Learning Objectives
Identify key components to consider in the preoperative planning for patients with hemophilia
Understand the goals of factor replacement during the perioperative period
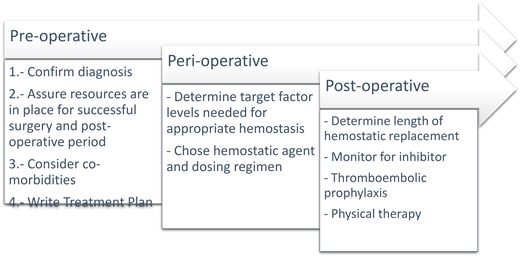
Overview of surgical management
Preoperative planning
The successful surgical management of patients with hemophilia requires advanced preoperative planning.1 A formal treatment plan should be determined and distributed well in advance of any invasive procedure or surgery and is best done under the guidance of a hemophilia treatment center (HTC).
Confirming the diagnosis and baseline factor levels
Prior to surgery, the patient's diagnosis should be confirmed with laboratory testing, rather than prior patient report. It is particularly important to differentiate severe von Willebrand's disease from hemophilia A. A preoperative history should include a review of baseline hemostatic needs that considers the frequency of breakthrough bleeding and the use of prophylactic and breakthrough hemostatic agents as well as surgical history, prior use of hemostatic support, and any bleeding complications. All patients with hemophilia should also be screened for the presence of an inhibitor to factor VIII (FVIII) or factor IX (FIX),2 and their prior factor exposure and inhibitor history should be reviewed, as the presence of an inhibitor has an impact on hemostatic treatment options. Sometimes patients with hemophilia A have discordance between one-stage clot-based FVIII assays and chromogenic FVIII3 ; thus both assays should be obtained preoperatively, if possible, especially if a chromogenic FVIII assay has not been performed in the past. In our practice we rely on the assay with the lowest FVIII for the baseline activity and subsequent monitoring, but not all facilities offer both assays on a 24-hour basis.
Assuring the necessary resources are in place
Access to advanced coagulation testing, clotting factor concentrates (CFCs), and hematology consultation must be available during the perioperative period and considered when patients decide where to receive surgery.4 Ideally, patients will be connected to their local HTC and have access to HTC support throughout the perioperative course.
Considering the postdischarge environment
Additionally, reviewing the patient's ability to self-infuse CFCs is key to planning postoperative CFC replacement if needed, depending on the severity of hemophilia and surgical bleeding risk. Patients with hemophilia who do not know how to self-infuse or have not recently self-infused CFCs are better served by receiving short-term intravenous access prior to surgery and may require appointments for CFC infusions postoperatively.
Considering comorbidities
To ensure successful hemostasis, it is critical to review whether the patient has any comorbidities or other exposures that increase bleeding or thrombotic risk. This includes underlying renal, cardiac, or liver disease as well as medications or supplements that cause acquired thrombocytopenia and/or platelet dysfunction (Table 1)5 ; ideally, these supplements/medications should be discontinued 7 to 14 days prior to surgery. Thrombotic risk assessment is also key to determining whether a patient needs postoperative prophylactic anticoagulation, which is not the standard of care for most patients with hemophilia in the postoperative setting.
