Abstract
The development of new drugs and subsequent novel combinations for the treatment of newly diagnosed multiple myeloma (NDMM) has resulted in a plethora of treatment options that can make the choice of initial induction therapy a challenge. A greater understanding of both patient- and disease-specific factors can provide a personalized approach to help design a treatment course. Historically, the choice of an induction regimen has been tethered to an initial impression of transplant eligibility at the time of diagnosis. As more effective and better-tolerated induction regimens have emerged, there has been increasing overlap in the induction strategies used for all patients with NDMM, which increasingly provide the ultimate goal of deep and durable remissions. The current treatment options and strategies for the management of NDMM are evaluated using the best available data to provide a rationale for these decisions.
Learning Objectives
Define the treatment options available to patients with newly diagnosed multiple myeloma (NDMM)
Understand the strategy for choosing treatment for patients with NDMM
CLINICAL CASE
A 63-year-old previously healthy African American woman presented with progressive dyspnea on exertion and lower back pain. Her initial laboratory evaluation revealed a hemoglobin of 7.4 g/dL, a calcium level of 9.6 mg/dL, a creatinine level of 1.7 mg/dL, and an elevated lactate dehydrogenase level. X-ray of the patient's lumbar spine demonstrated multiple lytic lesions and a moderate compression fracture at L2. Due to the suspicion for multiple myeloma, further diagnostic testing was performed. A serum protein electrophoresis showed a monoclonal paraprotein level of 3.7 g/dL that was identified as IgG λ by serum immunofixation. Quantitative immunoglobulins demonstrated a markedly elevated IgG level at 6730 mg/dL and low IgM and IgA levels. Serum free λ level was elevated at 335 mg/L with a markedly abnormal κ/λ ratio of 0.04. Urine protein electrophoresis revealed a free λ level of 355 mg/24 hours. The β2-microglobulin concentration was 4.1 mg/L, and her albumin was 3.2 g/dL. A positron emission tomography/computed tomography scan demonstrated multiple hypermetabolic osseous lesions without extramedullary disease. A bone marrow aspirate and biopsy specimen showed a hypercellular marrow with plasma cells accounting for 80% of the cellularity with λ restriction by flow cytometry. Fluorescence in situ hybridization revealed monosomy 13 in 38 of 50 cells. She received 1 unit of packed red blood cell transfusion, which improved her dyspnea.
Defining newly diagnosed multiple myeloma
Multiple myeloma is a neoplastic plasma cell disorder, characterized by clonal proliferation of malignant plasma cells in the bone marrow and usually a monoclonal protein in the blood and/or urine. It is associated with end-organ damage consisting of anemia, renal insufficiency, bone lesions, and/or hypercalcemia, and the International Myeloma Working Group updated the definition to include validated biomarkers present in patients without end-organ damage but associated with an 80% risk of progression to active disease with the first 2 years since diagnosis (clonal bone marrow plasma cell percentage ≥60%, involved/uninvolved serum free light chain ratio ≥100, or >1 focal lesion on magnetic resonance imaging or positron emission tomography/computed tomography) (Table 1).1
Multiple myeloma diagnostic criteria
| Clonal bone marrow plasma cells | ≥10% |
| OR | |
| Biopsy-proven plasmacytoma | |
| AND | |
| “CRAB” | Hypercalcemia: Serum calcium >11 mg/dL or >1 mg/dL above ULN |
| Renal insufficiency: Serum creatinine >2 mg/dL or CrCl <40 mL/min | |
| Anemia: Hemoglobin <10 g/dL or >2 g/dL below LLN | |
| Bone lesions: ≥1 osteolytic lesion (on WBLDCT or PET scan) | |
| OR | |
| “SLiM” criteria | ≥60% clonal bone marrow plasma cells |
| Involved to uninvolved FLC ratio ≥100 and involved FLC ≥10 mg/dL | |
| >1 focal lesion on MRI ≥5 mm in size |
| Clonal bone marrow plasma cells | ≥10% |
| OR | |
| Biopsy-proven plasmacytoma | |
| AND | |
| “CRAB” | Hypercalcemia: Serum calcium >11 mg/dL or >1 mg/dL above ULN |
| Renal insufficiency: Serum creatinine >2 mg/dL or CrCl <40 mL/min | |
| Anemia: Hemoglobin <10 g/dL or >2 g/dL below LLN | |
| Bone lesions: ≥1 osteolytic lesion (on WBLDCT or PET scan) | |
| OR | |
| “SLiM” criteria | ≥60% clonal bone marrow plasma cells |
| Involved to uninvolved FLC ratio ≥100 and involved FLC ≥10 mg/dL | |
| >1 focal lesion on MRI ≥5 mm in size |
FLC, free light chain; MRI, magnetic resonance imaging; PET, positron emission topography; WBLDCT, whole-body low-dose computed tomography.
What is the optimal treatment for patients with newly diagnosed multiple myeloma?
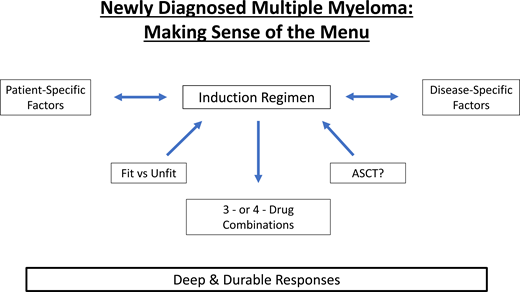
The emergence of novel agents, drug combinations, and therapeutic strategies has significantly improved outcomes in the past decade. Historical approaches to the management of newly diagnosed multiple myeloma (NDMM) have been categorized with a distinction between those patients deemed to be eligible or ineligible for high-dose chemotherapy and autologous stem cell transplantation at the time of diagnosis. Induction regimens were subsequently chosen based on a variety of patient- and disease-specific details. With limited treatment options and strategies available, the historic approach to the transplant-ineligible patient has largely been one of a “less is more” strategy by choosing fewer combined agents or by administering 2 to 3 drugs at lower doses, potentially sacrificing efficacy for safety. For transplant-eligible patients, the preferred intensity of induction has ranged widely from doublet regimens and transplant with modest clinical responses to Total Therapy regimens with deep responses despite high potential toxicity.2 The introduction of multiple agents and therefore multiple combinations allows the treatment intensity pendulum to continue to swing as we deepen our understanding of what our goals of therapy are. The question remains whether the disease course is a marathon that we aspire to finish with a slow and steady course or perhaps the treatment approach be aggressive from the time of diagnosis and use our best tools from the outset.
More therapeutic options have led to dramatic improvements in overall response rates and the depth of response, making the choice of the induction regimen solely based on transplant eligibility less relevant. It is well established that the depth of response is one of the most important prognostic factors in multiple myeloma (MM) and that the achievement of deep remissions represents a therapeutic goal for a large percentage of patients with MM.3 Ultimately, the optimal treatment approach for both fit and unfit patients with NDMM should incorporate the same strategy: provide a good balance of safety and efficacy while preserving the goal of eradicating as much disease as possible to provide deep and durable remissions, if not eventual cure.
What to consider when choosing an induction regimen?
The choice of an induction regimen for a patient with NDMM must take patient-specific, disease-specific, and therapy-related factors into consideration (Table 2). Importantly, shared decision- making between the physician and patient is paramount to meet differing treatment preferences and needs. The general approach to the management of any patient with NDMM is to administer initial induction therapy over a period of 4 to 6 cycles prior to possible high dose chemotherapy and autologous stem cell transplant (HDT-ASCT) or, alternatively, store stem cells and defer transplant. Patients who do not pursue HDT-ASCT, either because of fitness or choice, ideally receive continuous therapy until disease progression with the currently available data. Patients who undergo HDT-ASCT ideally receive maintenance therapy until progression.
