Abstract
Anticoagulants have been in use for nearly a century for the treatment and prevention of venous and arterial thromboembolic disorders. The most dreaded complication of anticoagulant treatment is the occurrence of bleeding, which may be serious and even life-threatening. All available anticoagulants, which target either multiple coagulation factors or individual components of the tissue factor (TF) factor VIIa or the common pathways, have the potential to affect hemostasis and thus to increase bleeding risk in treated patients. While direct oral anticoagulants introduced an improvement in care for eligible patients in terms of safety, efficacy, and convenience of treatment, there remain unmet clinical needs for patients requiring anticoagulant drugs. Anticoagulant therapy is sometimes avoided for fear of hemorrhagic complications, and other patients are undertreated due to comorbidities and the perception of increased bleeding risk.
Evidence suggests that the contact pathway of coagulation has a limited role in initiating physiologic in vivo coagulation and that it contributes to thrombosis more than it does to hemostasis. Because inhibition of the contact pathway is less likely to promote bleeding, it is an attractive target for the development of anticoagulants with improved safety.
Preclinical and early clinical data indicate that novel agents that selectively target factor XI or factor XII can reduce venous and arterial thrombosis without an increase in bleeding complications.
Learning Objectives
Understand the data in support of targeting the contact pathway for antithrombotic therapy and its potential advantages over existing anticoagulants
Become acquainted with the various factor XI and factor XII targeting agents under development and their prospective indications
CLINICAL CASE
A 75-year-old man is referred for a total knee arthroplasty (TKA) due to osteoarthrosis of the right knee. His medical history includes previous symptomatic distal deep vein thrombosis secondary to TKA in the contralateral knee, previous gastrointestinal bleeding associated with peptic ulcer disease, arterial hypertension controlled with an angiotensin II receptor blocker and hydrochlorothiazide, dyslipidemia treated with a statin, diabetes mellitus controlled with metformin and a sodium-glucose cotransporter 2 inhibitor, and diabetic nephropathy with baseline creatinine clearance (CrCl) of 32 mL/min.
The patient requires antithrombotic therapy for venous thromboembolism (VTE) prophylaxis. However, the risk of bleeding in this patient is a concern. Is there a safe and effective therapeutic option for VTE prophylaxis for this patient?
Introduction
Thromboembolic diseases are associated with significant morbidity and mortality, and anticoagulation is the mainstay of treatment.1,2 Currently available anticoagulants that target multiple coagulation factors or single components of the common pathway of coagulation can affect hemostasis, and bleeding complications may counterweight the benefits of antithrombotic treatment.3
Consequently, there remains a need for effective anticoagulants with a lower risk of bleeding complications. To accomplish this, focus has been placed on factor (F) XI and FXII, essential components of the contact (intrinsic) coagulation pathway, which have been proposed as new and promising targets for anticoagulant therapy with minimal or no bleeding risk.4,5
This review discusses the shortcomings of existing anticoagulants and the advances made in understanding the role of the contact system in thrombosis and hemostasis as a new target for anticoagulant treatment with improved safety, and it details the clinical data currently available and the ongoing studies with agents targeting FXI and FXII.
In search of the holy grail: effective drugs that minimize the risk of bleeding
A brief historical perspective of anticoagulant treatment
Anticoagulants have been in use for nearly a century for both the treatment and prevention of venous and arterial thromboembolic disorders. Unfractionated heparin, a naturally occurring glycosaminoglycan, was discovered in 1916. Low-molecular-weight heparin was developed in the 1980s, adding the advantages of less frequent subcutaneous administration, lower risk of bleeding and heparin-induced thrombocytopenia, better bioavailability, administration at fixed doses, and longer half-life. Through binding and activation of antithrombin, heparins inactivate thrombin, FXa, and other coagulation proteases.6 Fondaparinux is a synthetic heparin pentasaccharide with a sequence almost identical to the antithrombin binding pentasaccharide found in heparin. It targets FXa activity, but it does not inhibit thrombin activity or release tissue factor pathway inhibitor, offering an improved therapeutic index.7 Warfarin, discovered in 1941 and approved for medical use in humans in 1954, and other vitamin K antagonists (VKAs) competitively inhibit the vitamin K epoxide reductase complex 1, an enzyme essential for vitamin K activation, consequently reducing the synthesis of biologically active forms of the clotting factors II, VII, IX, and X, as well as the native anticoagulants protein C and protein S. VKAs have several disadvantages attributable to their unpredictable pharmacokinetics and pharmacodynamics, a narrow therapeutic index, slow onset of activity, numerous drug and food interactions, and, therefore, need for routine monitoring.8,9
Argatroban and bivalirudin are parenteral direct thrombin inhibitors developed in the 1990s for use in various antithrombotic indications. Unlike heparin, direct thrombin inhibitors do not require antithrombin as a cofactor for activity and can inhibit both soluble as well as clot-bound thrombin. These agents have a therapeutic index comparable to that of heparins but are invaluable in conditions where a contraindication to heparin exists, including heparin-induced thrombocytopenia or an allergy to heparin.10
The direct oral anticoagulants (DOACs) were approved in the 2010s and act by selectively targeting and inhibiting either thrombin (dabigatran) or FXa (apixaban, edoxaban, and rivaroxaban).11 The DOACs were shown to be as effective and safer than VKAs in patients with atrial fibrillation (AF) and VTE and offer practical advantages thanks to the oral administration at fixed doses with no need for laboratory monitoring. However, the DOACs also bear a nonnegligible bleeding risk, regardless of the indication for which they were prescribed.12,13 A timeline illustrating key landmarks in the development of anticoagulants is presented in Figure 1.
Key landmarks in the development of anticoagulants. FDA, Food and Drug Administration; LMWH, low-molecular-weight heparin; UFH, unfractionated heparin.
Key landmarks in the development of anticoagulants. FDA, Food and Drug Administration; LMWH, low-molecular-weight heparin; UFH, unfractionated heparin.
