRMM, with superior PFS demonstrated in the isatuximab arms (Table 1).17,18 In the GMMG-HD7 phase 3 study in newly diagnosed multiple myeloma (NDMM), isatuximab added to RVd improved MRD (10−5) negativity rates (50.1% vs 35.6%) prior to ASCT, further supporting the incorporation of an anti-CD38 MoAb in frontline treatment.19
Elotuzumab, a humanized IgG1 MoAb directed against SLAMF7, has shown improved PFS and OS in combination with Rd (phase 3 ELOQUENT-2 trial) and Pd (phase 3 ELOQUENT-3 trial) in RRMM.20,21 Disappointingly, when added to frontline RVd in transplant-eligible NDMM patients (phase 2 SWOG-1211 and phase 3 GMMG-HD6 trials) or to Rd (phase 3 ELOQUENT-1 trial) in transplant-ineligible patients, no improvements in outcomes were demonstrated.22–24
Antibody-drug conjugates
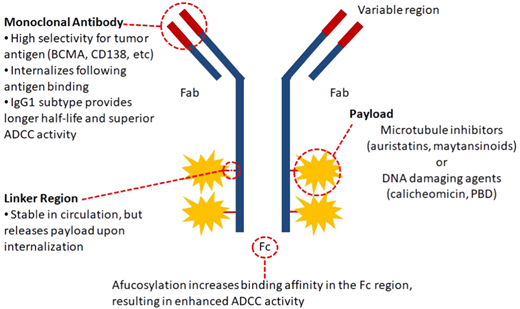
Despite the success of naked MoAbs, the vast majority of MM patients ultimately relapse and require novel interventions. Consequently, researchers have cleverly combined the specificity of MoAbs with a cytotoxic drug, creating a sophisticated delivery system that transports a lethal payload directly to the antigen-expressing cell (Figure 1). This technology has already shown success in other hematologic malignancies, including lymphoma (brentuximab vedotin) and acute myeloid leukemia (gemtuzumab ozogamicin).25
Characteristics of ADCs. The components of the ADC and its target antigen influence the efficacy and safety profile. Preferably, target antigens should only be found on malignant cells, be abundantly expressed, be capable of internalization, and not be shed from the cellular membrane. The cytotoxic drug (payload or warhead) is the ultimate effector component, inducing direct cell killing either by inhibiting microtubule formation or directly damaging cellular DNA. It should be highly potent in the subnanomolar range and preferably nonpermeable to avoid damage to surrounding tissues. The linker connects the warhead to the antibody. These should be stable in circulation and cleavable upon lysosomal degradation. The conjugation chemistry of the linker determines the drug:antibody ratio, which critically influences the ADC potency. ADCC, antibody-dependent T-cellular cytotoxicity, Fab, fragment antigen-binding region.
Characteristics of ADCs. The components of the ADC and its target antigen influence the efficacy and safety profile. Preferably, target antigens should only be found on malignant cells, be abundantly expressed, be capable of internalization, and not be shed from the cellular membrane. The cytotoxic drug (payload or warhead) is the ultimate effector component, inducing direct cell killing either by inhibiting microtubule formation or directly damaging cellular DNA. It should be highly potent in the subnanomolar range and preferably nonpermeable to avoid damage to surrounding tissues. The linker connects the warhead to the antibody. These should be stable in circulation and cleavable upon lysosomal degradation. The conjugation chemistry of the linker determines the drug:antibody ratio, which critically influences the ADC potency. ADCC, antibody-dependent T-cellular cytotoxicity, Fab, fragment antigen-binding region.
Several ADCs have been evaluated in clinical trials in RRMM (Table 2), the most promising of which target B-cell maturation antigen (BCMA). BCMA has emerged as an attractive target, as it is expressed at high levels on PCs and plasmablasts but not on other tissues.26 This selectivity, in addition to the fact that BCMA undergoes internalization, makes it an ideal target for ADCs.26
Summary of antibody-drug conjugates for MM
| Name . | Target . | Payload . | Combination . | Trial phase: number of patients (n) . | Response/activity . | Current status (ClinicalTrials.gov) . |
|---|---|---|---|---|---|---|
| Belantamab mafodotin (belamaf) | BCMA | MMAF | Monotherapy28 | Phase 1: n = 35 | ORR 60% | DREAMM-1 (completed) NCT02064387 |
| Phase 2: n = 196 | ORR 31% | DREAMM-2 (completed) NCT03525678 | ||||
| B (Q3W) vs Pd | Phase 3: n = 380 (E) | N/A | DREAMM-3 (recruiting) NCT04162210 | |||