Factors to be considered when choosing induction therapy
| Patient related . | Disease related . | Treatment related . |
|---|---|---|
| Age/Fitness | Bone marrow disease burden | Access to standard-of-care therapies |
| Caregiver support | Extramedullary disease | Costs and copays |
| Comorbidities | Molecular cytogenetics/genomics | Route of administration |
| Compliance | CRAB symptoms | Clinical trial availability |
| Lifestyle preferences | R-ISS/ISS | Toxicity |
| Performance status/Frailty |
| Patient related . | Disease related . | Treatment related . |
|---|---|---|
| Age/Fitness | Bone marrow disease burden | Access to standard-of-care therapies |
| Caregiver support | Extramedullary disease | Costs and copays |
| Comorbidities | Molecular cytogenetics/genomics | Route of administration |
| Compliance | CRAB symptoms | Clinical trial availability |
| Lifestyle preferences | R-ISS/ISS | Toxicity |
| Performance status/Frailty |
ISS, International Staging System; R-ISS, Revised International Staging System.
What is the optimal treatment of a fit patient with NDMM?
The menu of choices available to incorporate into the optimal induction therapy for fit patients with NDMM continues to grow. Recent trials have incorporated 3- and 4-drug combinations, using an immunomodulatory agent (IMiD), a proteasome inhibitor (PI), and a corticosteroid as a backbone. Several studies also support the use of minimal residual disease (MRD) for monitoring the response in MM because of its prognostic value. A recent meta-analysis demonstrated that the achievement of undetectable MRD improved progression-free survival (PFS) (hazard ratio [HR], 0.33) and overall survival (OS) (HR, 0.45) in comparison with the presence of MRD. Its prognostic impact was seen even in those patients with high-risk features such as high-risk cytogenetic abnormalities, demonstrating the importance of a deep response to therapy.4
Three-drug combinations
The combination of lenalidomide, bortezomib, and dexamethasone (VRd) has been a recent standard of care for initial treatment for NDMM. The SWOG S0777 study compared VRd with lenalidomide and dexamethasone, showing definitively that VRd was associated with an improved overall response rate, very good partial response (VGPR) or better rate, PFS, and OS.5 The combination of VRd with or without initial transplantation was also studied and confirmed the significant initial efficacy of this regimen in the IFM 2009 and DETERMINATION phase 3 studies.6,7
As MRD appears to be increasingly important as a goal of therapy to improve long-term outcomes, multiple other strategies to achieve MRD negativity have been evaluated. Gay and colleagues have presented results of the FORTE study, whose initial objectives were to compare induction with carfilzomib, lenalidomide, and dexamethasone (KRd) vs carfilzomib, cyclophosphamide, and dexamethasone (KCd) and to evaluate whether HDT-ASCT remained important in patients who received KRd induction. A second randomization assigned patients to receive either lenalidomide maintenance or doublet carfilzomib and lenalidomide (KR) maintenance.8,9 After KRd induction followed by HDT-ASCT and KRd consolidation (KRd-ASCT), the VGPR rate was 89% and the MRD-negative rate was 58%. A third arm of the study evaluated KRd for 12 cycles without transplant (KRd12) and demonstrated a VGPR rate of 90% and an MRD- negative rate of 54%, similar to what was seen in the KRd-ASCT arm. Survival analyses after a median follow-up of nearly 4 years subsequently have shown a significant improvement in PFS in the KRd-ASCT arm when compared to the KRd12 and KCd-ASCT arms (not reached vs 57 vs 53 months; KRd-ASCT vs KCd-ASCT: HR 0.53, P < .001; KRd-ASCT vs KRd12: HR 0.64, P = .0023; KRd12 vs KCd-ASCT: HR 0.82, P = .262) and was seen in all subgroups. The 3-year OS was 90% in both the KRd-ASCT and KRd12 arms vs 83% with KCd. These data further solidify the strategy of IMiD-PI-corticosteroid combinations and demonstrate the importance of deep responses to translate into improved outcomes. We now have several prospective studies solidifying an advantage when incorporating a proteasome inhibitor and an IMiD together as part of an induction regimen. Prolonged induction paired with consolidation strategies and maintenance provide deep responses and a PFS benefit and should be considered in any fit patient with NDMM.
Four-drug regimens
The emergence of CD38 monoclonal antibodies into frontline therapy offers an opportunity to improve upon the successes of 3-drug combinations. CD38-targeting monoclonal antibodies have shown marked activity and a favorable toxicity profile when first evaluated as a single agent in heavily pretreated patients with MM. The early experience with quadruplet regimens in NDMM was evaluated in 2 randomized phase 3 trials: ALCYONE and CASSIOPEIA.10,11 The ALCYONE study evaluated bortezomib-melphalan-prednisone with or without daratumumab in transplant-ineligible patients with NDMM. The primary end point of improved PFS was met when patients treated in the daratumumab arm achieved a deeper response and a 3-year PFS of 50% vs 18% compared with patients who were treated with bortezomib-melphalan-prednisone alone. There was a 40% reduction in the risk of death for those patients who received the quadruplet regimen, the first time that an improvement in OS was seen with a daratumumab-based combination therapy.10 The CASSIOPEIA study demonstrated that the addition of daratumumab to bortezomib-thalidomide-dexamethasone before and after transplantation improved the depth or response, PFS, and OS. A second randomization evaluated daratumumab monotherapy vs observation in those patients who achieved at least a partial response. The primary end point of the study was stringent complete response (sCR) after consolidation, and secondary end points included MRD-negative rate, PFS, and OS.11 This study was the first to show that the addition of a CD38 targeting monoclonal antibody to induction and a consolidation regimen is highly effective without adding significant additional toxicity.
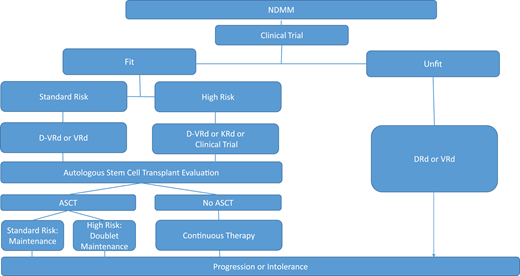
The GRIFFIN study was a randomized phase 2 study designed to evaluate the addition of daratumumab to RVd (D-RVd) in transplant-eligible patients. The primary end point was sCR by the end of consolidation with a secondary end point of PFS.12 The primary end point demonstrated improved sCR rates with D-RVd vs RVd (67.0% vs 48.0%; P = .0079) in the final analysis after a median follow-up of 49.6 months. MRD negativity rates were also higher in the D-RVd group vs control (10-5: 64.4% vs 30.1%, P < .0001; 10-6: 35.6% vs 15.5%, P = .0013). Sustained MRD negativity rates were higher in the D-RVd vs RVd groups after 12 months in all relevant subgroups, including high-risk patients, associated with improved PFS for all intention-to-treat patients.13 Estimated 48-month PFS rates were 87.2% for D-RVd and 70.0% for RVd. The phase 3 randomized PERSEUS trial that compared these same 2 arms and evaluated if daratumumab can be discontinued in patients with sustained MRD negativity is ongoing. The MASTER, MANHATTAN, and IFM2018-04 trials will all evaluate the role of daratumumab with KRd induction in patients with NDMM, the option of an MRD response-adapted approach, MRD negativity rates, and particularly the use of daratumumab with KRd induction in high-risk patients, the last of which is a glaring unmet need.14-16 Although no trial compares the strategy of CD38 antibody-based therapy at induction vs the time of first relapse, many favor the use of the best drugs, including daratumumab, for first-line therapy to induce the deepest response possible to translate into the best clinical outcomes. It is becoming increasingly clear that CD38 monoclonal antibodies play a significant role in achieving unprecedented responses in a patient with NDMM, and it is expected that 4-drug regimens will likely become a new standard of care (Figure 1, Table 3).
Treatment algorithm for newly diagnosed multiple myeloma. ASCT, autologous stem cell transplant; d, dexamethasone; D, daratumumab; R, lenalidomide; V, bortezomib.
Treatment algorithm for newly diagnosed multiple myeloma. ASCT, autologous stem cell transplant; d, dexamethasone; D, daratumumab; R, lenalidomide; V, bortezomib.