Patient categories at increased risk of bleeding with anticoagulant therapy
The most important, common, and dreaded complication of anticoagulant treatment is the occurrence of bleeding, which may be serious, life-threatening, or have devastating long-term consequences. Anticoagulant therapy is often avoided for fear of hemorrhagic complications, and many patients are undertreated due to comorbidities or a precepted unacceptable bleeding risk.3
All of the available anticoagulant drugs have the potential to affect hemostasis and, thus, to increase bleeding risk in treated patients. This risk is higher in certain patient populations.
The incidence of AF, stroke, and VTE increases with age, and so does the risk of bleeding complications with anticoagulant treatment. Although elderly patients have a higher absolute benefit from anticoagulant treatment, bleeding is a major concern, even at lower doses of DOACs, particularly in frail patients, those with renal dysfunction, and patients taking multiple medications with potential drug-drug interactions, who may also be prone to recurrent falls.14
Acute ischemic stroke in patients with AF is associated with a high risk for both hemorrhagic transformation and ischemic recurrence in the acute poststroke period. Large ischemic lesions, cerebral microbleeds, and thrombolytic therapy may all increase the risk of hemorrhagic transformation, whereas high CHA2DS2-VASc score [CHADS, Congestive heart failure, Hypertension, Age 75 or more, Diabetes, Stroke; VASc, Vascular disease, Age 65–74, Sex category (female sex)], high National Institutes of Health Stroke Scale score, and large ischemic lesions are all associated with a higher risk for both ischemic recurrence and bleeding.15 It is suggested that the best time for initiating anticoagulation for secondary stroke prevention is 4 to 14 days from stroke onset. There is, however, a concern that while early initiation increases the risk of hemorrhagic transformation, delayed initiation leaves the patient at risk for recurrent ischemic stroke.16 Moreover, the combined use of anticoagulants and antiplatelet agent in patients with AF and cardiovascular or cerebrovascular disease significantly increases the risk of major bleeding.17
Patients with cancer are at increased risk of VTE and recurrent events, but also at increased risk of major bleeding, regardless of the choice of anticoagulant.18 Metastatic disease, primary gastrointestinal malignancies, chronic kidney disease stage III or higher, and thrombocytopenia <100 000 × 109/L are all associated with increased risk of bleeding.19 The challenge of balancing competing risks of bleeding and recurrent VTE is greater in the setting of thrombocytopenia, which may require anticoagulant dose modifications.20,21
Patients with cirrhosis frequently have complex alterations in their hemostatic system in which standard coagulation laboratory test may not represent the actual hemostatic balance.22 Nevertheless, portal hypertension complicating liver cirrhosis may increase the risk of bleeding from esophageal varices.23
Finally, patients with chronic kidney disease have an increased risk of arterial and venous thrombosis, as well as an increased risk of bleeding, attributable to platelet dysfunction.24-26
All these patients may benefit from anticoagulants with an improved safety profile, preferably with minimal renal clearance, and an enhanced efficacy in patients with a high thrombotic risk.
The contact pathway of coagulation
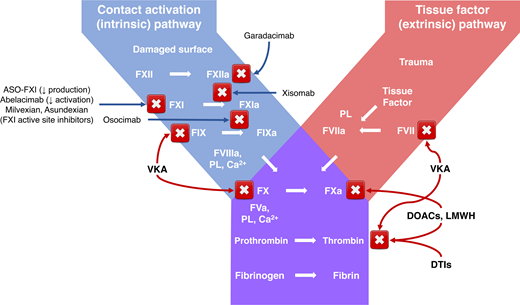
The contact pathway of coagulation consists of the proteins FXI, FXII, prekallikrein, and high-molecular-weight kininogen (HK) and is involved in procoagulant and proinflammatory reactions, thus linking inflammatory and coagulopathic responses. Evidence suggests that it has a limited role in initiating physiologic in vivo coagulation and that it contributes to pathologic thrombosis and inflammation more than it does to hemostasis.27-30
The contact pathway can be activated by exposure of blood to in vivo activators, including extracellular nucleic acids, neutrophil extracellular traps, long-chain polyphosphates from various infectious pathogens, and negatively charged artificial surfaces of indwelling medical devices. These negatively charged compounds bind FXII, inducing activation of the serine protease FXIIa, which in turn activates HK-bound prekallikrein, forming plasma kallikrein (PKa). PKa amplifies FXII activation by positive feedback, inducing subsequent downstream activation of both coagulation and inflammation. FXIIa activates FXI to FXIa and triggers the activation of FIX, FX, and prothrombin, whereas activation of PKa results in cleavage of HK and the release of the vasoactive peptide bradykinin.28,31
FXI deficiency is a rare autosomal recessive disorder. Since FXI is required for driving clot formation in the activated partial thromboplastin time assay, patients lacking FXI have markedly prolonged activated partial thromboplastin times. Despite this, patients with very low FXI levels may be asymptomatic, and bleeding in FXI-deficient patients is typically traumatic or surgical, involving tissues rich in fibrinolytic activity such as the mouth, nose, and urinary tract.32 Patients with severe FXI deficiency have a reduced incidence of deep vein thrombosis and ischemic stroke, but FXI deficiency does not confer protection against acute myocardial infarction.33
FXII deficiency is inherited as an autosomal recessive disorder. It is not associated with any bleeding risk. The relationship between FXII and thrombosis is not well established, yet evidence suggests FXII deficiency is not protective against thrombosis.34,35 Nonetheless, due to its proinflammatory and prothrombotic properties, FXII may be targeted in disease conditions in which these downstream cascades are dysregulated, such as hereditary angioedema, neuroinflammatory and neurodegenerative disorders,34-36 and indwelling medical device–related thrombosis.37
Differentiating thrombosis from hemostasis
Hemostasis and thrombosis share similar enzymatic reactions but yield different outcomes.