| B (Q3W) + Pemb30 | Phase 1/2: n = 41 | ORR 47% | DREAMM-4 (active, not recruiting) NCT03848845 | |||
| B + novel agent31 | Phase 1/2: n = 464 (E) | ORR 53% with feladilimab | DREAMM-5 (recruiting) NCT04126200 | |||
| B-Vd OR Rd32 | Phase 1/2: n = 152 (E) | ORR 78% in BVd arm | DREAMM-6 (active, not recruiting) NCT03544281 | |||
| B-Pd34 | Phase 1/2: n = 96 (E) | ORR 88.9% | ALGONQUIN (recruiting) NCT03715478 | |||
| B-Rd (transplant-ineligible NDMM) | Phase 1/2: n = 66 (E) | N/A | NCT04808037 (recruiting) | |||
| B-Vd vs D-Vd | Phase 3: n = 575 (E) | N/A | DREAMM-7 (active, not recruiting) NCT04246047 | |||
| B-Pd vs V-Pd | Phase 3: n = 450 (E) | N/A | DREAMM-8 (recruiting) NCT04484623 | |||
| B-VRd (transplant-ineligible NDMM33 | Phase 1: n = 144 (E) | ORR 100% (n = 12) | DREAMM-9 (recruiting) NCT04091126 | |||
| Monotherapy in renal impairment | Phase 1: n = 36 (E) | N/A | DREAMM-12 (recruiting) NCT04398745 | |||
| Monotherapy in liver impairment | Phase 1: n = 28 (E) | N/A | DREAMM-13 (recruiting) NCT04398680 | |||
| Monotherapy (varying doses and schedules) | Phase 2: n = 180 (E) | N/A | DREAMM-14 (recruiting) NCT05064358 | |||
| AMG 224 | BCMA | Mertansine | Monotherapy36 | Phase 1: n = 42 (E) | ORR 27% (3 mg/kg) | NCT02561962 (active, not recruiting) |
| CC 99712 | BCMA | Maytansinoid-like | Monotherapy | Phase 1: n = 160 (E) | N/A | NCT04036461 (recruiting) |
| MEDI2228 | BCMA | PBD | Monotherapy37 | Phase 1: n = 82 | ORR 66% at 0.14 mg/kg | NCT03489525 (completed) |
| Indatuximab ravtansine | CD138 | Maytansinoid DM4 | In combination with R or P | Phase 1/2: n = 64 | ORR 71.7% with R and 70.6% with P | NCT01001442 (completed) |
| Lorvotuzumab mertansine | CD56 | Mertansine | Monotherapy | Phase 1: n = 37 | ORR 5.7% | NCT00346255 (completed) |
| Milatuzumab | CD74 | Doxorubicin | Monotherapy | Phase 1: n = 25 | 26% SD | NCT00421525 (completed) |
| STRO-001 | CD74 | MMAF | Monotherapy | Phase 1: n = N/A | N/A | NCT03424603 (recruiting) |
| DFRF4539A | FcRH5 | MMAE | Monotherapy | Phase 1: n = 39 | ORR 5%; 49% SD | NCT01432353 (completed) |
| SGN-CD48A | CD48 | MMAE | Monotherapy | Phase 1: n = 14 | N/A | NCT03379584 (terminated) |
| ABBV-838 | SLAMF7 | MMAE | Monotherapy | Phase 1/1b: n = 75 | ORR 10.7% | NCT02462525 (terminated) |
| Name . | Target . | Payload . | Combination . | Trial phase: number of patients (n) . | Response/activity . | Current status (ClinicalTrials.gov) . |
|---|---|---|---|---|---|---|
| Belantamab mafodotin (belamaf) | BCMA | MMAF | Monotherapy28 | Phase 1: n = 35 | ORR 60% | DREAMM-1 (completed) NCT02064387 |
| Phase 2: n = 196 | ORR 31% | DREAMM-2 (completed) NCT03525678 | ||||
| B (Q3W) vs Pd | Phase 3: n = 380 (E) | N/A | DREAMM-3 (recruiting) NCT04162210 | |||
| B (Q3W) + Pemb30 | Phase 1/2: n = 41 | ORR 47% | DREAMM-4 (active, not recruiting) NCT03848845 | |||
| B + novel agent31 | Phase 1/2: n = 464 (E) | ORR 53% with feladilimab | DREAMM-5 (recruiting) NCT04126200 | |||
| B-Vd OR Rd32 | Phase 1/2: n = 152 (E) | ORR 78% in BVd arm | DREAMM-6 (active, not recruiting) NCT03544281 | |||
| B-Pd34 | Phase 1/2: n = 96 (E) | ORR 88.9% | ALGONQUIN (recruiting) NCT03715478 | |||
| B-Rd (transplant-ineligible NDMM) | Phase 1/2: n = 66 (E) | N/A | NCT04808037 (recruiting) | |||
| B-Vd vs D-Vd | Phase 3: n = 575 (E) | N/A | DREAMM-7 (active, not recruiting) NCT04246047 | |||
| B-Pd vs V-Pd | Phase 3: n = 450 (E) | N/A | DREAMM-8 (recruiting) NCT04484623 | |||
| B-VRd (transplant-ineligible NDMM33 | Phase 1: n = 144 (E) | ORR 100% (n = 12) | DREAMM-9 (recruiting) NCT04091126 | |||
| Monotherapy in renal impairment | Phase 1: n = 36 (E) | N/A | DREAMM-12 (recruiting) NCT04398745 | |||
| Monotherapy in liver impairment | Phase 1: n = 28 (E) | N/A | DREAMM-13 (recruiting) NCT04398680 | |||
| Monotherapy (varying doses and schedules) | Phase 2: n = 180 (E) | N/A | DREAMM-14 (recruiting) NCT05064358 | |||
| AMG 224 | BCMA | Mertansine | Monotherapy36 | Phase 1: n = 42 (E) | ORR 27% (3 mg/kg) | NCT02561962 (active, not recruiting) |
| CC 99712 | BCMA | Maytansinoid-like | Monotherapy | Phase 1: n = 160 (E) | N/A | NCT04036461 (recruiting) |