Summary of select data from randomized trials for induction therapy for transplant-eligible NDMM
| Reference . | Patients: total/arms, No. . | Median follow-up, mo . | Median age, y . | Regimen . | PFS . | OS . |
|---|---|---|---|---|---|---|
| GIMEMA62 | 236 vs 238 | 43 | 57 | VTd vs Td | 3-y PFS 68% vs 56% | 3-y 86% vs 84% |
| IFM2013-0463 | 169 vs 169 | NR | 59.5 | VTd vs VCd | NR | NR |
| EMNO2/HO9523 | 495 vs 702 | 60.3 | 58 | VCd-ASCT vs VCd-VMP | Median PFS 56.7 vs 41.9 mo (HR, 0.73, P = .0001) | 5-y OS 75.1% vs 71.6% (HR, 0.90; 95% CI 0.71-1.13; P = .35) |
| STAMINA24 | 247 vs 254 vs 257 | 38 | 56 | ASCT-R vs ASCT-VRd-R vs Tandem ASCT-R | 38-mo PFS rate: ASCT/ASCT-R: 58.5% (95% CI, 51.7%-64.6%) ASCT-VRd-R: 57.8% (95% CI, 51.4%-63.7%) ASCT-R: 53.9% (95% CI, 47.4%-60%) | OS: ASCT+ R: 81.8% (95% CI, 76.2%-86.2%) ASCT-VRd-R: 85.4% (95% CI, 80.4%-89.3%) ASCT-R: 83.7% (95% CI, 78.4%-87.8%) |
| IFM 200925 | 350 vs 350 | 44 | 59.5 | RVd-ASCT-R RVd-R | Median 50 vs 36 mo (HR, 0.65; 95% CI, 0.53-0.80; P < .001) | 4 y: 81% vs 82% (HR, 1.16; 95% CI, 0.80-1.68; P = .87) |
| DETERMINATION7 | 365 vs 357 | 76 | 56 | RVd-ASCT-R RVd-R | Median: 67.5 vs 46.2 mo (HR, 1.53; 95% CI, 1.23-1.91; P < .001) | 5 y: 79% vs 81% (HR, 1.10; 95% CI, 0.73-1.65; P > .99) |
| FORTE8,9 | 158 vs 157 vs 159 | 50.9 | 57 | KRd-ASCT-KRd KRd12 KCd-ASCT-KCd | 3 y: 68.8% vs 68.5% vs 67.2% (P = .86) 4 y: 69% (KRd-ASCT) vs 56% (KRd12) vs 51% (KCd-ASCT) HR for KRd-ASCT vs KCd-ASCT, 0.54; 95% CI, 0.38-0.78; P = .0008 HR for KRd-ASCT vs KRd12, 0.61; 95% CI, 0.43-0.88; P = .0084 | 4 y: 86% (KRd-ASCT) vs 85% (KRd12) vs 76% (KCd-ASCT) |
| GRIFFIN12 | 104 vs 103 | 22.1 | 60 (27%> 65 y) | D-RVd-ASCT-DR RVd-ASCT-R | 3 y: 88.9% vs 81.2% (HR, 0.46; 95% CI, 0.21-1.01) | 3 y: 92.6% vs 92.2% (HR, 0.90; 95% CI, 0.32-2.57) |
| CASSIOPEIA11 | 543 vs 542 | 18.8 | 58.5 | R1: D-VTd-ASCT-D-VTd VTd-ASCT-VTd R2: D maint vs obs | Median: NR in either arm (HR, 0.47; 95% CI, 0.33-0.67; P < .0001) | Median: NR in either arm (HR, 0.43; 95% CI, 0.23-0.80) |
| GMMG-HD652 | 139 vs 141 vs 137 vs 142 | 49.8 | 59 | RVd-ASCT-R RVd-ASCT-EloR Elo-RVd-ASCT-R Elo-RVd-ASCT-EloR | 3 y: 68.8% vs 68.5% vs 66.2% vs 67.2% (P = .86) | 3 y: 89.4% vs 89.1% vs 92.5% vs 89.7% (P = .43) |
| Reference . | Patients: total/arms, No. . | Median follow-up, mo . | Median age, y . | Regimen . | PFS . | OS . |
|---|---|---|---|---|---|---|
| GIMEMA62 | 236 vs 238 | 43 | 57 | VTd vs Td | 3-y PFS 68% vs 56% | 3-y 86% vs 84% |
| IFM2013-0463 | 169 vs 169 | NR | 59.5 | VTd vs VCd | NR | NR |
| EMNO2/HO9523 | 495 vs 702 | 60.3 | 58 | VCd-ASCT vs VCd-VMP | Median PFS 56.7 vs 41.9 mo (HR, 0.73, P = .0001) | 5-y OS 75.1% vs 71.6% (HR, 0.90; 95% CI 0.71-1.13; P = .35) |
| STAMINA24 | 247 vs 254 vs 257 | 38 | 56 | ASCT-R vs ASCT-VRd-R vs Tandem ASCT-R | 38-mo PFS rate: ASCT/ASCT-R: 58.5% (95% CI, 51.7%-64.6%) ASCT-VRd-R: 57.8% (95% CI, 51.4%-63.7%) ASCT-R: 53.9% (95% CI, 47.4%-60%) | OS: ASCT+ R: 81.8% (95% CI, 76.2%-86.2%) ASCT-VRd-R: 85.4% (95% CI, 80.4%-89.3%) ASCT-R: 83.7% (95% CI, 78.4%-87.8%) |
| IFM 200925 | 350 vs 350 | 44 | 59.5 | RVd-ASCT-R RVd-R | Median 50 vs 36 mo (HR, 0.65; 95% CI, 0.53-0.80; P < .001) | 4 y: 81% vs 82% (HR, 1.16; 95% CI, 0.80-1.68; P = .87) |
| DETERMINATION7 | 365 vs 357 | 76 | 56 | RVd-ASCT-R RVd-R | Median: 67.5 vs 46.2 mo (HR, 1.53; 95% CI, 1.23-1.91; P < .001) | 5 y: 79% vs 81% (HR, 1.10; 95% CI, 0.73-1.65; P > .99) |
| FORTE8,9 | 158 vs 157 vs 159 | 50.9 | 57 | KRd-ASCT-KRd KRd12 KCd-ASCT-KCd | 3 y: 68.8% vs 68.5% vs 67.2% (P = .86) 4 y: 69% (KRd-ASCT) vs 56% (KRd12) vs 51% (KCd-ASCT) HR for KRd-ASCT vs KCd-ASCT, 0.54; 95% CI, 0.38-0.78; P = .0008 HR for KRd-ASCT vs KRd12, 0.61; 95% CI, 0.43-0.88; P = .0084 | 4 y: 86% (KRd-ASCT) vs 85% (KRd12) vs 76% (KCd-ASCT) |
| GRIFFIN12 | 104 vs 103 | 22.1 | 60 (27%> 65 y) | D-RVd-ASCT-DR RVd-ASCT-R | 3 y: 88.9% vs 81.2% (HR, 0.46; 95% CI, 0.21-1.01) | 3 y: 92.6% vs 92.2% (HR, 0.90; 95% CI, 0.32-2.57) |
| CASSIOPEIA11 | 543 vs 542 | 18.8 | 58.5 | R1: D-VTd-ASCT-D-VTd VTd-ASCT-VTd R2: D maint vs obs | Median: NR in either arm (HR, 0.47; 95% CI, 0.33-0.67; P < .0001) | Median: NR in either arm (HR, 0.43; 95% CI, 0.23-0.80) |
| GMMG-HD652 | 139 vs 141 vs 137 vs 142 | 49.8 | 59 | RVd-ASCT-R RVd-ASCT-EloR Elo-RVd-ASCT-R Elo-RVd-ASCT-EloR | 3 y: 68.8% vs 68.5% vs 66.2% vs 67.2% (P = .86) | 3 y: 89.4% vs 89.1% vs 92.5% vs 89.7% (P = .43) |
ASCT, autologous stem cell transplant; D, daratumumab; DR, daratumumab, lenalidomide; Elo, elotuzumab; maint, maintenance; NR, not reported; obs, observation; R, lenalidomide; Td, thalidomide, dexamethasone; V, bortezomib; VCd, bortezomib, cyclophosphamide, dexamethasone; VMP, bortezomib, melphalan, prednisone; VTd, bortezomib, thalidomide, dexamethasone; VRd, bortezomib, lenalidomide, dexamethasone.
High-risk patients
The definition of high-risk MM (HRMM) continues to evolve, and a number of clinical, laboratory, and genetic features can be used to identify patients with high-risk biology.17 Historical therapeutic approaches have recommended the use of a PI-based treatment, but it is expected that multidrug combinations that offer multiple mechanisms of targeting the active malignant clone will lead to the greatest depth of response. The FORTE study did include approximately one-third of patients who had at least 1 high-risk cytogenetic feature. Subgroup analyses showed that patients with high-risk disease as defined by Revised International Staging System III had improved outcomes with KRd alone or KRd with transplantation compared with KCd.18 This prompted several groups to start using KRd and transplantation in high-risk patients as a follow-up of the KRd-ASCT and KRd-12 groups showed similar results for high-risk (4-year PFS 62% and 45%) and standard-risk (4-year PFS 80% and 67%) patients. This benefit was also seen in patients with double-hit high-risk disease defined by the presence of ≥2 high-risk cytogenetic abnormalities (4-year PFS 55% and 31%).19 During the second randomization of this trial, more MRD+ patients turned negative in the KR arm vs the single-agent lenalidomide arm (46% vs 32%, P = .04) after a median follow-up of 31 months. Patients with high-risk disease have inferior outcomes overall and are a clear unmet need. FORTE offers a glimpse into the role of prolonged inductions with or without transplant, each achieving deep responses, although still demonstrating the benefit of ASCT.
The phase 3 ENDURANCE trial is the only randomized trial to prospectively compare KRd to VRd in NDMM.20 The trial was designed for standard-risk NDMM patients not intended for immediate transplant and specifically excluded high-risk patients. At a median follow-up of 9 months, the median PFS was 34.6 months in the KRd group and 34.4 months in the VRd group (P = .74; HR, 1.04). Median OS was not reached in either group. Of note, a composite of grade 3 to 5 treatment-related cardiac, pulmonary, and renal toxicity was higher in the KRd vs VRd group (16% vs 5%, P < .0001) largely caused by a higher frequency of dyspnea and heart failure in the KRd group. A subsequent retrospective study of KRd vs RVd followed by ASCT in high-risk NDMM showed similar overall response rate pre-ASCT (98% vs 93%, P = .659), 100 days post-ASCT (100% vs 94.8%, P = .51), and final best response (100% vs 94.8%, P = .79).21 MRD assessment was performed pre- and post-ASCT, and there was no significant difference between both groups (pre: 18% vs 12%; post: 41% vs 43%) at either time point (P = .45 and 1.00, respectively).
As discussed above, the GRIFFIN study showed a significant benefit when daratumumab was included as part of initial induction therapy, but the study included few high-risk patients. Ongoing interest remains as to whether daratumumab-based induction is beneficial in the high-risk population as well. A meta-analysis was performed to evaluate the role of daratumumab for the treatment of MM in patients with high-risk cytogenetic factors. While this analysis did include trials of patients with NDMM or HRMM, among patients with newly diagnosed HRMM, the addition of daratumumab to backbone regimens was associated with improved PFS (pooled HR, <0.67; 95% confidence interval [CI], 0.47-0.95; P = .02) with little evidence of heterogeneity (Cochran Q, P = .77; I2 = 0%).22 At this time, it is not clear that one combination is better than another specifically for high-risk NDMM, and we anxiously await ongoing trials designed to answer this question.
Role of transplant
The role of ASCT for NDMM continues to evolve. Transplant remains an option on the menu for fit patients to achieve a deep response. As mentioned above, multiple studies show the benefit of transplant to continually deepen the response and achieve higher rates of MRD negativity.6,7,23,24 The DETERMINATION and IFM-09 trials offer data from transplant trials in the era of novel agents.6,7 Although these trials by design are not intended to be a “transplant or no transplant” trial, as opposed to a “transplant now or later” trial, the primary end point of PFS supports early transplant in the fit patient. The median PFS was 46 months in the RVd-alone group and 67.5 months in the transplant group. An important note was that patients with high-risk features had a median duration of PFS of 55.5 months in the transplant group vs 17 months in the RVd-alone group. The OS, however, is similar among the 2 groups after a median follow-up of 76 months. The RVd + ASCT arm resulted in a higher percentage of patients in whom MRD was not detected (54% vs 40%), suggesting a strategy to drive deep and durable responses. Patients who achieved MRD negativity either before or after maintenance therapy had an improved 5-year PFS compared with those who remained MRD positive, regardless of whether they received transplantation. The IFM-09 had a nearly identical approach to clinical trial design but discontinued lenalidomide maintenance after 12 months. The median PFS in this trial, however, was 36 months in the RVd-alone group vs 50 months in the transplant arm, perhaps at least in part due to the limited exposure to lenalidomide maintenance. Importantly, PFS was particularly improved in patients who achieved MRD negativity, no matter which assigned treatment arm. This suggests that if deep response is a goal, the treatment selection from the menu should give patients the best chance of obtaining that goal, with or without transplant. These modern transplant trials demonstrate the feasibility of both pursuing or deferring transplant, the successes of continuous therapy to achieve deep responses, and the importance of the elimination of MRD.
Maintenance therapy
Post-ASCT maintenance strategies are a key component to delaying disease recurrence in NDMM and aim to prolong OS. Four randomized phase 3 studies have compared lenalidomide maintenance with observation or placebo post-ASCT.25-28 While there were inherent differences among the patient populations and study designs, each trial demonstrated a clear improvement in median time to progression/PFS in the lenalidomide arms. A meta-analysis demonstrated a median PFS of 52.8 months for the lenalidomide group compared to 23.5 months for the placebo/observation group (HR, 0.48; 95% CI, 0.41-0.55). Although none of the evaluated trials were powered to evaluate OS as a primary endpoint, the meta-analysis demonstrated a significant OS benefit (median not reached vs 86.0 months; HR, 0.75; 95% CI, 0.63-0.90; P = .001) for lenalidomide vs placebo/observation. A second meta-analysis, including data from the maintenance portion of the Myeloma XI trial, confirmed this PFS and OS benefit and specifically regardless of age, sex, International Staging System stage, response after ASCT, or whether patients received a lenalidomide-containing induction regimen.29 The optimal duration of maintenance therapy remains to be determined, but the recent IFM-09 and DETERMINATION studies showed the inherent difference in outcomes that resulted when maintenance was discontinued prior to disease progression. Based on the existing body of evidence available, lenalidomide maintenance should ideally continue until disease progression. Multiple other drugs and drug combinations have been or are currently being evaluated in the maintenance setting with the goal to tailor maintenance therapy based on an individual's disease characteristics, cytogenetic risk group, and tolerance, thereby maximizing disease response and survival outcomes while minimizing treatment burden (Table 4).30
Summary of select data from randomized trials for post-ASCT maintenance therapy
| Reference . | Patients: total/arms, No. . | Median follow-up, mo . | Regimen . | Dosing schedule . | Key efficacy outcomes . | Key safety data . |
|---|---|---|---|---|---|---|
| Myeloma IX42 | 245 vs 247 | 38 | T vs obs | T 50-100 mg/d continuous | Median PFS: 30 vs 23 mo (HR, 1.42) 3-y OS: 75% vs 80% | Discontinuation due to AEs: 52.2%; SAE: 8.5% |
| 1098 vs 1333 | NR | Meta-analysis, T vs no T maint, including non-ASCT | Various doses/durations | OS: HR 0.75 7-y OS: 12.3% difference in rate, in favor of T maint | NR | |
| HOVON-5043,44 | 268 vs 268 | Initial analysis: 52 Follow-up: 129 | TAd-ASCT-T vs VAd-ASCT-IFN | T 50 mg/d until PD | Median EFS: 34 vs 22 mo (HR, 0.60); HR 0.62 at follow-up Median PFS: 34 vs 25 mo (HR, 0.67) OS: HR 0.96 OS from relapse 20 vs 31 mo (HR, 1.50) | T maint: discontinuation due to AEs: 33%; 42% at follow-up (vs 27% IFN) Grade 3-4 PN: 10% |
| NCIC-CTG Myeloma 1045 | 166 vs 166 | 4.1 y | TP vs obs | T 200 mg/d, P 50 mg q2d; up to 4 y | 4-y PFS: 32% vs 14% (HR, 0.55) 4-y OS: 68% vs 60% (HR, 0.77) Median OS postrelapse: 27.7 vs 34.1 mo | Grade 3/4 thromboembolism: 7.3% vs 0 Grade 3/4 PN: 9.6% vs 1.2% |
| IMWG meta-analysis46 | 1276 vs 1510 | NR | T vs no T maint including non-ASCT | T various doses/durations | PFS: HR 0.65 OS: HR 0.84 | NR |
| IFM 2005-0225 | 307 vs 307 | 45 | R vs placebo | 10 mg continuous, increase up to 15 mg; until progression | Median PFS: 41 vs 23 mo (HR, 0.50; P < .001) 4-y PFS: 43 vs 22% (P < .001) 4-y OS: 73% vs 75% (P = .7) | Discontinued for AEs: 27% vs 15%; 2.4 times risk of SPMs with R |
| CALGB 10010426,27 | 231 vs 229 | Initial report: 34 Follow-up: 91 | R vs placebo post-ASCT | 10 mg continuous, increase up to 15 mg; until progression | Initial: Median PFS/TTP 46 vs 27 mo (HR, 0.48) 3-y OS: 88% vs 80% (HR, 0.62) Follow-up: Median PFS/TTP: 57 vs 29 mo (HR, 0.57; P < .0001) Median OS: 114 vs 84 mo (P = .0004) | Discontinuation due to AEs: 10% Grade 3/4 neutropenia: 32%/13% vs 12%/3% Heme/solid SPMs: 8%/6% vs 1%/4% |
| RV-MM-20928 | 162 vs 125 (67 vs 68 post-ASCT) | 51.2 | R vs obs post-MPR (n = 132) or ASCT (n = 141) consolidation | 10 mg (21 out of 28 days); until progression | ASCT-R vs ASCT: Median PFS: 54.7 vs 37.4 mo 5-y OS: 78.4% vs 66.6% R maint CR rate improvement: 15.7% to 35.7% R vs no maint, post-MPR/ASCT Median PFS: 41.9 vs 21.6 mo (HR, 0.47) OS: HR 0.64 | R vs no maint, post-MPR/ASCT Grade 3/4 AEs: Neutropenia 23.3% vs 0% Infections 6.0% vs 1.7% Discontinuation due to AEs: 5.2% vs 0% |
| Phase 3 meta-analysis26 | 605 vs 603 | 79.5 | R vs placebo/obs post-ASCT | R doses varied | Median PFS: 52.8 vs 23.5 mo (HR, 0.48) Median PFS2: 73.3 vs 56.7 mo (HR, 0.72) 7-year OS: 62% vs 50% (HR, 0.75) | Discontinuation due to AEs: 29.1% vs 12.2% Heme/solid SPMs prior to PD: 5.3%/5.8% vs 0.8%/2.0% |
| Myeloma XI29 | 730 vs 518 | 31 | R vs obs post-ASCT | R 10 mg (21 out of 28 days); until progression | Median PFS: 57 vs 30 mo (HR, 0.48; P < .0001) 3-y OS: 88% vs 80% (HR, 0.69; P = .014) | Grade 3/4 neutropenia: 28%/5% Discontinuations due to AEs: 28% SPMs: 5.3% vs 3.1% |
| NCT0109183131 | 60 vs 57 | 41 vs 42.3 | RP vs R alone post-ASCT | RP (R 10 mg, days 1-21, 28-d cycles; P 50 mg, q2d); until progression | Median PFS: 37.6 vs 31.5 mo 4-year OS: 77% vs 75% | Grade 3/4 AEs: Neutropenia: 8% vs 13% Infections: 8% vs 5% Discontinuations due to AEs: 5% vs 8% |
| GMMG-MM547 | PAd-R → 2 y vs VCd-R → 2 y vs PAd-R → CR vs VCd-R → CR: 125 vs 126 vs 126 vs 125 | 60.1 | R → 2 y vs R CR post-PAd/VCd + ASCT | R 10-15 mg/d continuous both arms | Median PFS: 43.2 vs 40.9 vs 35.9 vs 35.7 mo 36-mo OS: 83% vs 85% vs 75% vs 77% | AEs during maintenance (R → 2 y vs R → CR): 77.6% vs 58.2% Grade ≥2 infections (R → 2 y vs R → CR): 52.7% vs 32.3% |
| HOVON-65/GMMG-HD48,49 | 413 vs 414 (270 vs 230 maint) | Initial analysis: 41 Updated analysis: 96 | PAd-ASCT-V vs VAd-ASCT-T | V 1.3 mg/m2 IV q2w, up to 2 y T 50 mg/d, up to 2 y | Initial: Median PFS: 35 vs 28 mo (HR, 0.75) Median PFS from last ASCT: 31 vs 26 mo 5-y OS: 61% vs 55% (HR, 0.81) Updated: Median PFS: 34 vs 28 mo (HR, 0.76) Median OS: 91 vs 82 mo (HR, 0.89) Median OS from relapse: 43 vs 40 mo (HR, 1.02) | SPMs: 7% in both arms |
| GEM05MENOS6550 | 91 vs 88 vs 92 | 58.6 | VT vs T vs IFN | VT (V 1.3 mg/m2 IV days 1, 4, 8, 11, q3m; T 100 mg/d) vs T (T 100 mg/d) vs IFN (3 MU × 3 per wk) post-ASCT, up to 3 y | Median PFS: 50.6 vs 40.3 vs 32.5 mo 5-year OS: 78% vs 72% vs 70% | Grade 2-3 PN: 48.8% vs 34.4% vs 1% Discontinuation due to toxicity: 21.9% vs 39.7% vs 20% |
| Tourmaline-MM351 | 395 vs 261 | 31 | Ixazomib vs placebo post-ASCT | Ixazomib 3-4 mg, days 1, 8, 15, 28-d cycles; up to 2 y | Median PFS: 26.5 vs 21.3 mo (HR, 0.72) | Grade ≥3 AEs: 42% vs 26% Grade ≥3 infections/infestations: 15% vs 8% Grade ≥3 GI disorders: 6% vs 1% Discontinuation due to AEs: 7% vs 5% |
| Reference . | Patients: total/arms, No. . | Median follow-up, mo . | Regimen . | Dosing schedule . | Key efficacy outcomes . | Key safety data . |
|---|---|---|---|---|---|---|
| Myeloma IX42 | 245 vs 247 | 38 | T vs obs | T 50-100 mg/d continuous | Median PFS: 30 vs 23 mo (HR, 1.42) 3-y OS: 75% vs 80% | Discontinuation due to AEs: 52.2%; SAE: 8.5% |
| 1098 vs 1333 | NR | Meta-analysis, T vs no T maint, including non-ASCT | Various doses/durations | OS: HR 0.75 7-y OS: 12.3% difference in rate, in favor of T maint | NR | |
| HOVON-5043,44 | 268 vs 268 | Initial analysis: 52 Follow-up: 129 | TAd-ASCT-T vs VAd-ASCT-IFN | T 50 mg/d until PD | Median EFS: 34 vs 22 mo (HR, 0.60); HR 0.62 at follow-up Median PFS: 34 vs 25 mo (HR, 0.67) OS: HR 0.96 OS from relapse 20 vs 31 mo (HR, 1.50) | T maint: discontinuation due to AEs: 33%; 42% at follow-up (vs 27% IFN) Grade 3-4 PN: 10% |
| NCIC-CTG Myeloma 1045 | 166 vs 166 | 4.1 y | TP vs obs | T 200 mg/d, P 50 mg q2d; up to 4 y | 4-y PFS: 32% vs 14% (HR, 0.55) 4-y OS: 68% vs 60% (HR, 0.77) Median OS postrelapse: 27.7 vs 34.1 mo | Grade 3/4 thromboembolism: 7.3% vs 0 Grade 3/4 PN: 9.6% vs 1.2% |
| IMWG meta-analysis46 | 1276 vs 1510 | NR | T vs no T maint including non-ASCT | T various doses/durations | PFS: HR 0.65 OS: HR 0.84 | NR |
| IFM 2005-0225 | 307 vs 307 | 45 | R vs placebo | 10 mg continuous, increase up to 15 mg; until progression | Median PFS: 41 vs 23 mo (HR, 0.50; P < .001) 4-y PFS: 43 vs 22% (P < .001) 4-y OS: 73% vs 75% (P = .7) | Discontinued for AEs: 27% vs 15%; 2.4 times risk of SPMs with R |
| CALGB 10010426,27 | 231 vs 229 | Initial report: 34 Follow-up: 91 | R vs placebo post-ASCT | 10 mg continuous, increase up to 15 mg; until progression | Initial: Median PFS/TTP 46 vs 27 mo (HR, 0.48) 3-y OS: 88% vs 80% (HR, 0.62) Follow-up: Median PFS/TTP: 57 vs 29 mo (HR, 0.57; P < .0001) Median OS: 114 vs 84 mo (P = .0004) | Discontinuation due to AEs: 10% Grade 3/4 neutropenia: 32%/13% vs 12%/3% Heme/solid SPMs: 8%/6% vs 1%/4% |
| RV-MM-20928 | 162 vs 125 (67 vs 68 post-ASCT) | 51.2 | R vs obs post-MPR (n = 132) or ASCT (n = 141) consolidation | 10 mg (21 out of 28 days); until progression | ASCT-R vs ASCT: Median PFS: 54.7 vs 37.4 mo 5-y OS: 78.4% vs 66.6% R maint CR rate improvement: 15.7% to 35.7% R vs no maint, post-MPR/ASCT Median PFS: 41.9 vs 21.6 mo (HR, 0.47) OS: HR 0.64 | R vs no maint, post-MPR/ASCT Grade 3/4 AEs: Neutropenia 23.3% vs 0% Infections 6.0% vs 1.7% Discontinuation due to AEs: 5.2% vs 0% |
| Phase 3 meta-analysis26 | 605 vs 603 | 79.5 | R vs placebo/obs post-ASCT | R doses varied | Median PFS: 52.8 vs 23.5 mo (HR, 0.48) Median PFS2: 73.3 vs 56.7 mo (HR, 0.72) 7-year OS: 62% vs 50% (HR, 0.75) | Discontinuation due to AEs: 29.1% vs 12.2% Heme/solid SPMs prior to PD: 5.3%/5.8% vs 0.8%/2.0% |
| Myeloma XI29 | 730 vs 518 | 31 | R vs obs post-ASCT | R 10 mg (21 out of 28 days); until progression | Median PFS: 57 vs 30 mo (HR, 0.48; P < .0001) 3-y OS: 88% vs 80% (HR, 0.69; P = .014) | Grade 3/4 neutropenia: 28%/5% Discontinuations due to AEs: 28% SPMs: 5.3% vs 3.1% |
| NCT0109183131 | 60 vs 57 | 41 vs 42.3 | RP vs R alone post-ASCT | RP (R 10 mg, days 1-21, 28-d cycles; P 50 mg, q2d); until progression | Median PFS: 37.6 vs 31.5 mo 4-year OS: 77% vs 75% | Grade 3/4 AEs: Neutropenia: 8% vs 13% Infections: 8% vs 5% Discontinuations due to AEs: 5% vs 8% |
| GMMG-MM547 | PAd-R → 2 y vs VCd-R → 2 y vs PAd-R → CR vs VCd-R → CR: 125 vs 126 vs 126 vs 125 | 60.1 | R → 2 y vs R CR post-PAd/VCd + ASCT | R 10-15 mg/d continuous both arms | Median PFS: 43.2 vs 40.9 vs 35.9 vs 35.7 mo 36-mo OS: 83% vs 85% vs 75% vs 77% | AEs during maintenance (R → 2 y vs R → CR): 77.6% vs 58.2% Grade ≥2 infections (R → 2 y vs R → CR): 52.7% vs 32.3% |
| HOVON-65/GMMG-HD48,49 | 413 vs 414 (270 vs 230 maint) | Initial analysis: 41 Updated analysis: 96 | PAd-ASCT-V vs VAd-ASCT-T | V 1.3 mg/m2 IV q2w, up to 2 y T 50 mg/d, up to 2 y | Initial: Median PFS: 35 vs 28 mo (HR, 0.75) Median PFS from last ASCT: 31 vs 26 mo 5-y OS: 61% vs 55% (HR, 0.81) Updated: Median PFS: 34 vs 28 mo (HR, 0.76) Median OS: 91 vs 82 mo (HR, 0.89) Median OS from relapse: 43 vs 40 mo (HR, 1.02) | SPMs: 7% in both arms |
| GEM05MENOS6550 | 91 vs 88 vs 92 | 58.6 | VT vs T vs IFN | VT (V 1.3 mg/m2 IV days 1, 4, 8, 11, q3m; T 100 mg/d) vs T (T 100 mg/d) vs IFN (3 MU × 3 per wk) post-ASCT, up to 3 y | Median PFS: 50.6 vs 40.3 vs 32.5 mo 5-year OS: 78% vs 72% vs 70% | Grade 2-3 PN: 48.8% vs 34.4% vs 1% Discontinuation due to toxicity: 21.9% vs 39.7% vs 20% |
| Tourmaline-MM351 | 395 vs 261 | 31 | Ixazomib vs placebo post-ASCT | Ixazomib 3-4 mg, days 1, 8, 15, 28-d cycles; up to 2 y | Median PFS: 26.5 vs 21.3 mo (HR, 0.72) | Grade ≥3 AEs: 42% vs 26% Grade ≥3 infections/infestations: 15% vs 8% Grade ≥3 GI disorders: 6% vs 1% Discontinuation due to AEs: 7% vs 5% |
AE, adverse event; CR, complete response; EFS, event-free survival; GI, gastrointestinal; heme, hematologic; IMWG, International Myeloma Working Group; IFN, interferon; IV, intravenous; MPR, melphalan, prednisone, lenalidomide; MU, million units; P, prednisone; PAd, bortezomib, doxorubicin, dexamethasone; PD, progressive disease; PFS2, progression-free survival from start of treatment to progression on next line of treatment; PN, peripheral neuropathy; q2d every other day; q2w, every 2 weeks; q3m, every 3 months; R, lenalidomide; RP, lenalidomide, prednisone; SAE, serious adverse event; SPM, second primary malignancy; T, thalidomide; TAd, thalidomide, doxorubicin, dexamethasone; TP, thalidomide, prednisone; TTP, time to progression; V, bortezomib; VAd, vincristine, doxorubicin, dexamethasone; VT, bortezomib, thalidomide.
What about treatment for unfit patients?
It is important to remember that fitness for transplant may be fluid around the time of diagnosis and after the initiation of induction, which can often significantly improve performance status due to rapid improvement in disease burden. Transplant consultation is an important component to determining eligibility, and all potentially eligible patients should be referred to a transplant center for evaluation early in the induction course. However, once transplant ineligibility has been determined, a tailored approach to ensure that the treatment benefit is balanced carefully with safety, while maintaining the goal of deep and durable responses, must be employed.
Although age has previously been used as an inclusion criterion for ASCT,28,31-33 this has become less relevant due to an aging, yet fit, population that may be otherwise excluded. Comorbidity scores have been validated and can help evaluate transplant eligibility and frailty, including a myeloma-specific comorbidity index score.34-36 Preventing and managing treatment side effects is of significant importance in the frail population that can allow for continued therapy that is tolerable and can provide deep responses.
Two- vs 3-drug regimens
We now have numerous randomized trials that confirm the benefit of 3-drug regimens in a transplant-ineligible or transplant-deferred population (Table 5). We have previously reviewed the SWOG 0777 phase 3 trial that confirmed the PFS and OS benefit of VRd when compared to Rd and solidified the importance of a triplet induction regimen.5 This was further substantiated in the MAIA trial, which added daratumumab to Rd (DRd) in this randomized controlled phase 3 trial.37 The primary end point was PFS with a secondary end point of OS. An interim analysis after a median follow-up of 56.2 months showed the median PFS was not reached (95% CI, 54.8 months to not reached) in the daratumumab group vs 34.4 months (95% CI, 29.6-39.2 months) in the control group (HR, 0.53; 95% CI, 0.43-0.66; P < .0001); estimated PFS at 60 months was 52.5% (95% CI, 46.7%-58.0%) vs 28.7% (95% CI, 23.1%-34.6%). Estimated 60-month overall survival was 66.3% (95% CI, 60.8%-71.3%) in the daratumumab group vs 53.1% (95% CI, 47.2%-58.6%) in the control group.38 MRD negativity rates were also significantly higher in the daratumumab group than in the control group after 48 months (31% vs 10%). The most common (>15%) grade 3 or higher treatment-emergent adverse events were neutropenia, pneumonia, anemia, and lymphopenia, all higher in the triplet arm, with no new safety concerns. We now have 2 large randomized, controlled clinical trials, ALCYONE and MAIA, that incorporate daratumumab for transplant- ineligible patients and demonstrated a significant improvement in OS. With longer follow up, DRd is expected to result in an unprecedented median PFS in this patient population and supports its frontline use as a new standard of care for induction in this transplant-ineligible population (Table 5).
Summary of select data from randomized trials for induction therapy for transplant-ineligible NDMM
| Reference . | Patients: total/arms, No. . | Median follow-up, mo . | Median age (range), y . | Regimen . | PFS . | OS . | Primary end point/comments . |
|---|---|---|---|---|---|---|---|
| PETHEMA53 | 260 patients; 130/130 | 32 | 73 (68-77) 73 (69-76) | VMP vs VTP Maint VT or VP | 31 mo, all patients | 3-y OS: 70% (64%-76%), all patients | ORR VTP 81%, VMP 80%, VTP more serious AEs (40 [31% vs 20 [15%], P = .01) and drug discontinuations |
| GIMEMA 030535,54 | 511 patients; 254/257 | 23.2 | 71 | VMPT-VT vs VMP | 3-y PFS: VMPT-VT 56%; VMP 41%; HR 0.67 (95% CI, 0.50-0.90; P = .008) | 5-y OS: VMPT-VT 61% VMP 51% HR 0.70 (P = .01) | PFS VMPT-VT arm: more frequent grade 3/4 AEs: neutropenia (38%), thrombocytopenia (22%), peripheral neuropathy (11%), cardiac events (11%) |
| MRC-IX (59)55 | 859 patients; 423/426 | 44 | 73 | MP vs CTd | MP 12.4 mo; CTd 13 mo HR 0.82 (95% CI, 0.70-0.96; P = .01) | MP 30.6 mo; CTd 33.2 mo; HR 0.89 (95% CI, 0.74-1.08; P = .24) | ORR/PFS/OS ORR: MP 32.6%; CTd 63.8% (P < . 0001) CTDa arm: higher rates of thromboembolic events, constipation, infection, and neuropathy |
| FIRST56 | 1623 patients; 535/541/547 | 67 | 73 (40-92) | Rd vs Rd18 vs MPT | Rd 25.5 mo; Rd18 20.7 mo; MPT 21.2 mo; HR 0.72, Rd vs MPT HR 0.70, Rd vs Rd18; P < .001 for both. Rd was superior to MPT for all secondary efficacy end points, including OS. | 4-y OS: Rd 59%; Rd18 56%; MPT 51% | PFS Grade 3/4 AEs more frequent with Rd than MPT (70% vs 78%) |
| UPFRONT57 | 502 patients; 168/167/167 | 42.7 | 74.5 (67-69) Vd + V 73 (66-77) VTd + V 72 (68-77) VMP + V | Vd 14.7 mo; VTd 15.4 mo; VMP 17.3 mo (P = NS) | Vd 49.8 mo VTd 51.5 mo VMP 53.1 mo (P = NS) | PFS Peripheral neuropathy near 50% in all arms. Early drug discontinuation (29%-38%). QoL scores decreased during induction and improved or stabilized thereafter. | |
| HOVON-8758 | 668 patients 318/319 | 36 | 72 (60-91) | MPT-T vs MPR-R | MPT-T 20 mo; MPR-R 23 mo; HR 0.87 (95% CI, 0.72-1.04; P = .12) | 4-y OS: MPT-T 52% MPR-R 56% | PFS Grade 3/4 hematologic toxicity with MPR-R vs clinically significant neuropathy with MPT-T |
| SWOG S07775,59 | 525 patients; 264/261 | 84 | 43% ≥ 65 | VRd vs Rd | VRd 41 mo Rd 29 mo HR 0.742 (95% CI 0.594-0.9028; 1-sided P = .003) | VRd NR; Rd 69 mo; HR 0.709 (95% CI, 0.543-0.926; 2-sided P = .0114) | PFS VRd (23%) and Rd (10%) discontinued induction treatment due to AE |
| MAIA38 | 737 patients: 368/369 | 56.2 | 73 (50-90) D-Rd continuous 74 (45-89) Rd continuous 43%-44% ≥ 75 | D-Rd NR Rd 34.4 mo HR 0.53 (95% CI, 0.43-0.66; P < .0001) | NR in either arm. HR 0.68 (95% CI, 0.53-0.86; P = .0013) | PFS Median duration on continuous treatment 47.5 mo (D-Rd) and 22.6 mo (Rd) | |
| HOVON-126/NMSG60 | 143 patients | 23.4 | 73 (64-90) | ITd → maintenance ixazomib vs placebo | PFS-R: ITd-I 9.5 mo; ITd- P 8.4 mo | OS-R at 18 mo, all patients 96% (88%-99%) | PFS Early mortality 8% age >75, only 55% randomly assigned to maintenance therapy |
| ALCYONE10 | 706 patients; 350/356 | 40.1 | 71 (40-93) 71 (50-91) | D-VMP-D VMP | D-VMP-D 36.4 mo; VMP 19.3 mo; HR 0.42 (95% CI, 0.34-0.51; P < .0001) | 3-y OS: D-VMP-D 78%; VMP 67.9%; HR 0.60 (95% CI, 0.46-0.80; P = .0003) | PFS Common |
| ECOG E1A1120 | 1087 patients; 542/545 | 9 | 65 (57-71) 64 (59-71) | VRd-R KRd-R | VRd 34.4 mo KRd 34.6 mo | Median OS NR either arm | PFS, OS; excluded high-risk disease; 17.3% discontinued VRd early due to AEs |
| TOURMALINE-MM261 | 705 patients; 351;354 | IRd 53.3 Rd 55.8 | 73 (48-90) 74 (48-88) | IRd-IR Rd-R | IRd 35.3 mo Rd 21.8 mo (HR, 0.830; 95% CI, 0.676-1.018; P = .073) | Median OS NR either arm at 58 mo. HR 0.998 (95% CI, 0.790-1.261; P = .988) | PFS PFS not improved in age ≥75 in IRd |
| Reference . | Patients: total/arms, No. . | Median follow-up, mo . | Median age (range), y . | Regimen . | PFS . | OS . | Primary end point/comments . |
|---|---|---|---|---|---|---|---|
| PETHEMA53 | 260 patients; 130/130 | 32 | 73 (68-77) 73 (69-76) | VMP vs VTP Maint VT or VP | 31 mo, all patients | 3-y OS: 70% (64%-76%), all patients | ORR VTP 81%, VMP 80%, VTP more serious AEs (40 [31% vs 20 [15%], P = .01) and drug discontinuations |
| GIMEMA 030535,54 | 511 patients; 254/257 | 23.2 | 71 | VMPT-VT vs VMP | 3-y PFS: VMPT-VT 56%; VMP 41%; HR 0.67 (95% CI, 0.50-0.90; P = .008) | 5-y OS: VMPT-VT 61% VMP 51% HR 0.70 (P = .01) | PFS VMPT-VT arm: more frequent grade 3/4 AEs: neutropenia (38%), thrombocytopenia (22%), peripheral neuropathy (11%), cardiac events (11%) |
| MRC-IX (59)55 | 859 patients; 423/426 | 44 | 73 | MP vs CTd | MP 12.4 mo; CTd 13 mo HR 0.82 (95% CI, 0.70-0.96; P = .01) | MP 30.6 mo; CTd 33.2 mo; HR 0.89 (95% CI, 0.74-1.08; P = .24) | ORR/PFS/OS ORR: MP 32.6%; CTd 63.8% (P < . 0001) CTDa arm: higher rates of thromboembolic events, constipation, infection, and neuropathy |
| FIRST56 | 1623 patients; 535/541/547 | 67 | 73 (40-92) | Rd vs Rd18 vs MPT | Rd 25.5 mo; Rd18 20.7 mo; MPT 21.2 mo; HR 0.72, Rd vs MPT HR 0.70, Rd vs Rd18; P < .001 for both. Rd was superior to MPT for all secondary efficacy end points, including OS. | 4-y OS: Rd 59%; Rd18 56%; MPT 51% | PFS Grade 3/4 AEs more frequent with Rd than MPT (70% vs 78%) |
| UPFRONT57 | 502 patients; 168/167/167 | 42.7 | 74.5 (67-69) Vd + V 73 (66-77) VTd + V 72 (68-77) VMP + V | Vd 14.7 mo; VTd 15.4 mo; VMP 17.3 mo (P = NS) | Vd 49.8 mo VTd 51.5 mo VMP 53.1 mo (P = NS) | PFS Peripheral neuropathy near 50% in all arms. Early drug discontinuation (29%-38%). QoL scores decreased during induction and improved or stabilized thereafter. | |
| HOVON-8758 | 668 patients 318/319 | 36 | 72 (60-91) | MPT-T vs MPR-R | MPT-T 20 mo; MPR-R 23 mo; HR 0.87 (95% CI, 0.72-1.04; P = .12) | 4-y OS: MPT-T 52% MPR-R 56% | PFS Grade 3/4 hematologic toxicity with MPR-R vs clinically significant neuropathy with MPT-T |
| SWOG S07775,59 | 525 patients; 264/261 | 84 | 43% ≥ 65 | VRd vs Rd | VRd 41 mo Rd 29 mo HR 0.742 (95% CI 0.594-0.9028; 1-sided P = .003) | VRd NR; Rd 69 mo; HR 0.709 (95% CI, 0.543-0.926; 2-sided P = .0114) | PFS VRd (23%) and Rd (10%) discontinued induction treatment due to AE |
| MAIA38 | 737 patients: 368/369 | 56.2 | 73 (50-90) D-Rd continuous 74 (45-89) Rd continuous 43%-44% ≥ 75 | D-Rd NR Rd 34.4 mo HR 0.53 (95% CI, 0.43-0.66; P < .0001) | NR in either arm. HR 0.68 (95% CI, 0.53-0.86; P = .0013) | PFS Median duration on continuous treatment 47.5 mo (D-Rd) and 22.6 mo (Rd) | |
| HOVON-126/NMSG60 | 143 patients | 23.4 | 73 (64-90) | ITd → maintenance ixazomib vs placebo | PFS-R: ITd-I 9.5 mo; ITd- P 8.4 mo | OS-R at 18 mo, all patients 96% (88%-99%) | PFS Early mortality 8% age >75, only 55% randomly assigned to maintenance therapy |
| ALCYONE10 | 706 patients; 350/356 | 40.1 | 71 (40-93) 71 (50-91) | D-VMP-D VMP | D-VMP-D 36.4 mo; VMP 19.3 mo; HR 0.42 (95% CI, 0.34-0.51; P < .0001) | 3-y OS: D-VMP-D 78%; VMP 67.9%; HR 0.60 (95% CI, 0.46-0.80; P = .0003) | PFS Common |
| ECOG E1A1120 | 1087 patients; 542/545 | 9 | 65 (57-71) 64 (59-71) | VRd-R KRd-R | VRd 34.4 mo KRd 34.6 mo | Median OS NR either arm | PFS, OS; excluded high-risk disease; 17.3% discontinued VRd early due to AEs |
| TOURMALINE-MM261 | 705 patients; 351;354 | IRd 53.3 Rd 55.8 | 73 (48-90) 74 (48-88) | IRd-IR Rd-R | IRd 35.3 mo Rd 21.8 mo (HR, 0.830; 95% CI, 0.676-1.018; P = .073) | Median OS NR either arm at 58 mo. HR 0.998 (95% CI, 0.790-1.261; P = .988) | PFS PFS not improved in age ≥75 in IRd |
CTd, cyclophosphamide, thalidomide, dexamethasone; IRd, ixazomib, lenalidomide, dexamethasone; IRd-IR, ixazomib, lenalidomide, dexamethasone followed by ixazomib, lenalidomide continuous therapy; ITd, ixazomib, thalidomide, dexamethasone; ITd-I, ixazomib, thalidomide, dexamethasone induction and ixazomib continuous therapy; ITd-P, ixazomib, thalidomide, dexamethasone and placebo continuous therapy; MP, melphalan, prednisone; MPR, melphalan, prednisone, lenalidomide; MPT, melphalan, prednisone, thalidomide; ORR, overall response rate; QoL, quality of life; Rd, lenalidomide, dexamethasone; Rd18, lenalidomide, dexamethasone for 18 months; Vd, bortezomib, dexamethasone; VMPT, bortezomib, melphalan, prednisone, thalidomide; VTd, bortezomib, thalidomide, dexamethasone.
Studies have shown the benefit of continuous therapy and that responses can deepen over time and lead to survival improvements.39,40 Continuous dexamethasone, however, can lead to excess toxicity in this population, and the opportunity to discontinue it should be considered as data suggest that maintenance therapy can be equally as effective without prolonged usage.41 The treatment of HRMM remains an unmet challenge for the unfit population, and the optimal treatment approach continues to evolve.
CLINICAL CASE (Continued)
The patient was diagnosed with Revised International Staging System II disease without high-risk features and received 4 cycles of D-RVd followed by high-dose melphalan and autologous stem cell transplant. She achieved MRD+ sCR after transplant and started lenalidomide maintenance. She is now 2 years post-transplant and remains in sCR.
Future perspectives
We now have multiple combinations available for the management of NDMM, with 4-drug combinations particularly showing deeper and more durable remissions than have previously been seen. The early successes of immunotherapeutic options in relapsed/refractory MM have led to ongoing studies looking to incorporate these therapies, including chimeric antigen receptor T-cell therapies and bispecific antibodies, into induction strategies for NDMM as a means to harness the potential of a less impaired immune system. Modern trials that have used overall response rate, PFS, and OS as primary end points are now seeing contemporary trials include MRD negativity as an end point and an opportunity for treatment-free intervals, a significant paradigm shift from the maintenance strategies we have become familiar with. Novel therapies, strategic combinations, and new end points for trials will continue to improve the already unprecedented achievements that have been seen in the management of NDMM to date.
Conflict-of-interest disclosure
Caitlin L. Costello: research funding: BMS, Janssen, Takeda; consultancy: BMS, Janssen, Takeda, Pfizer.
Off-label drug use
Caitlin L. Costello: nothing to disclose.