While hemostasis is the natural process that halts bleeding at injury sites by formation of a local hemostatic plug, thrombosis is generally pathologic, in which interruption of blood flow may lead to organ damage. Differentiating these processes is key to the development of antithrombotic agents with minimal effect on hemostasis.38
With some evidence of its contribution to thrombosis, the contact pathway is an attractive target for the development of anticoagulants with an enhanced safety profile, because due to its nature, inhibition is less likely to promote bleeding compared with anticoagulants that target multiple coagulation factors or the tissue factor (TF)-FVIIa or the common pathways.38,39 Both FXI and FXII have received attention as potential targets in the development of new anticoagulants with improved safety. Nevertheless, it is uncertain whether FXI or FXII represents a better therapeutic target, weighing efficacy vs safety. While severe FXI deficiency was found to confer protection from thrombotic events, data for FXII are inconclusive. On the other hand, because congenital deficiency of FXII is not associated with a bleeding diathesis, FXII may be perceived a safer target than FXI, although efficacy may be hindered since FXI activation by TF-FVIIa and thrombin may still occur.4,39
Development of agents to target FXI and FXII
Preclinical studies indicated that knockout mice deficient in one of the contact proteins were protected against artificially induced thrombosis. Moreover, inhibition of the contact pathway by monoclonal antibodies, antisense oligonucleotides, and small molecules targeting either FXI or FXII were found to prevent both venous and arterial thrombosis in various animal models in mammals.29,30 The main advantage of these new anticoagulants is their selectivity to targeting single molecules, which reduces the likelihood of off-target adverse effects.4 Subsequent studies in human participants confirmed the safety of these agents in phase 1 and phase 2 clinical trials.
Agents directed against FXI include antisense oligonucleotides (ASO)40,41 and DNA aptamers,42,43 which inhibit the biosynthesis of FXI, as well as FXI targeting monoclonal antibodies and small molecules. ASOs bind to their complementary messenger RNA (mRNA) targets, thereby allowing for selective and catalytic degradation of the targeted mRNA, which leads to a reduction in the corresponding protein levels, with a high degree of target selectivity. FXI is synthesized primarily in the liver, an optimal target tissue for ASO therapy.40,44 ASOs targeting FXI demonstrated effectiveness in various animal models,40,45-47 as well as in humans.48-50 Numerous monoclonal antibodies are under development. These directly inhibit the activation of FXI (abelacimab)51-53 or the activation of FIX by FXIa (osocimab),54-57 they or block FXIIa-mediated activation of FXI (xisomab, O1A6 and 14E11).58-61 Notably, xisomab does not inhibit FXI activation mediated by thrombin, and although it binds FXI and not FXII, it has no effect on FXIa activity and thus may be considered a backdoor inhibitor of FXIIa.59,60 Orally available small-molecule inhibitors of FXI include milvexian (BMS-986177/JNJ-70033093)62-66 and asundexian (BAY 2433334).67,68 Parenteral small molecules directed at the active site or exosites of FXI (FXI BMS-262084, BMS-654457, and ONO-8610539) were mostly studied in animal models.69,70 Naturally occurring inhibitors of FXIa include molecules derived from snakes (fasxiator), vampire bats (desmolaris), and ticks (boophilin), none of which were studied in human participants.71-73
Fewer agents in development target FXII. These include the monoclonal antibody garadacimab (formerly known as CSL 312)36,74-77 and small molecules and ASOs, which so far have been investigated only in animal models.74
FXI and FXII inhibitors differ in their pharmacologic properties, which will define their clinical application. ASOs (IONIS FXI-Rx and fesomersen) and antibodies (abelacimab, osocimab, xisomab, and garadacimab) require parenteral administration by subcutaneous or intravenous injection. Small molecules (milvexian and asundexian) can be delivered orally or parenterally. ASOs that target FXI mRNA, causing its degradation, have a slow onset of action since reduction levels into the therapeutic range require 3 to 4 weeks, thus disqualifying them for use in acute settings. By contrast, rapid inhibition of FXI can be achieved by antibodies, DNA aptamers, and small molecules, which enables their use in both acute and chronic settings.4,39 Last, ASOs and antibodies have long half-lives (mean elimination half-life of 52 hours for FXI-ASO,40 25-30 days for abelacimab, and 30-44 days for osocimab),56 which may allow for less frequent administration but may also require reversal strategies.78 On the other hand, small-molecule inhibitors milvexian and asundexian have much shorter half-lives and are expected to be administered once or twice daily, similar to DOACs.64-68 The pharmacologic properties of these FXI and FXII inhibitors are presented in Table 1.
Characteristics, mechanism of action, and pharmacologic properties of FXI and FXII inhibitors
| . | Mechanism of action . | Route of administration . | Onset of action . | Half-life . | Administration frequency . | Renal excretion . | Metabolism by CYP . | Potential for food and drug interactions . |
|---|---|---|---|---|---|---|---|---|
| DNA aptamers: 11.16, 12.7, and FELIAP | Bind over large surface areas on a target protein and block specific macromolecular interactions | IV or SC | Rapid (minutes to hours) | Short (minutes to hours) | Daily | No | No | No |
| ASOs: IONIS FXI-Rx (ISIS 416858) and fesomersen | Inhibit the biosynthesis of FXI | SC | Slow (weeks) | Long (weeks) | Once weekly to once monthly | No | No | No |
| Antibodies | ||||||||
| Abelacimab (MAA868) | Binds to the catalytic domain of FXI and locks it in the inactive zymogen conformation, preventing its activation by FXII/thrombin | IV or SC | Rapid (hours) | Long (weeks) | Once monthly | No | No | No |
| Osocimab (BAY 1213790) | Binds next to the active site of FXIa and inhibits the activation of factor IX | IV or SC | Rapid (hours) | Long (30-44 days) | Once monthly | No | No | No |
| Xisomab (AB023) | Inhibits FXIIa- mediated activation of FXI but not FXI activation by thrombin | IV | Rapid (hours) | Days to weeks, half-life increases with high doses | Once monthly | No | No | No |
| Garadacimab (CSL312) | Binds to the catalytic domain of FXIIa and inhibits its protease activity | IV or SC | Rapid (hours) | Long (weeks) | Once monthly | No | No | No |
| Small molecules | ||||||||
| Milvexian (BMS-986177/JNJ-70033093) | Active site-directed inhibitor of FXI | Oral | Rapid (minutes to hours) Saturable absorption with doses ≥300 mg | Short (terminal half-life 8.3-13.8 hours) | Once or twice daily | Yes, <20% | Yes | Yes |
| Asundexian (BAY 2433334) | Active site-directed inhibitor of FXI | Oral | Rapid (minutes to hours) | Short (terminal half-life 15.8-17.8 hours) | Once daily | Yes, <15% | Yes | Yes |
| . | Mechanism of action . | Route of administration . | Onset of action . | Half-life . | Administration frequency . | Renal excretion . | Metabolism by CYP . | Potential for food and drug interactions . |
|---|---|---|---|---|---|---|---|---|
| DNA aptamers: 11.16, 12.7, and FELIAP | Bind over large surface areas on a target protein and block specific macromolecular interactions | IV or SC | Rapid (minutes to hours) | Short (minutes to hours) | Daily | No | No | No |
| ASOs: IONIS FXI-Rx (ISIS 416858) and fesomersen | Inhibit the biosynthesis of FXI | SC | Slow (weeks) | Long (weeks) | Once weekly to once monthly | No | No | No |
| Antibodies | ||||||||
| Abelacimab (MAA868) | Binds to the catalytic domain of FXI and locks it in the inactive zymogen conformation, preventing its activation by FXII/thrombin | IV or SC | Rapid (hours) | Long (weeks) | Once monthly | No | No | No |
| Osocimab (BAY 1213790) | Binds next to the active site of FXIa and inhibits the activation of factor IX | IV or SC | Rapid (hours) | Long (30-44 days) | Once monthly | No | No | No |
| Xisomab (AB023) | Inhibits FXIIa- mediated activation of FXI but not FXI activation by thrombin | IV | Rapid (hours) | Days to weeks, half-life increases with high doses | Once monthly | No | No | No |
| Garadacimab (CSL312) | Binds to the catalytic domain of FXIIa and inhibits its protease activity | IV or SC | Rapid (hours) | Long (weeks) | Once monthly | No | No | No |
| Small molecules | ||||||||
| Milvexian (BMS-986177/JNJ-70033093) | Active site-directed inhibitor of FXI | Oral | Rapid (minutes to hours) Saturable absorption with doses ≥300 mg | Short (terminal half-life 8.3-13.8 hours) | Once or twice daily | Yes, <20% | Yes | Yes |
| Asundexian (BAY 2433334) | Active site-directed inhibitor of FXI | Oral | Rapid (minutes to hours) | Short (terminal half-life 15.8-17.8 hours) | Once daily | Yes, <15% | Yes | Yes |
CYP, cytochrome P450; IV intravenous; SC, subcutaneous.
In case of bleeding complications, growing evidence suggests that patients with FXI deficiency rarely require FXI replacement during surgery and may be typically managed with antifibrinolytics and occasionally recombinant FVIIa. Therefore, these strategies are expected to be effective for patients taking therapeutic FXI inhibitors with bleeding or who require urgent surgery.78
Clinical trials with FXI and FXII inhibitors
A few FXI and FXII inhibitors are at various stages of clinical development, with data from phase 2 studies available for some. None of these agents has completed evaluations in phase 3 studies. We hereby provide an overview of selected clinical data currently available and ongoing studies with agents targeting FXI and FXII. Clinical data from phase 2 trials are summarized in Table 2, and ongoing clinical trials are presented in Table 3.
Clinical data from completed phase 2 trials with FXI and FXII inhibitors
| Agent . | Registry number (name) . | Clinical trial phase and indication . | No. of patients . | Comparator . | Efficacy outcome . | Safety outcome . | Remarks . |
|---|---|---|---|---|---|---|---|
| IONIS FXI-Rx (ISIS 416858) | NCT01713361 | Phase 2 VTE prevention in patients undergoing TKA48 | 315 | SC enoxaparin 40 mg OD | Composite of asymptomatic DVT (via bilateral venography) and symptomatic VTE, fatal PE, and unexplained death | Major bleeding or CRNMB | IONIS FXI-Rx 200 mg noninferior efficacy, 300 mg superior efficacy, comparable safety |
| NCT02553889 | Phase 2 Patients with ESRD on hemodialysis50 | 49 | Placebo | PK | Safety and tolerability; frequency and severity of adverse events | PK in patients with ESRD consistent with previous reports in healthy volunteers Less category 3 or 4 clots in the air traps and dialysis membranes | |
| Abelacimab (MAA868) | EudraCT 2019-003756-37 (ANT-005 TKA) | Phase 2 VTE prevention in patients undergoing TKA53 | 412 | SC enoxaparin 40 mg OD | Confirmed VTE (via ipsilateral venography) and symptomatic VTE | Major bleeding or CRNMB | 30 mg abelacimab noninferior efficacy, 75 mg and 150 mg superior efficacy, comparable safety |
| Osocimab (BAY 1213790) | NCT03276143 (FOXTROT) | Phase 2 VTE prevention in patients undergoing TKA57 | 813 | SC enoxaparin 40 mg OD or oral apixaban 2.5 mg BID | Composite of asymptomatic DVT (via bilateral venography) and symptomatic VTE, fatal PE, and unexplained death | Major bleeding or CRNMB | Postoperative osocimab 0.6-1.2 mg/kg noninferior to enoxaparin, preoperative osocimab 1.8 mg/kg superior, but ↑ bleeding |
| Xisomab (AB023) | NCT03612856 | Phase 2 Patients with ESRD on hemodialysis61 | 24 | Placebo | Hemodialysis efficiency as measured by frequency of clotting on the dialysis filters and circuit and levels of potassium and blood urea nitrogen | Treatment-related AEs, bleeding | Xisomab well tolerated, less occlusive events |
| Garadacimab (CSL312) | NCT03712228 | Phase 2 Prevention of attacks in HAE-C1-INH36 | 32 | Placebo | Time-normalized number of HAE-C1-INH attacks during treatment period | AEs and serious AEs, anaphylaxis, thromboembolic events, and bleeding events | Garadacimab significantly reduced the number of monthly attacks vs placebo and was well tolerated during the study |
| Milvexian (BMS-986177/JNJ-70033093) | NCT03891524 (AXIOMATIC-TKR) | Phase 2 VTE prevention in patients undergoing TKA66 | 1242 | SC enoxaparin 40 mg OD | Confirmed VTE (via ipsilateral venography) and symptomatic VTE | Major bleeding or CRNMB | Met proof-of-efficacy criteria; VTE incidence 12% significantly lower than the prespecified benchmark of 30% (1-sided P < .001) |
| Asundexian (BAY 2433334) | NCT04218266 (PACIFIC-AF) | Phase 2 Stroke prevention in AF68 | 753 | Apixaban 5 mg BID | NA | Major bleeding or CRNMB | Lower bleeding rates with asundexian 20 mg and 50 mg OD vs apixaban |
| Agent . | Registry number (name) . | Clinical trial phase and indication . | No. of patients . | Comparator . | Efficacy outcome . | Safety outcome . | Remarks . |
|---|---|---|---|---|---|---|---|
| IONIS FXI-Rx (ISIS 416858) | NCT01713361 | Phase 2 VTE prevention in patients undergoing TKA48 | 315 | SC enoxaparin 40 mg OD | Composite of asymptomatic DVT (via bilateral venography) and symptomatic VTE, fatal PE, and unexplained death | Major bleeding or CRNMB | IONIS FXI-Rx 200 mg noninferior efficacy, 300 mg superior efficacy, comparable safety |
| NCT02553889 | Phase 2 Patients with ESRD on hemodialysis50 | 49 | Placebo | PK | Safety and tolerability; frequency and severity of adverse events | PK in patients with ESRD consistent with previous reports in healthy volunteers Less category 3 or 4 clots in the air traps and dialysis membranes | |
| Abelacimab (MAA868) | EudraCT 2019-003756-37 (ANT-005 TKA) | Phase 2 VTE prevention in patients undergoing TKA53 | 412 | SC enoxaparin 40 mg OD | Confirmed VTE (via ipsilateral venography) and symptomatic VTE | Major bleeding or CRNMB | 30 mg abelacimab noninferior efficacy, 75 mg and 150 mg superior efficacy, comparable safety |
| Osocimab (BAY 1213790) | NCT03276143 (FOXTROT) | Phase 2 VTE prevention in patients undergoing TKA57 | 813 | SC enoxaparin 40 mg OD or oral apixaban 2.5 mg BID | Composite of asymptomatic DVT (via bilateral venography) and symptomatic VTE, fatal PE, and unexplained death | Major bleeding or CRNMB | Postoperative osocimab 0.6-1.2 mg/kg noninferior to enoxaparin, preoperative osocimab 1.8 mg/kg superior, but ↑ bleeding |
| Xisomab (AB023) | NCT03612856 | Phase 2 Patients with ESRD on hemodialysis61 | 24 | Placebo | Hemodialysis efficiency as measured by frequency of clotting on the dialysis filters and circuit and levels of potassium and blood urea nitrogen | Treatment-related AEs, bleeding | Xisomab well tolerated, less occlusive events |
| Garadacimab (CSL312) | NCT03712228 | Phase 2 Prevention of attacks in HAE-C1-INH36 | 32 | Placebo | Time-normalized number of HAE-C1-INH attacks during treatment period | AEs and serious AEs, anaphylaxis, thromboembolic events, and bleeding events | Garadacimab significantly reduced the number of monthly attacks vs placebo and was well tolerated during the study |
| Milvexian (BMS-986177/JNJ-70033093) | NCT03891524 (AXIOMATIC-TKR) | Phase 2 VTE prevention in patients undergoing TKA66 | 1242 | SC enoxaparin 40 mg OD | Confirmed VTE (via ipsilateral venography) and symptomatic VTE | Major bleeding or CRNMB | Met proof-of-efficacy criteria; VTE incidence 12% significantly lower than the prespecified benchmark of 30% (1-sided P < .001) |
| Asundexian (BAY 2433334) | NCT04218266 (PACIFIC-AF) | Phase 2 Stroke prevention in AF68 | 753 | Apixaban 5 mg BID | NA | Major bleeding or CRNMB | Lower bleeding rates with asundexian 20 mg and 50 mg OD vs apixaban |
AEs, adverse events; BID, twice daily; CRNMB, clinically relevant nonmajor bleeding; DVT, deep venous thrombosis; HAE-C1-INH, C1-esterase inhibitor-deficient hereditary angioedema; NA, not available; OD, once daily; PE, pulmonary emboli; PK, pharmacokinetics.
Ongoing clinical trials with FXI and FXII inhibitors
| Agent . | Registry number (name) . | Clinical trial phase and indication . | No. of patients . | Comparator . | Additional therapies . | Efficacy outcome . | Safety outcomes . | Study recruitment status* . |
|---|---|---|---|---|---|---|---|---|
| IONIS FXI-Rx (ISIS 416858) | NCT03358030 (EMERALD) | Phase 2 Patients with ESRD on hemodialysis | 315 | Placebo | NA | PK, PD | Major bleeding or CRNMB, treatment- emergent AEs | Completed, awaiting results |
| Fesomersen (IONIS-FXI-LRx and BAY2976217) | NCT03582462 | Phase 1 Healthy volunteers | 66 | Placebo | NA | PK, PD | Treatment- emergent AEs | Completed, awaiting results |
| NCT04534114 (RE-THINc ESRD) | Phase 2 Prevention of cardiovascular events in patients with ESRD | 307 | Placebo | NA | PK, PD | Major bleeding or CRNMB, treatment- emergent AEs | Active, not recruiting | |
| Antibodies | ||||||||
| Abelacimab (MAA868) | NCT04213807 | Phase 2 Atrial fibrillation | 28 | Placebo | NA | Dose-range finding study—PK, PD | Safety, tolerability, and immunogenicity | Completed, awaiting results |
| NCT04755283 (AZALEA-TIMI 71) | Phase 2 Atrial fibrillation | Estimated enrollment 1200 | Rivaroxaban 15 mg and 20 mg OD | NA | NA | Safety and tolerability, major bleeding, and CRNMB | Active, not recruiting | |
| NCT05171049 (ASTER) | Phase 3 Treatment of cancer- associated VTE | Estimated enrollment 1655 | Apixaban 10 mg BID followed by 5 mg BID | NA | Centrally adjudicated VTE recurrence | Major bleeding or CRNMB | Active, recruiting | |
| NCT05171075 (MAGNOLIA) | Phase 3 Treatment of GI and GU cancer-associated VTE | Estimated enrollment 1020 | Dalteparin 200 IU/kg/d followed by 150 IU/kg/d | NA | Centrally adjudicated VTE recurrence | Major bleeding or CRNMB | Active, recruiting | |
| Osocimab (BAY 1213790) | NCT03787368 | Phase 1 Safety in patients with ESRD | 55 | Placebo | NA | PK, PD | Major bleeding or CRNMB | Completed, awaiting results |
| NCT04523220 (CONVERT) | Phase 2 Safety in patients with ESRD | 686 | Placebo | NA | PK, PD | Major bleeding or CRNMB | Active, not recruiting | |
| Xisomab (AB023) | NCT04465760 | Phase 2 Prevention of CAT in patients with cancer receiving chemotherapy | Estimated enrollment 50 | None | NA | Incidence of CAT | Major bleeding or CRNMB | Active, recruiting |
| Garadacimab (CSL312) | NCT04281524 | Phase 1/2 Prevention of CAT in patients with cancer receiving chemotherapy | 0 (study withdrawn) | Placebo | NA | Incidence of CAT | Treatment- emergent AEs | Withdrawn (business decision, not safety related) |
| NCT04409509 | Phase 2 COVID-19 | 124 | Placebo | NA | Endotracheal intubation or death prior to intubation | Completed, awaiting results | ||
| NCT05130970 | Phase 2 Idiopathic pulmonary fibrosis | Estimated enrollment 80 | Placebo | NA | PK, PD | Treatment-emergent AEs | Active, recruiting | |
| NCT04656418 | Phase 3 HAE-C1-INH | Estimated enrollment 60 | Placebo | NA | Time-normalized number of HAE-C1-INH attacks during treatment | Treatment-emergent AEs | Active, not recruiting | |
| Small molecules | ||||||||
| Milvexian (BMS-986177/JNJ-70033093) | NCT03766581 (AXIOMATIC-SSP) | Phase 2 Secondary stroke prevention | 2366 | Placebo | Aspirin, clopidogrel | Composite of new ischemic stroke during the treatment period and new covert brain infarction on MRI | Bleeding according to BARC criteria | Active, not recruiting |
| Asundexian (BAY 2433334) | NCT04304534 (PACIFIC-AMI) | Phase 2 Acute MI | 1592 | Placebo | Aspirin, clopidogrel | CV events: MI, stroke, stent thrombosis, and death | Bleeding according to BARC criteria | Completed, awaiting results |
| NCT04304508 (PACIFIC-STROKE) | Phase 2 Acute noncardioembolic ischemic stroke | 1808 | Placebo | NA | Symptomatic ischemic stroke or covert brain infarcts on MRI | Major bleeding or CRNMB | Completed, awaiting results | |
| NCT04510987 | Phase 1 Patients with ESRD on hemodialysis | 48 | None | NA | PK, PD | Treatment-emergent AEs | Completed, awaiting results | |
| Agent . | Registry number (name) . | Clinical trial phase and indication . | No. of patients . | Comparator . | Additional therapies . | Efficacy outcome . | Safety outcomes . | Study recruitment status* . |
|---|---|---|---|---|---|---|---|---|
| IONIS FXI-Rx (ISIS 416858) | NCT03358030 (EMERALD) | Phase 2 Patients with ESRD on hemodialysis | 315 | Placebo | NA | PK, PD | Major bleeding or CRNMB, treatment- emergent AEs | Completed, awaiting results |
| Fesomersen (IONIS-FXI-LRx and BAY2976217) | NCT03582462 | Phase 1 Healthy volunteers | 66 | Placebo | NA | PK, PD | Treatment- emergent AEs | Completed, awaiting results |
| NCT04534114 (RE-THINc ESRD) | Phase 2 Prevention of cardiovascular events in patients with ESRD | 307 | Placebo | NA | PK, PD | Major bleeding or CRNMB, treatment- emergent AEs | Active, not recruiting | |
| Antibodies | ||||||||
| Abelacimab (MAA868) | NCT04213807 | Phase 2 Atrial fibrillation | 28 | Placebo | NA | Dose-range finding study—PK, PD | Safety, tolerability, and immunogenicity | Completed, awaiting results |
| NCT04755283 (AZALEA-TIMI 71) | Phase 2 Atrial fibrillation | Estimated enrollment 1200 | Rivaroxaban 15 mg and 20 mg OD | NA | NA | Safety and tolerability, major bleeding, and CRNMB | Active, not recruiting | |
| NCT05171049 (ASTER) | Phase 3 Treatment of cancer- associated VTE | Estimated enrollment 1655 | Apixaban 10 mg BID followed by 5 mg BID | NA | Centrally adjudicated VTE recurrence | Major bleeding or CRNMB | Active, recruiting | |
| NCT05171075 (MAGNOLIA) | Phase 3 Treatment of GI and GU cancer-associated VTE | Estimated enrollment 1020 | Dalteparin 200 IU/kg/d followed by 150 IU/kg/d | NA | Centrally adjudicated VTE recurrence | Major bleeding or CRNMB | Active, recruiting | |
| Osocimab (BAY 1213790) | NCT03787368 | Phase 1 Safety in patients with ESRD | 55 | Placebo | NA | PK, PD | Major bleeding or CRNMB | Completed, awaiting results |
| NCT04523220 (CONVERT) | Phase 2 Safety in patients with ESRD | 686 | Placebo | NA | PK, PD | Major bleeding or CRNMB | Active, not recruiting | |
| Xisomab (AB023) | NCT04465760 | Phase 2 Prevention of CAT in patients with cancer receiving chemotherapy | Estimated enrollment 50 | None | NA | Incidence of CAT | Major bleeding or CRNMB | Active, recruiting |
| Garadacimab (CSL312) | NCT04281524 | Phase 1/2 Prevention of CAT in patients with cancer receiving chemotherapy | 0 (study withdrawn) | Placebo | NA | Incidence of CAT | Treatment- emergent AEs | Withdrawn (business decision, not safety related) |
| NCT04409509 | Phase 2 COVID-19 | 124 | Placebo | NA | Endotracheal intubation or death prior to intubation | Completed, awaiting results | ||
| NCT05130970 | Phase 2 Idiopathic pulmonary fibrosis | Estimated enrollment 80 | Placebo | NA | PK, PD | Treatment-emergent AEs | Active, recruiting | |
| NCT04656418 | Phase 3 HAE-C1-INH | Estimated enrollment 60 | Placebo | NA | Time-normalized number of HAE-C1-INH attacks during treatment | Treatment-emergent AEs | Active, not recruiting | |
| Small molecules | ||||||||
| Milvexian (BMS-986177/JNJ-70033093) | NCT03766581 (AXIOMATIC-SSP) | Phase 2 Secondary stroke prevention | 2366 | Placebo | Aspirin, clopidogrel | Composite of new ischemic stroke during the treatment period and new covert brain infarction on MRI | Bleeding according to BARC criteria | Active, not recruiting |
| Asundexian (BAY 2433334) | NCT04304534 (PACIFIC-AMI) | Phase 2 Acute MI | 1592 | Placebo | Aspirin, clopidogrel | CV events: MI, stroke, stent thrombosis, and death | Bleeding according to BARC criteria | Completed, awaiting results |
| NCT04304508 (PACIFIC-STROKE) | Phase 2 Acute noncardioembolic ischemic stroke | 1808 | Placebo | NA | Symptomatic ischemic stroke or covert brain infarcts on MRI | Major bleeding or CRNMB | Completed, awaiting results | |
| NCT04510987 | Phase 1 Patients with ESRD on hemodialysis | 48 | None | NA | PK, PD | Treatment-emergent AEs | Completed, awaiting results | |
AEs, adverse events; BARC, Bleeding Academic Research Consortium; BID, twice daily; CAT, catheter-associated thrombosis; COVID-19, coronavirus disease 2019; CRNMB, clinically relevant nonmajor bleeding; CV, cardiovascular; HAE-C1-INH, C1-esterase inhibitor-deficient hereditary angioedema; GI, gastrointestinal; GU, genitourinary; MI, myocardial infarction; MRI, magnetic resonance imaging; NA, not applicable; OD, once daily; PD, pharmacodynamics; PK, pharmacokinetics.
Study recruitment status as of July 2022.
Antisense oligonucleotides
IONIS-FXIRx (alternative names: BAY-2306001; FXI ASO; ISIS 404071; ISIS-416858; ISIS-FXIRX) was the first FXI-targeting agent to be developed. In a phase 2 trial comparing the safety and efficacy of IONIS-FXIRx with those of enoxaparin for postoperative thromboprophylaxis in 315 patients undergoing elective total knee arthroplasty, IONIS-FXIRx showed either noninferior (200-mg regimen) or superior (300-mg regimen) efficacy over enoxaparin, with no increase in bleeding rates.48
Fesomersen (formerly known as ION-957943, IONIS-FXI-LRx, and BAY2976217) is a newly developed N-acetyl galactosamine (GN3) ligand-conjugated (LICA) conjugated 2′-MOE chimeric ASO targeting FXI, with enhanced affinity to hepatocytes, allowing less frequent administration and lower ASO doses, thus reducing the potential for local adverse reactions.41 The ongoing RE-THINc ESRD trial (NCT04534114) investigates the safety, pharmacokinetics, and pharmacodynamics of fesomersen for the prevention of cardiovascular events in patients with end-stage renal disease (ESRD) undergoing hemodialysis, and its results are expected shortly.
Abelacimab (MAA868)
An open-label, phase 2, prospective trial (ANT-005 TKA) randomized 412 patients who were undergoing total knee arthroplasty to receive 1 of 3 regimens of abelacimab (30 mg, 75 mg, or 150 mg) administered postoperatively as a single intravenous dose or to receive 40 mg enoxaparin administered subcutaneously once daily. The primary efficacy outcome was confirmed VTE, detected by mandatory venography of the operated leg involved in the operation or objective confirmation of symptomatic events, whereas the principal safety outcome was a composite of major or clinically relevant nonmajor bleeding up to 30 days after surgery. All 3 abelacimab regimens met the criterion for noninferiority to enoxaparin, with the 75-mg and 150-mg regimens being superior to enoxaparin (P < .001 for superiority for both regimens). Rates of bleeding and serious adverse events were comparably low with all 4 treatment regimens.53
Studies comparing the safety and tolerability of abelacimab to those of rivaroxaban for the prevention of stroke and systemic emboli in patients with AF (AZALEA-TIMI 71, NCT04755283), apixaban for the treatment of cancer-associated VTE (ASTER, NCT05171049), and dalteparin for the treatment of gastrointestinal and genitourinary cancer–associated VTE (MAGNOLIA, NCT05171075) are currently ongoing.
Osocimab (BAY 1213790)
The phase 2 noninferiority FOXTROT trial (NCT03276143) compared the safety and efficacy of 4 different doses of osocimab given before or after surgery with those of enoxaparin and apixaban for the prevention of VTE in 813 adult patients undergoing elective primary total knee arthroplasty. The efficacy of postoperative osocimab doses of 0.6 to 1.8 mg/kg, assessed by mandatory bilateral venography performed 10 to 13 days after surgery, was noninferior to that of enoxaparin. Furthermore, the preoperative dose of 1.8 mg/kg of osocimab met criteria for superiority compared with enoxaparin, but it was associated with higher bleeding rates.57
The currently ongoing CONVERT trial (NCT04523220) evaluates 2 dose regimens of osocimab given as a once-monthly subcutaneous injection for the prevention of thromboembolic events in patients with ESRD who have a high cardiovascular risk and require hemodialysis.
Xisomab (AB023)
A randomized, double-blind, phase 2 study compared xisomab with placebo in 24 patients with ESRD undergoing heparin-free hemodialysis (NCT03612856). Preliminary results suggest xisomab treatment to be well tolerated, with less frequent occlusive events requiring hemodialysis circuit exchange.61 Another phase 2 trial is currently evaluating xisomab for the prevention of catheter-associated thrombosis in patients with cancer receiving chemotherapy (NCT04465760).
Garadacimab (CSL312)
Since FXII is involved in proinflammatory responses and activation of the kallikrein-kinin system, the role of the FXII- targeting antibody garadacimab was studied for the prevention of attacks in 32 patients with C1-esterase inhibitor- deficient hereditary angioedema, in whom it significantly reduced the number of monthly attacks.36 Additional studies currently investigate its role in preventing respiratory deterioration in patients with coronavirus disease 2019 (NCT04409509) and in the treatment of patients with idiopathic pulmonary fibrosis (NCT05130970). A phase 2 clinical study to assess the efficacy and safety of garadacimab for the prevention of catheter- associated thrombosis in patients with cancer receiving chemotherapy through a central catheter was planned but withdrawn by the sponsor.
Milvexian (BMS-986177/JNJ-70033093)
In a parallel-group, phase 2 trial (the AXIOMATIC-TKR study, NCT03891524), postoperative administration of oral milvexian was effective for the prevention of VTE in 1242 patients undergoing knee arthroplasty and was associated with a low risk of bleeding or serious adverse events.66 The currently ongoing phase 2 AXIOMATIC-SSP trial (NCT03766581) is aimed to determine whether the addition of milvexian to dual-antiplatelet therapy with aspirin and clopidogrel is more effective than standard therapy for secondary stroke prevention.
Asundexian (BAY 2433334)
The multicenter, randomized, double-blind, phase 2 PACIFIC-AF trial (NCT04218266) compared the efficacy and safety of oral asundexian with those of apixaban in 753 patients with AF, in whom daily asundexian doses of either 20 mg or 50 mg resulted in lower rates of bleeding compared with apixaban, with near-complete in vivo FXIa inhibition.68
The PACIFIC-AMI (NCT04304534) and PACIFIC-STROKE (NCT04304508) trials compared oral asundexian with placebo on top of dual-antiplatelet therapy for the prevention of cardiovascular events in patients with acute myocardial infarction or following noncardioembolic stroke, respectively. Both trials have been recently completed, and their results are pending.
Conclusion
While DOACs introduced an improvement in care for eligible patients in terms of safety, efficacy, and convenience of treatment, there remain unmet clinical needs for patients requiring anticoagulant drugs.
With evidence of its contribution to thrombosis, the contact pathway of coagulation is an attractive target for the development of anticoagulants with an enhanced safety profile because, due to its nature, inhibition is less likely to promote bleeding compared with anticoagulants that target multiple coagulation factors or the TF-FVIIa or the common pathways.
Preclinical and early clinical data indicate that novel agents that selectively target FXI or FXII can reduce venous and arterial thrombosis without an increase in bleeding complications. Proving their superiority or noninferiority compared with existing anticoagulants is the ultimate challenge. Since current event rates are gratefully low, this would require the designing of large-scale clinical trials, which carry significant costs.
Like with DOACs, development of these agents started with trials on VTE prevention in high VTE risk orthopedic surgery and prevention of stroke and systemic emboli in patients with AF. Next steps could be focusing on clinical scenarios in which DOACs have not been tested or are contraindicated.
With many agents under development, the prospect of FXI and FXII inhibition as a novel approach to safer anticoagulation looks promising.
CLINICAL CASE (Continued)
The patient was considered for recruitment to a clinical trial evaluating a FXI-targeting monoclonal antibody, but a CrCl of 32 mL/min precluded his participation due to an exclusion criterion of CrCl <60 mL/min. The patient eventually received VTE prophylaxis with enoxaparin 40 mg once daily, starting 12 hours following surgery, which was switched to a direct FXa inhibitor upon his discharge from the hospital. The patient arrived at the emergency department on postoperative day 3 with recurrence of upper gastrointestinal bleeding and a reduction in hemoglobin level of 2.3 g/dL. Anticoagulant therapy was stopped and the patient received 2 units of packed red blood cells. A gastroscopy revealed bleeding stigmata from peptic ulcer disease. He was managed conservatively with a high-dose proton pump inhibitor and discharged 3 days later.
Conflict-of-interest disclosure
Omri Cohen received research funding from Pfizer and participated in an advisory board for PlasFree.
Walter Ageno received research funding from Bayer and participated in advisory boards for Bayer, Sanofi, Viatris, Leo Pharma, and Norgine.
Off-label drug use
Omri Cohen: none of the drugs discussed (anti-Factor XI and anti-Factor XII) are currently approved for clinical use.
Walter Ageno: none of the drugs discussed (anti-Factor XI and anti-Factor XII) are currently approved for clinical use.