| MEDI2228 | BCMA | PBD | Monotherapy37 | Phase 1: n = 82 | ORR 66% at 0.14 mg/kg | NCT03489525 (completed) |
| Indatuximab ravtansine | CD138 | Maytansinoid DM4 | In combination with R or P | Phase 1/2: n = 64 | ORR 71.7% with R and 70.6% with P | NCT01001442 (completed) |
| Lorvotuzumab mertansine | CD56 | Mertansine | Monotherapy | Phase 1: n = 37 | ORR 5.7% | NCT00346255 (completed) |
| Milatuzumab | CD74 | Doxorubicin | Monotherapy | Phase 1: n = 25 | 26% SD | NCT00421525 (completed) |
| STRO-001 | CD74 | MMAF | Monotherapy | Phase 1: n = N/A | N/A | NCT03424603 (recruiting) |
| DFRF4539A | FcRH5 | MMAE | Monotherapy | Phase 1: n = 39 | ORR 5%; 49% SD | NCT01432353 (completed) |
| SGN-CD48A | CD48 | MMAE | Monotherapy | Phase 1: n = 14 | N/A | NCT03379584 (terminated) |
| ABBV-838 | SLAMF7 | MMAE | Monotherapy | Phase 1/1b: n = 75 | ORR 10.7% | NCT02462525 (terminated) |
Note: This list is not exhaustive for all ADCs developed for MM.
B, belantamab; belamaf, belantamab mafodotin; D, daratumumab; d, dexamethasone; E, estimate; MMAE, monomethyl auristatin E; N/A, not available; Pemb, pembrolizumab; P, pomalidomide; Q3W, once every 3 weeks; R, lenalidomide; SD, stable disease; V, bortezomib.
Belantamab mafodotin (GSK2857916), a first-in-class humanized IgG1, afucosylated ADC conjugated to monomethyl auristatin-F (MMAF), was the first ADC to demonstrate significant therapeutic benefit in MM. Preclinical studies demonstrate that belantamab mafodotin eliminates myeloma cells through several mechanisms of action: direct cell killing via the inhibition of microtubule polymerization, classical IgG effector functions through an enhanced fragment crystallizable region (Fc) domain, and immunogenic cell death, a process in which dying cells elicit an adaptive immune response.27
Belantamab mafodotin was evaluated in the pivotal DREAMM-2 study.28 This phase 2 study explored doses of 2.5 mg/kg or 3.4 mg/kg administered intravenously every 3 weeks until progression. In patients receiving the US Food and Drug Administration–approved 2.5-mg/kg dose (n = 97), all were TCR and had a median of 7 (3-12) prior lines of therapy. The ORR was 31% (97.5% CI, 20.8–42.6), and the median PFS and OS were 2.8 (95% CI, 1.6–3.6) and 13.7 months (95% CI, 9.9-not reached), respectively. For responding patients, the DOR was 11 months (95% CI, 4.2-not reached). Post hoc analyses demonstrated similar response and OS in patients with high-risk cytogenetics and those with impaired renal function, although patients with extramedullary disease did not appear to derive the same benefit.28 Consistent with MMAF-containing ADCs, the most common AEs (any grade/grade ≥3) were keratopathy (72%/46%), change in best corrected visual acuity (54%/31%), thrombocytopenia (38%/22%), anemia (27%/21%), and blurred vision (25%/4%). Dose reductions and delays due to toxicity occurred in 54% and 35%, respectively, and were less common in the 2.5-mg/kg arm of the study.
Belantamab mafodotin is US Food and Drug Administration approved for use in RRMM patients who have received 4 or more lines of therapy. The approval came with a boxed warning, indicating that toxicity to the cornea may result in vision loss, corneal ulcers, or dry eyes. Although the mechanism is yet unclear, preclinical data suggest that ocular toxicity is related to the receptor- independent uptake of the intact ADC into the epithelial limbal stem cells of the cornea.29 In DREAMM-2, 72% of patients in the 2.5-mg/kg cohort demonstrated keratopathy on ophthalmologic exam; however, only 56% experienced symptoms (most commonly blurred vision and/or dry eyes), and only 3 patients (3%) discontinued therapy for corneal AEs.28 Importantly, experience from DREAMM-2 indicates that patients recover from corneal toxicity with dose holds for grade 2 events or higher, with the majority of patients (88%) maintaining responses despite prolonged dose delays. Based on this experience, the recommendations for management of corneal events include the frequent use of preservative-free lubricant eye drops, eye care professional assessments before each dose, and timely dose holds and modifications.
Numerous studies are now evaluating belantamab mafodotin in different drug combinations, dosing schedules, and treatment settings (Table 2). This includes studies combining belantamab mafodotin with pembrolizumab (DREAMM-4),30 with novel agents (DREAMM-5),31 with lenalidomide or bortezomib (DREAMM-6),32 and with RVd in NDMM (DREAMM-9).33 Notably, in the Algonquin study, belantamab mafodotin was combined with Pd to identify the optimal dose and schedule in pomalidomide-naive patients.34 Across cohorts of patients receiving belantamab mafodotin, 1.92 mg/kg every 4 weeks or 2.5 mg/kg every 4, 8, or 12 weeks (n = 56), the ORR was 88.9% (≥ very good partial response [VGPR], 72%) and PFS, 17 months (14.5-not reached); notably, 48% of patients were TCR.34 Additionally, studies exploring lower doses and extended schedules of belantamab mafodotin as a strategy to reduce the incidence and severity of corneal toxicity are ongoing (DREAMM-9, DREAMM-14, and NCT04808037). Emerging data from these studies are encouraging, demonstrating a reduction in the incidence and severity of corneal toxicity with good clinical efficacy.34,35
Several other ADCs targeting BCMA have been developed. AMG 224 is a humanized IgG1 anti-BCMA mertansine-conjugated ADC. In a phase 1 study, at the expansion dose of 3 mg/kg (n = 11) the ORR was 27%.36 Mild ocular events were observed in 30% of patients with no reports of keratopathy. MEDI2228 is another humanized pyrrolobenzodiazepine (PBD)-conjugated anti-BCMA ADC. In a first-in-human study, an ORR of 65.9% was reported at the maximum tolerated dose (MTD) of 0.14 mg/kg (n = 41).37 The safety profile was consistent with PBD-containing ADCs and included rash (31.7%), thrombocytopenia (31.7%), pleural effusions (24.4%), and increased gamma- glutamyl transferase (24.4%). Unexpectedly, ocular AEs in the form of photophobia were reported in 58.5% of patients. Phase 1 studies of CC-99712, an anti-BCMA ADC conjugated to 4 maytansinoid molecules, and HDP-101, an anti-BCMA ADC conjugated to an amanitin derivative, are recruiting (NCT04036461 and NCT04879043, respectively).
ADCs targeting antigens other than BCMA have shown less promising efficacy in MM. Table 2 summarizes the current state of development of indatuximab ravtansine (anti-CD138), lorvotuzumab mertansine (anti-CD56), STRO-001 (anti-CD74), and several other ADCs with novel targets, most having had their development halted due to disappointing efficacy.
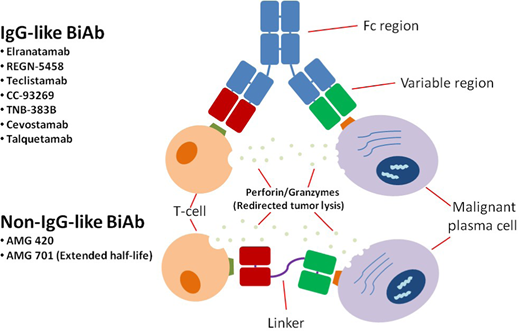
Bispecific antibodies
BsAbs are unique antibody constructs that simultaneously bind 2 antigens. In cancer therapeutics, this usually involves targeting an antigen on the tumor and a molecule on an immune cell, resulting in immune effector cell activation and tumor lysis (Figure 2). Similar to the ADCs, BCMA has been the selected antigen target for the development of BsAbs, although others have emerged (Table 3).
Characteristics of BsAbs. IgG-like BsAbs include an Fc region, while non–IgG-like BsAbs consist of only Fab (fragment antigen-binding) variable regions and linkers. Since the Fc portion provides stability and longevity to the molecule in circulation, most non–IgG-like BsAbs require more frequent dosing to maintain therapeutic plasma levels; they also lack the Fc-mediated effector functions such as antibody-dependent T-cellular cytotoxicity and complement-dependent cytotoxicity. Several BsAbs, both IgG-like and non–IgG-like, are under development for the treatment of MM. BiAb, bispecific antibodies.
Characteristics of BsAbs. IgG-like BsAbs include an Fc region, while non–IgG-like BsAbs consist of only Fab (fragment antigen-binding) variable regions and linkers. Since the Fc portion provides stability and longevity to the molecule in circulation, most non–IgG-like BsAbs require more frequent dosing to maintain therapeutic plasma levels; they also lack the Fc-mediated effector functions such as antibody-dependent T-cellular cytotoxicity and complement-dependent cytotoxicity. Several BsAbs, both IgG-like and non–IgG-like, are under development for the treatment of MM. BiAb, bispecific antibodies.