Drugs, supplements, and comorbidities affecting platelet functiona
| Drugs affecting platelet function6 . | |
|---|---|
| Class . | Examples . |
| Aspirin and other nonsteroidal inflammatory drugs | Ibuprofen, naproxen, indomethacin, diclofenac, meclofenamic acid, sulindac, Pepto Bismol |
| Glycoprotein IIb/IIIa inhibitors | Abciximab, eptifibatide, tirofiban |
| Phosphodiesterase inhibitors | Sildenafil |
| β-lactam antibiotics | Penicillins, cephalosporins |
| P2Y12 antagonists | Ticlopidine, clopidogrel, prasugrel, ticagrelor |
| Selective serotonin reuptake inhibitors | Fluoxetine, paroxetine, sertraline |
| Tricyclic antidepressants | Amitriptyline, doxepin, nortriptyline |
| Dietary supplements and foods7,8 | |
| Dietary supplements such as fish oils containing omega-3 fatty acids, vitamin E, vitamin B3 | |
| Herbal supplements such as ginkgo biloba, ginseng, turmeric, cinnamon, tarragon, willow, feverfew, dong quai, meadowsweet, pycnogenol (pine bark extract), guarana | |
| Ethanol and nonalcoholic beer | |
| Foods (likely only when consumed in large quantities or as a supplement) such as cumin, cloves, onion, garlic, black tree fungus, ginger, aspartame, red/purple grapes (including grape juice, raisins, red wine, grape seeds), green tea, kiwi fruit, tomatoes, blueberries | |
| Medical conditions affecting platelet function7 | |
| Hematologic disorders such as myeloproliferative neoplasm, myelodysplastic syndrome, paraproteinemia, Gaucher disease | |
| Renal failure | |
| Liver disease | |
| Abnormal cardiac anatomy such as valvular heart disease, left ventricular assistive device, cardiopulmonary bypass | |
| Connective tissue diseases such as Ehlers-Danlos | |
| Drugs affecting platelet function6 . | |
|---|---|
| Class . | Examples . |
| Aspirin and other nonsteroidal inflammatory drugs | Ibuprofen, naproxen, indomethacin, diclofenac, meclofenamic acid, sulindac, Pepto Bismol |
| Glycoprotein IIb/IIIa inhibitors | Abciximab, eptifibatide, tirofiban |
| Phosphodiesterase inhibitors | Sildenafil |
| β-lactam antibiotics | Penicillins, cephalosporins |
| P2Y12 antagonists | Ticlopidine, clopidogrel, prasugrel, ticagrelor |
| Selective serotonin reuptake inhibitors | Fluoxetine, paroxetine, sertraline |
| Tricyclic antidepressants | Amitriptyline, doxepin, nortriptyline |
| Dietary supplements and foods7,8 | |
| Dietary supplements such as fish oils containing omega-3 fatty acids, vitamin E, vitamin B3 | |
| Herbal supplements such as ginkgo biloba, ginseng, turmeric, cinnamon, tarragon, willow, feverfew, dong quai, meadowsweet, pycnogenol (pine bark extract), guarana | |
| Ethanol and nonalcoholic beer | |
| Foods (likely only when consumed in large quantities or as a supplement) such as cumin, cloves, onion, garlic, black tree fungus, ginger, aspartame, red/purple grapes (including grape juice, raisins, red wine, grape seeds), green tea, kiwi fruit, tomatoes, blueberries | |
| Medical conditions affecting platelet function7 | |
| Hematologic disorders such as myeloproliferative neoplasm, myelodysplastic syndrome, paraproteinemia, Gaucher disease | |
| Renal failure | |
| Liver disease | |
| Abnormal cardiac anatomy such as valvular heart disease, left ventricular assistive device, cardiopulmonary bypass | |
| Connective tissue diseases such as Ehlers-Danlos | |
Please note this list is not all inclusive and does not include medications and supplements that can cause thrombocytopenia.
Hemostatic treatment plan
After confirming diagnosis and reviewing the bleeding and thrombotic risks of the patient and the upcoming surgery, a finalized hemostasis treatment plan is critical for communicating with both the patient and the surgical team and ensuring successful perioperative hemostasis. Hemostasis treatment plans should include the diagnosis, type of surgery, timing and need for lab monitoring, and plans for CFC support both preoperatively and postoperatively, including frequency, duration, and location of CFC infusions.
Perioperative management
The clinical effectiveness of hemostasis for surgical interventions can be assessed as per criteria defined by the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis.9
Target factor levels
The World Federation of Hemophilia (WFH; Table 2) and the National Hemophilia Foundation's Medical and Scientific Advisory Council (Table 3) have guidelines for target factor levels and duration of postoperative hemostatic support depending on whether the surgery is minor or major.
WFH recommendations for peak factor levels and length of factor replacement
| . | Minor procedures or surgeries . | Major surgeries . | ||
|---|---|---|---|---|
| . | Examples include tooth extraction, invasive biopsies . | Examples include orthopedic, cardiovascular, intra-abdominal, intracanal surgeries . | ||
| . | Hemophilia A . | