Abstract
Bacterial contamination of platelet units has been one of the most common transfusion-transmitted infections. Approximately 4 to 7 fatalities are being reported to the US Food and Drug Administration (FDA) annually, which cites bacterially contaminated platelet units as the cause. Over the past 3 decades, different mitigation strategies have been introduced to minimize the risk of morbidity and mortality related to contaminated platelet units. The process of platelet collection and manufacturing as well as storage at 20°C to 24°C contributes to higher prevalence of contaminated units. The risk of transfusing bacterially contaminated platelets can be lowered using different types of interventions. Prevention of bacterial contamination can be done by strict adherence to techniques that minimize contamination during unit collection. The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve large volume and delayed sampling. Finally, pathogen reduction can inactivate bacteria or other pathogens present in the unit.
This article describes different strategies that blood centers and transfusion services have undertaken since October 2021 to meet FDA guidance requirements. Market forces as well as feasibility of different FDA-proposed approaches have limited the number of practical solutions to just a few. In addition, the blood product availability required hospitals to adopt more progressive strategies to provide patients with needed platelet products.
Learning Objectives
Describe causes of bacterial contamination of platelet components and principles of mitigation strategies
Discuss the impact of US Food and Drug Administration guidance on mitigation of bacterial contamination in platelet components and their effect on hospitals
CLINICAL CASE
A 25-year-old patient with history of aplastic anemia came to the infusion clinic to receive a unit of red blood cells and a unit of platelets. The infusion of red blood cells was uneventful. Approximately 20 minutes after the platelet transfusion was initiated, the patient developed fever, rigors, chills, and transient hypotension. The infusion was stopped, the unit was sent to the transfusion service, and a transfusion reaction investigation was initiated. One hour later, the patient continued to be febrile, tachycardic, and hypotensive despite fluid resuscitation. The patient was admitted, and blood cultures were obtained before starting empiric broad-spectrum antimicrobial therapy. Cultures of the implicated unit and posttransfusion samples from the patient demonstrated growth of Staphylococcus saprophyticus. The patient completed antibiotic therapy and was discharged home after full recovery.
This example is based on a real case of transfusion-associated septic reaction and illustrates the risk of bacterial contamination of platelet units.1 The US Food and Drug Administration (FDA) has developed a new guidance document for collection centers and transfusion services intended to mitigate the risk of bacterial contamination of platelet units.2 This article describes the transfusion services perspective in implementing this new guidance.
Introduction
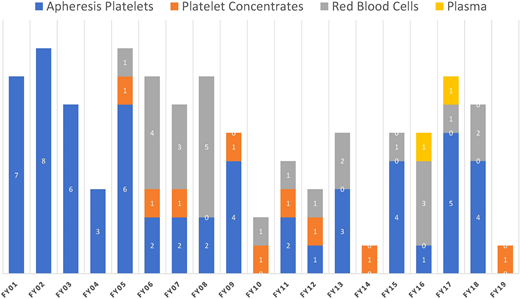
Platelet concentrates are at an increased risk of bacterial contamination due to storage under conditions permissive to accelerated bacterial growth. The FDA mortality data, even if not precise and likely suffering from underreporting, identify fatalities due to transfusion of contaminated units as summarized in Figure 1.3 The actual number of reported fatalities due to bacterial contamination of platelet components, both apheresis and whole blood derived, continues to decrease and remains relatively low. It is possible that some deaths may be not reported due to complexities of patient care and missing causal attribution.
Reported fatalities to the FDA suspected to be a result of microbial contamination. The number of blood components transfused per fiscal year (FY) in these time periods varied between 10 and 20 million per year.26
Reported fatalities to the FDA suspected to be a result of microbial contamination. The number of blood components transfused per fiscal year (FY) in these time periods varied between 10 and 20 million per year.26
Since the introduction of component therapy, it has been recognized that platelet components stored at room temperature provide a better milieu for bacterial growth. The bacteria inoculum, even if small, can enter logarithmic growth and ultimately result in a product with millions of viable organisms as well as debris derived from dying organisms. The transfusion of such a product to a patient may result in a septic reaction and, on rare occasions, in fatality.
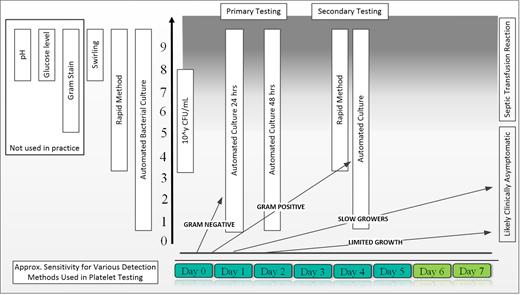
Recognition of this risk has led to introduction of several interventions over the past 30 years, which resulted in a significantly decreased risk of contamination and ultimately a decreased risk of septic complications (Figure 2). These interventions are focused on donor and/or product. The donor-focused mitigation strategies include improved donor interview and examination, deferral of donors with increased risk of bacteremia (eg, recent dental procedures, unhealed wounds), thorough cleaning of the phlebotomy site, diversion of the initial 20 to 40 mL of blood after needle insertion to avoid a skin plug entering the collection system, and postdonation self-deferral due to symptoms suggestive of infection. The product-related interventions included testing of the product for changes indicative of potential contamination (eg, pH, glucose level, swirling), direct detection of bacterial contamination (eg, a microbial culture), the length of storage postcollection (ie, 3 to 7 days), and treatment of the product with substances that are bactericidal and/or bacteriostatic (Figure 2). Some of these interventions have been introduced and ultimately required by voluntary accrediting organizations (eg, College of American Pathologists; Association for the Advancement of Blood and Biotherapies [AABB]) since 2004 and driven by consideration for safety of blood components. There has been extensive research involved in identifying the most sensitive and specific approaches. At the same time, there has been a growing concern that only certain interventions seemed to be successful in lowering the risk of detectable contamination (eg, diversion of blood decreased the contamination rate by around 70% and in combination with improved arm preparation by 77%) while others were unhelpful (eg, pH, glucose testing). The detection of the presence and/or growth of bacteria seemed particularly promising as it was not affecting the platelets and was very sensitive, especially if performed later during the storage (ie, at least 24 hours postcollection and 12 hours after obtaining bacterial cultures). Figure 2 illustrates several important considerations when successful and timely detection of contamination is to be considered. All potential points of entry of bacteria into the blood products are summarized in Figure 3. Identification of these points is important in determining the most effective strategies for bacterial contamination mitigation strategies.
Bacterial growth and detection in platelet products. The initial bacterial inoculum can be very small and is also infrequent, with an estimated 0.1% to 0.03% of products being microbially contaminated. Different microbial organisms have different growth curves, which affect the likelihood of detection with primary testing (unless delayed). Gram-positive, slow-growing, and limited-growth organisms can be missed on primary testing. The Gram-positive bacteria can reach concentrations above 10e5 colony-forming units (CFU/mL), which are associated with symptomatic septic reactions. The secondary testing is more likely to detect such organisms.
Bacterial growth and detection in platelet products. The initial bacterial inoculum can be very small and is also infrequent, with an estimated 0.1% to 0.03% of products being microbially contaminated. Different microbial organisms have different growth curves, which affect the likelihood of detection with primary testing (unless delayed). Gram-positive, slow-growing, and limited-growth organisms can be missed on primary testing. The Gram-positive bacteria can reach concentrations above 10e5 colony-forming units (CFU/mL), which are associated with symptomatic septic reactions. The secondary testing is more likely to detect such organisms.
Elements to consider in mitigation of bacterial contamination of platelets. IV, intravenous.
Elements to consider in mitigation of bacterial contamination of platelets. IV, intravenous.
At the same time, another method for significantly decreasing the risk of bacterial contamination has been shown to be successful—namely, pathogen reduction (PR).4,5 In this approach, the component is treated with a chemical and/or UV light that either inhibits growth and/or destroys the organism. Thus, at this point, 2 competing approaches (ie, detection and elimination) became available and blood centers started to consider these different approaches.
As expected, both approaches have advantages and disadvantages. The testing pathway has evolved over the past 30 years.6,7 The recognition of the risks combined with increasing feasibility of laboratory testing provided for greater sensitivity of testing. The initial tests that used swirling, pH, and glucose measurements were extremely inefficient and carried low sensitivity.8 These tests were replaced by more specific and sensitive bacterial cultures and detection systems using antigen and genetic material focused on the presence of bacterial-derived material in a blood product. From a practical perspective, bacterial culture needed to address 2 possibly competing objectives: first, to provide for increased sensitivity of detecting uncommon events with possibly only few organisms present and, second, to optimize the volume and timing of testing so the product continues to be clinically useful.
Multiple studies showed that an increase in the volume of material tested and a longer growth phase result in a better detection rate.9 These observations led to 2 major changes to bacterial detection—namely, large-volume delayed sampling (ie, LVDS 36 and 48 hours postcollection) and the use of both anaerobic and aerobic culture media.6 These new approaches also require a delayed release for transfusion (12 or 24 hours) after the initial culture (36 or 48 hours from collection) to allow for the automated detection system to increase the sensitivity of testing (ie, small colony-forming unit load may take several hours to be detected). The LVDS 48 paradigm, obtaining bacterial cultures 48 hours postcollection, allowed for testing to be limited to blood donor centers and, based on the UK and US data, qualify such products for 7-day storage7 (see below).
Another method of bacterial detection relies on rapid testing (eg, Pan Genera Detection testing), which is performed prior to issue of the product for transfusion.10,11 This method detects the presence of material derived from Gram-positive and Gram-negative bacteria. It has been used for over 15 years in many transfusion services and provided for the ability to extend the outdate of apheresis platelets up to 7 days.11–13 This approach requires involvement of transfusion service personnel, and its limit of detection is several fold higher (eg, depending on the organism starting at 10e3-10e5 colony-forming units/mL) than bacterial culture (1-10 colony-forming units/mL).14,15
The second pathway is based on PR.4,5,14 This technology has been in development for over 30 years but only more recently has been approved for use in the United States while being more widely used in other countries. PR is achieved by inactivation of viruses, bacteria, and parasites using UV light with or without a photosensitizer. It is a proactive approach as it treats all platelet products irrespective of their contamination status. This technology has an impact on the physiology of platelets, making them less effective (eg, lower corrected count increment and increased frequency of transfusion) in exchange for increased safety. Some authors believe it is still a good trade-off for increased safety, especially against new pathogens.5 Interestingly, PR platelets can still be bacterially contaminated, although less frequently than untreated platelets.1,14,16 These products have 5-day expiration in the United States at the time of this writing.
The source of bacterial contamination can be also environmental. A recent review triggered by discovery of Acinetobacter spp as a cause of several septic reactions revealed that container damage may lead to bacterial contamination with environmental organisms.16 These contaminants may enter the bag after the inactivation process and grow to concentrations sufficient to cause septic reactions.
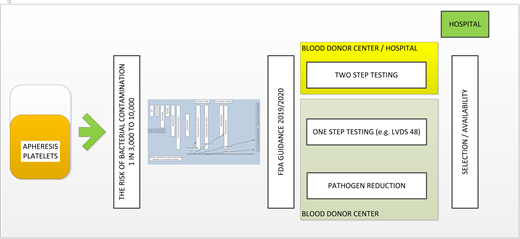
Regulatory framework—2019/2020 FDA guidance
In this context of uncertainty, the field has moved in multiple directions partially driven by price and partially driven by available scientific data. Over the course of several years, the FDA has worked on a guidance document based on scientific data.
The regulatory framework had to address several different types of bacterial contamination mitigation strategies. Primarily, the concerns were raised about the impact on platelet availability as well as their quality based on the different pathways of mitigation strategies. This concern could be addressed by using PR systems that deliver products with a lower risk of bacterial contamination. At the same time, platelet components that undergo chemical treatments show some significant changes in their function and clinical efficacy, including lower recovery posttransfusion and, arguably, a shorter shelf-life compared with platelets undergoing bacterial culture. The 2-step mitigation strategies combine the features of longer storage time up to 7 days and the lack of impact on platelet quality. However, the 2-step approach raises other issues due to required collaboration between the blood centers providing the platelets with the first-step testing and the transfusion services and hospitals needing to perform the second-step testing. It has also become apparent that many facilities were not ready or not willing to participate in a 2-step mitigation strategy.17
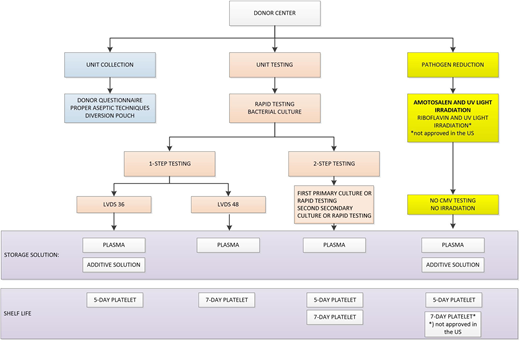
In this context, the FDA submitted a few draft guidance documents that attempted to address different concerns from stakeholders. There was robust discussion in the field related to optimal approaches. In September 2019, the guidance document entitled “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion” was published.2 The implementation date was set for 2020 and extended to October 1, 2021, due to the ongoing pandemic. The final document is a compromise trying to address and, to some extent, accommodate different strategies for increasing platelet safety. There are 9 different pathways allowed to achieve this goal (Figure 4). Some pathways included 1-step testing while others included 2-step testing. Finally, there was recognition that these testing approaches are considered an improvement to previous testing to reduce the risk of bacterial contamination. However, the multiple options were not equivalent in the context of price and logistics. It became quickly apparent that only a couple of options would be pursued due to these concerns. The selection of the optimal options should balance the increased cost of any of the approaches and complex implementation logistics with longer shelf life and availability.
Summary of steps in bacterial contamination mitigation strategies. CMV, cytomegalovirus.
Summary of steps in bacterial contamination mitigation strategies. CMV, cytomegalovirus.
The AABB in its Association Bulletin interpreted this guidance document as providing for “a) all mitigation strategies improve safety compared to current practice; b) all mitigation strategies are approved by the FDA and are deemed therapeutically equivalent; c) hospitals should be prepared to accept a range of products to maximize platelet availability; and d) hospitals should be prepared to perform secondary testing to maximize platelet availability.”18,19 Although this seems a very reasonable interpretation, market forces relatively quickly narrowed down the initially offered options. Large blood centers decided to provide a limited number of options to their customers, as discussed below. Figure 4 illustrates the complexity of options as well as different limitations of each solution.
Blood collection center response
The implementation of the new guidance represented a significant challenge for blood collection facilities. Blood centers connected with their hospitals (clients) to understand their preferences and needs. The final results of such conversations needed to ensure cost-effective production of platelets while providing adequate supply. Securing client satisfaction was not an easy task for the blood centers, and it continues to be an issue, especially when there is only 1 supplier of platelet products available.
New products require new standard operating procedures, training, updating and validating codes, new labels, lengthy licensing processes, and, in some instances, major changes in their computer system. For example, collection of LVDS platelet products requires increased sampling volumes resulting in decreasing collection yield and available platelet units for transfusion. Also, false-positive microbial testing results decrease the availability of products. These limitations may create logistical challenges and require “overcollection” of platelet units to address these losses. In terms of PR, given a set of specifications related to the volume and platelet yield needed for chemical treatment, not all collected units may qualify for treatment by PR, with the subsequent concern of diverting units that do not qualify for PR to other testing strategies.
Transfusion service and hospital response
The response of transfusion services to the new guidance document by the FDA has been variable.17 The hospitals and transfusion medicine services have become concerned with new products as well as their availability. For these reasons, it was also practical to continue some of the previous approaches to bacterial mitigation strategies. Several factors affected the response of hospitals, including (1) the size of the hospital as well as the number of platelet units transfused, (2) the distance to the closest blood supplier, (3) the availability and feasibility of rapid testing, (4) the willingness to perform 2-step testing, and (5) clinical considerations regarding the patient population served and types of platelet products best suited to address patients' needs. Briefly, we discuss these concerns below.
Hospital size and patient population
The overall volume of transfused components had a significant impact on how the hospitals perceived new changes. Large hospitals had difficulties implementing a 2-step strategy; therefore, only 2 practical options remained: LVDS 48 and/or PR platelets. Both options had their shortcomings, and some hospitals decided to pursue a mixed inventory containing both types of products.
Patient population
Hospital-based transfusion services have different needs based on their size and patient mix: large centers might benefit from platelets ready to use (ie, LVDS 48, PR), smaller centers might benefit from having platelets with the longest practical shelf life (PR, LVDS 48), and trauma centers might benefit from readily available platelets stored in plasma with the highest potency (LVDS 48). Cancer centers supporting patients who require multiple transfusions over long periods of time might benefit from using platelets stored in additive solution, which reduces the incidence of allergic reactions and allows for ABO substitutions.20,21 Platelets stored in additive solution can be obtained by LVDS 36 and PR but cannot be extended to 7-day storage. PR platelets do not require irradiation and do not need to be tested for cytomegalovirus status; these characteristics might be attractive for cancer and pediatric centers as well.
Economic considerations
Many transfusion services have been concerned with the financial impact of moving to PR technology. The economic impact of such a transition has been modeled by a group of investigators in a study sponsored by the PR technology manufacturer. They compared 3 different scenarios: PR platelets only, LVDS 48 only, and a mix of both. The authors relied on published data and survey results from 27 institutions. The authors concluded that there is a small, unclear if economically relevant difference between all 3 approaches. Despite some important limitations of this study (eg, the authors assumed existence of a 7-day PR platelet product that had the lowest cost but is not approved by the FDA), one can use it as an example of budgetary analysis prior to committing to a particular approach.22
Some other publications have been less supportive of the financial superiority of PR platelets and considered other detection methods as financially more advantageous (eg, LVDS 48).23,24 These financial considerations have limited the impact on blood suppliers once the largest blood collector made a strategic decision to move to PR products exclusively. However, customers, including large hospitals, may use these evaluations for their interactions with smaller blood suppliers who might be willing to recognize and consider customer preferences in their business decisions. These analyses can also strengthen the argument for “vendor shopping” to minimize the cost to transfusion services and hospitals with equivalent, if not preferred, clinical outcomes to the patients.
The financial impact of the implementation of the FDA guidance is different for blood centers and their customers. The blood centers may consider this new product requirement as a potential margin-generating initiative. This cannot be said of their customers who pay a higher price for at best an equivalent if not an inferior product. This tension is noted in an AABB News publication in 2021.25
Our approach
Now, as we are approaching 1 year since implementation of the FDA guidance, it has become apparent that there are primarily 2 preferred options by both blood supplier and hospitals. These are PR platelets with a 5-day expiration and LVDS 48 platelets with a 7-day expiration. They have their limitations, but they have improved logistics and simplicity of the approach.18
Some transfusion services still retain the ability for secondary testing. Such testing continues to be used in the transition phase as suppliers decide on their best pathway forward or offer products that will be eventually discontinued. The transition initially led to “vendor shopping” as hospitals attempted to obtain platelet components with particular characteristics.
Conclusions
Platelet units are now tested using different methods and stored in different conditions. Platelet products manufactured following the FDA guidance are deemed to have adequate safety, potency, and purity for transfusion in different clinical scenarios. Blood centers and transfusions services should team up to ensure enough flexibility to allow for cost-effective strategies that allow for a robust platelet inventory. The best platelet product is the one that is available for the patient.
Conflict-of-interest disclosure
Zbigniew M. Szczepiorkowski: Fresenius Kabi: Consultancy, Scientific Advisory Board, Research Funding; Grifols Inc: Consultancy, Honoraria; Novartis: Consultancy; Erydel: Research Funding.
Monica B. Pagano: no competing financial interests to declare.
Off-label drug use
Zbigniew M. Szczepiorkowski: nothing to disclose.
Monica B. Pagano: nothing to disclose.