Summary of bispecific T-cell antibodies for MM
| Name . | Target . | Antibody construct . | Triple-class refractory (median LoT) . | Trial phase . | Schedule . | Preliminary response/activity . | Safety . | Current status (ClinicalTrials.gov) . |
|---|---|---|---|---|---|---|---|---|
| AMG 42038 | BCMA-CD3 | BiTE® | N/A (29% prior anti-CD38, median 5 LoT) | Phase 1 | Continuous infusion for 4 wk (out of 6) | ORR = 31% ORR MTD = 70% | 38% CRS (6.25% ≥ gr 3) 5% ≥ gr 3 polyneuropathy 24% ≥ gr 3 infection | Active, not recruiting NCT03836053 |
| AMG 70139 | BCMA-CD3 | HLE-BiTE® | 68% (median 6 LoT) | Phase 1/2 | Weekly IV | ORR = 36% ORR = 83% at 9 mg | 75% CRS (10.5% ≥ gr 3) 8% neurotoxicity (gr 1-2) 13% ≥ gr 3 infection | Recruiting NCT03287908 |
| Elranatamab41 | BCMA-CD3 | Humanized IgG2a Fc | 91% (median 6 LoT; 22% prior anti-BCMA) | Phase 1 | Weekly or every 2 wk Sc | ORR = 64% for doses ≥215 µg/kg | 67% CRS (gr 1-2) | MagnetisMM-1 Recruiting NCT03269136 |
| REGN545842 | BCMA-CD3 | Fc Fab arms | 97.1% (median 5 LoT) | Phase 1/2 | Weekly IV | ORR = 73.3% at 96-200-mg doses | 38.2% CRS (gr 1-2) 4% neurotoxicity (gr 1-2) 23% pneumonia (11% ≥ gr 3) | Recruiting NCT03761108 |
| Teclistamab40 | BCMA-CD3 | Humanized IgG4 Fc | 77.8% (median 5 LoT; prior anti-BCMA not permitted) | Phase 1/2 | Weekly Sc | ORR = 63% | 72.1% CRS (gr 3, 0.6%; no gr 4) 14.5% neurotoxicity (1 gr 4 event) 44.8% ≥ gr 3 infection | MajestTEC-1 Recruiting NCT03145181 |
| CC-9326944 | BCMA-CD3 | Asymmetric 2-arm IgG | 66.7% (median 6 LoT) | Phase 1 | Weekly IV | ORR = 83.3% in 10 pts with doses ≥6 mg | 89.5% CRS (1 gr 5 event) 26.3% infection | Recruiting NCT03486067 |
| TNB-383B44 | BCMA-CD3 | IgG4 Fc CD3 activating T effector cells | 62% (median 5 LoT) | Phase 1 | Q21d IV | ORR = 79% at doses ≥40 mg | 52% CRS (3% ≥ gr 3 at RP2D) 28% infection | Recruiting NCT03933735 |
| Cevostamab45 | FcRH5-CD3 | Humanized IgG1 Fc | 85% (median 6 LoT; 33.5% prior anti-BCMA) | Phase 1 | Q21d IV | ORR = 54.5% at 160-mg-dose level | 80.7% CRS (1.3% ≥ gr 3) 18.8% ≥ gr 3 infection 14.3% neurotoxicity (0.3% ≥ gr 3) | Recruiting NCT03275103 |
| Talquetamab46 | GPRC5D-CD3 | Humanized IgG4 Fc | Weekly: 77% (median 6 LoT; 30% prior anti-BCMA) Biweekly: 65% (median 5 LoT; 17% prior anti-BCMA) | Phase 1/2 | Weekly or biweekly Sc | Weekly: ORR = 70% Biweekly: ORR = 71% | Weekly: 73% CRS (1 gr 3) Biweekly: 78% CRS (gr 1-2) | MonumenTal-1 Recruiting NCT03399799 |
| Name . | Target . | Antibody construct . | Triple-class refractory (median LoT) . | Trial phase . | Schedule . | Preliminary response/activity . | Safety . | Current status (ClinicalTrials.gov) . |
|---|---|---|---|---|---|---|---|---|
| AMG 42038 | BCMA-CD3 | BiTE® | N/A (29% prior anti-CD38, median 5 LoT) | Phase 1 | Continuous infusion for 4 wk (out of 6) | ORR = 31% ORR MTD = 70% | 38% CRS (6.25% ≥ gr 3) 5% ≥ gr 3 polyneuropathy 24% ≥ gr 3 infection | Active, not recruiting NCT03836053 |
| AMG 70139 | BCMA-CD3 | HLE-BiTE® | 68% (median 6 LoT) | Phase 1/2 | Weekly IV | ORR = 36% ORR = 83% at 9 mg | 75% CRS (10.5% ≥ gr 3) 8% neurotoxicity (gr 1-2) 13% ≥ gr 3 infection | Recruiting NCT03287908 |
| Elranatamab41 | BCMA-CD3 | Humanized IgG2a Fc | 91% (median 6 LoT; 22% prior anti-BCMA) | Phase 1 | Weekly or every 2 wk Sc | ORR = 64% for doses ≥215 µg/kg | 67% CRS (gr 1-2) | MagnetisMM-1 Recruiting NCT03269136 |
| REGN545842 | BCMA-CD3 | Fc Fab arms | 97.1% (median 5 LoT) | Phase 1/2 | Weekly IV | ORR = 73.3% at 96-200-mg doses | 38.2% CRS (gr 1-2) 4% neurotoxicity (gr 1-2) 23% pneumonia (11% ≥ gr 3) | Recruiting NCT03761108 |
| Teclistamab40 | BCMA-CD3 | Humanized IgG4 Fc | 77.8% (median 5 LoT; prior anti-BCMA not permitted) | Phase 1/2 | Weekly Sc | ORR = 63% | 72.1% CRS (gr 3, 0.6%; no gr 4) 14.5% neurotoxicity (1 gr 4 event) 44.8% ≥ gr 3 infection | MajestTEC-1 Recruiting NCT03145181 |
| CC-9326944 | BCMA-CD3 | Asymmetric 2-arm IgG | 66.7% (median 6 LoT) | Phase 1 | Weekly IV | ORR = 83.3% in 10 pts with doses ≥6 mg | 89.5% CRS (1 gr 5 event) 26.3% infection | Recruiting NCT03486067 |
| TNB-383B44 | BCMA-CD3 | IgG4 Fc CD3 activating T effector cells | 62% (median 5 LoT) | Phase 1 | Q21d IV | ORR = 79% at doses ≥40 mg | 52% CRS (3% ≥ gr 3 at RP2D) 28% infection | Recruiting NCT03933735 |
| Cevostamab45 | FcRH5-CD3 | Humanized IgG1 Fc | 85% (median 6 LoT; 33.5% prior anti-BCMA) | Phase 1 | Q21d IV | ORR = 54.5% at 160-mg-dose level | 80.7% CRS (1.3% ≥ gr 3) 18.8% ≥ gr 3 infection 14.3% neurotoxicity (0.3% ≥ gr 3) | Recruiting NCT03275103 |
| Talquetamab46 | GPRC5D-CD3 | Humanized IgG4 Fc | Weekly: 77% (median 6 LoT; 30% prior anti-BCMA) Biweekly: 65% (median 5 LoT; 17% prior anti-BCMA) | Phase 1/2 | Weekly or biweekly Sc | Weekly: ORR = 70% Biweekly: ORR = 71% | Weekly: 73% CRS (1 gr 3) Biweekly: 78% CRS (gr 1-2) | MonumenTal-1 Recruiting NCT03399799 |
Note: This list is not exhaustive for all bispecific T-cell antibodies developed for MM.
Fab, fragment antigen-binding; gr, grade; HLE, half-life extended; IV, intravenous; LoT, lines of therapy; pts, patients; Q21d, every 21 days; RP2D, recommended phase 2 dose; Sc, subcutaneous; wk, week.
AMG 420, which targets BCMA on PCs and CD3 on T cells, was the first BsAb to show efficacy in human trials. Coined a BiTE® (bispecific T-cell engager) because it lacks an Fc region, AMG 420 justified the development of bispecifics in MM, demonstrating a 70% ORR at the MTD (400 µg/d; n = 10).38 The BiTE® format offers the advantage of better tissue penetrance and access to epitopes but with the caveat of a short half-life, necessitating a continuous intravenous infusion. This has been remedied by the development of an extended half-life version, AMG 701,39 and numerous Fc-containing BsAbs that can be administered every 1 to 3 weeks, with the majority moving to subcutaneous formulations (Table 3).40–46 Fc-containing bispecifics are larger and therefore more stable in circulation, while the Fc function may or may not be silenced depending on the agent. Thus far, BsAbs have been evaluated in heavily pretreated patients, with the majority being TCR; a good number have also included older patients (>80 years). All have demonstrated encouraging clinical activity (Table 3), although data on durability of responses are still immature.
Teclistamab, an anti-BCMA/CD3 BsAb, is furthest along in clinical development, with an anticipated commercial approval in 2022. In the phase 1/2 MajesTEC-1 study, 165 patients received teclistamab (77.8% TCR) at the recommended target dose of 1.5 mg/kg.40 The ORR was 63% (≥complete remission, 39.4%), with 44 patients (26.7%) achieving MRD (10−5). Responses were maintained across different subgroups, including poor-risk groups, with the exception of those with extramedullary disease, those with stage III disease, or those with PC marrow involvement of 60% or greater. The median DOR was 18.4 months (95% CI, 14.9-not estimable), while the PFS was 11.3 months (95% CI, 8.8-17.1). CRS, neutropenia, infection, and neurotoxicity of any grade/grade higher than or equal to 3 occurred in 72.1%/0.6%, 70.9%/64.2%, 76.4%/44.8%, and 14.5%/0%, respectively; 19 patients died from AEs, including 12 deaths due to COVID-19.
In the phase 1 MagnetisMM-1 study, elranatamab (PF-06863135), another anti-BCMAxCD3 BsAb, demonstrated an ORR of 64% among 55 patients (91% TCR) receiving doses of 215 µg/kg or higher.41 Remarkably, 7 of 10 patients treated with prior BCMA-targeted therapy achieved a partial response or better.41 The incidence of CRS at the recommended dose (1000 µg/kg or 76 mg) was 67% (all grade 1/2).41 Studies of teclistamab and elranatamab in combination with immunomodulatory drugs and anti-CD38s and in earlier lines of treatment are ongoing (Table 4).
Summary of bispecific T-cell antibody combinations for MM
| Name . | Patient population . | Trial phase . | Combination drugs . | Current status (ClinicalTrials.gov) . |
|---|---|---|---|---|
| Elranatamab | RRMM | Phase 1b/2 | Arm 1: elranatamab + nirogacestat (GSI) | MagnetisMM-4 (recruiting) NCT05090566 |
| Arm 2: elranatamab + lenalidomide + dexamethasone | ||||
| Phase 3 | Arm 1: elranatamab | MagnetisMM-5 (recruiting) NCT05020236 | ||
| Arm 2: elranatamab + daratumumab | ||||
| Arm 3: earatumumab + pomalidomide + dexamethasone | ||||
| Teclistamab + talquetamab | RRMM | Phase 3 | Arm 1: teclistamab + daratumumab | MajestTEC-3 (recruiting) NCT05083169 |
| Arm 2: daratumumab + pomalidomide + dexamethasone | ||||
| Arm 3: daratumumab + bortezomib + dexamethasone | ||||
| Phase 1 | Arm 1: teclistamab + talquetamab | NCT04586426 (recruiting) | ||
| Arm 2: teclistamab + talquetamab + daratumumab | ||||
| Phase 1b | Arm 1: daratumumab + teclistamab | TRIMM-2 (recruiting) NCT04108195 | ||
| Arm 2: daratumumab + talquetamab | ||||
| Arm 3: daratumumab + talquetamab + pomalidomide | ||||
| Arm 4: daratumumab + teclistamab + pomalidomide | ||||
| Cevostamab | RRMM | Phase 1 | Arm 1: cevostamab | Recruiting NCT04910568 |
| Arm 2: cevostamab + pomalidomide + dexamethasone | ||||
| Arm 3: cevostamab + daratumumab + dexamethasone |
| Name . | Patient population . | Trial phase . | Combination drugs . | Current status (ClinicalTrials.gov) . |
|---|---|---|---|---|
| Elranatamab | RRMM | Phase 1b/2 | Arm 1: elranatamab + nirogacestat (GSI) | MagnetisMM-4 (recruiting) NCT05090566 |
| Arm 2: elranatamab + lenalidomide + dexamethasone | ||||
| Phase 3 | Arm 1: elranatamab | MagnetisMM-5 (recruiting) NCT05020236 | ||
| Arm 2: elranatamab + daratumumab | ||||
| Arm 3: earatumumab + pomalidomide + dexamethasone | ||||
| Teclistamab + talquetamab | RRMM | Phase 3 | Arm 1: teclistamab + daratumumab | MajestTEC-3 (recruiting) NCT05083169 |
| Arm 2: daratumumab + pomalidomide + dexamethasone | ||||
| Arm 3: daratumumab + bortezomib + dexamethasone | ||||
| Phase 1 | Arm 1: teclistamab + talquetamab | NCT04586426 (recruiting) | ||
| Arm 2: teclistamab + talquetamab + daratumumab | ||||
| Phase 1b | Arm 1: daratumumab + teclistamab | TRIMM-2 (recruiting) NCT04108195 | ||
| Arm 2: daratumumab + talquetamab | ||||
| Arm 3: daratumumab + talquetamab + pomalidomide | ||||
| Arm 4: daratumumab + teclistamab + pomalidomide | ||||
| Cevostamab | RRMM | Phase 1 | Arm 1: cevostamab | Recruiting NCT04910568 |
| Arm 2: cevostamab + pomalidomide + dexamethasone | ||||
| Arm 3: cevostamab + daratumumab + dexamethasone |
Note: This list is not exhaustive for all bispecific T-cell antibody combinations developed for MM.
GSI, gamma secretase inhibitor.
Other notable anti-BCMA BsAbs include REGN5458, CC-93269, and TNB-383B (Table 3). In a phase 1/2 trial of REGN5458, the ORR was 73.3% among patients treated at the 96-mg- and 200-mg-dose levels; no patients experienced CRS of grade 3 or higher.42 Of the 12 patients treated with 6 mg or more of CC-93269, the ORR was 83.3%.43 CRS was reported in 89.5% of patients, mostly grade 1/2 (57.9%/26.3%); however, one patient died in the setting of CRS, with infection as a potential contributor.43 In the dose-escalation cohort of TNB-383B (doses ≥40 mg every 3 weeks), the ORR was 79% (n = 19/24) with CRS reported as mainly grade 1/2.44
Fc receptor homolog 5 (FcRH5) is a cell surface antigen of unknown function whose expression is restricted to B cells, with the highest expression on PCs. BFCR4350A (cevostamab) is a humanized IgG Fc antibody targeting FcRH5 and CD3. In an ongoing phase 1 study, cevostamab was administered for a fixed duration of 17 cycles, unlike other BsAb trials that treat to progression.45 Among 160 patients (85% TCR), the ORR was 56.7% and 36.1% for those patients receiving doses of 132 to 198 mg (n = 60) or 20 to 90 mg (n = 83), respectively. The ORR among patients who had prior CAR T-cell or anti-BCMA therapy was 44.4% and 36.4%, respectively. CRS was observed in 80.7% (128/160) of patients (mainly grade 1/2; 2 cases grade 3). In the single step-up dose cohorts (n = 86), the median DOR was 11.5 months (95% CI, 6.0, 18.4) at a follow-up of 14.3 months. Thus, cevostamab appears to be a beneficial treatment option, with a unique therapeutic target, for RRMM patients.
Talquetamab is an IgG4 Fc-containing BsAb targeting G- protein–coupled receptor family C group 5 member D (GPRC5D).46 GPRC5D is highly expressed on PCs but also on keratinized tissues. In the MonumenTal-1 study, patients received subcutaneous talquetamab at doses of 405 µg/kg (n = 30) weekly or 800 µg/kg biweekly (n = 23).44 Unique AEs, owing to the expression of GPRC5D on keratinized tissues, include dysgeusia, palmar/plantar desquamation, nail dystrophy, and systemic rash, which were reported in 75% of patients (mostly grade 1/2; 7.5% grade 3).46 Mitigation strategies for these toxicities included the use of emollient creams and, for oral AEs, saliva-substitute sprays and rinses at the onset of symptoms. The ORR and CRS in patients receiving the 405-µg/kg or 800-µg/kg dose was 70% and 73% (1 grade 3) and 71% and 78% (all grade 1/2), respectively.46 Trials exploring talquetamab in combination with teclistamab, as well as with SOC antimyeloma agents, are actively recruiting (Table 4).
CLINICAL CASE (Continued)
The patient responds to belantamab mafodotin; however, she experiences multiple dose interruptions for corneal AEs and progresses after 9 months. She is then referred to an academic center and is enrolled in a clinical trial evaluating talquetamab (anti-GPRC5D) monotherapy. In cycle 1 she experiences grade 1 CRS but no neurotoxicity. After 6 months of therapy, she continues to have an ongoing response with manageable grade 1 dysgeusia and dermatologic symptoms.
Conclusion
Alongside CAR T-cell therapy, antibody-based immunotherapies have emerged as important off-the-shelf therapeutic options for all patients with RRMM (Table 5). The sequencing and duration of these therapies remain ongoing clinical questions, although emerging data are encouraging. In a heavily pretreated RRMM population relapsing on a BsAb (n = 64), the ORR to subsequent treatment (second BsAb, n = 20; CAR T, n = 15) was 58%, with an OS of 17.6 months (95% CI, 21.6-not reached).47 Further, in the MagnetisMM-1 study, 7 of 13 patients previously treated with an anti-BCMA targeted therapy (5/7 for those treated with anti-BCMA ADCs) responded to the BCMAxCD3 BsAb, elranatamab.41 Meanwhile in the ongoing phase 1 study of cevostamab (FCRH5xCD3 BsAb) that limited treatment to 17 cycles, 6 patients continued to maintain responses for 6 months or longer after the cessation of treatment.45
Comparison of novel immunotherapy approaches in multiple myeloma
| . | Antibody drug conjugates . | Bispecific T-cell engagers . | CAR T-cell therapy . |
|---|---|---|---|
| Advantages | Off-the-shelf therapy | Off-the-shelf therapy | - |
| Immune and nonimmune mechanisms of action | - | - | |
| Infrequent dosing (every 3 wk-12 wk) | - | One-time therapy | |
| Encouraging response rates | Deep responses | Deep responses | |
| No CRS/ICANS | Mostly grade 1-2 CRS/ICANS | - | |
| Outpatient administration | Only initial dosing as inpatient | Vacation from continuous therapy | |
| Disadvantages | Continuous therapy until progression | Continuous therapy until progression | - |
| Frequent dose interruptions | Weekly or biweekly dosing | Administration delays due to manufacturing time | |
| Ocular toxicity | Significant immunosuppression | Potential for severe CRS/ICANS; prolonged cytopenias | |
| Ophthalmic exams prior to dosing | Specialized centers required | Complex infrastructure required | |
| Cost ($$) | Cost ($$) | Cost ($$$) |
| . | Antibody drug conjugates . | Bispecific T-cell engagers . | CAR T-cell therapy . |
|---|---|---|---|
| Advantages | Off-the-shelf therapy | Off-the-shelf therapy | - |
| Immune and nonimmune mechanisms of action | - | - | |
| Infrequent dosing (every 3 wk-12 wk) | - | One-time therapy | |
| Encouraging response rates | Deep responses | Deep responses | |
| No CRS/ICANS | Mostly grade 1-2 CRS/ICANS | - | |
| Outpatient administration | Only initial dosing as inpatient | Vacation from continuous therapy | |
| Disadvantages | Continuous therapy until progression | Continuous therapy until progression | - |
| Frequent dose interruptions | Weekly or biweekly dosing | Administration delays due to manufacturing time | |
| Ocular toxicity | Significant immunosuppression | Potential for severe CRS/ICANS; prolonged cytopenias | |
| Ophthalmic exams prior to dosing | Specialized centers required | Complex infrastructure required | |
| Cost ($$) | Cost ($$) | Cost ($$$) |
ICANS, immune effector cell-associated neurotoxicity syndrome; wk, week.
While ADCs and BsAbs demonstrate promising efficacy, future efforts need to focus on the optimal timing and duration of therapy, on combinational strategies, on mitigation of the risk of immunosuppression/infection (BsAbs) and corneal toxicity (ADC), on the mechanisms of resistance, and on equitable access. Although much work is still to be done, these novel approaches offer new hope for a yet incurable disease.
Conflict-of-interest disclosure
Christopher Cipkar: no competing financial interests to declare.
Christine Chen: consultancy: Forus Therapeutics; advisory board: Amgen, Bristol Myers Squibb, Janssen Pharmaceuticals.
Suzanne Trudel: research funding: Amgen, Bristol Myers Squibb, Genentech, GlaxoSmithKline, Janssen Pharmaceuticals, Pfizer, Roche; consultancy: Bristol Myers Squibb, Forus, GlaxoSmithKline, K36 Therapeutics, Roche; advisory board: Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen Pharmaceuticals, Pfizer, Sanofi.
Off-label drug use
Christopher Cipkar: nothing to disclose.
Christine Chen: nothing to disclose.
Suzanne Trudel: nothing to disclose.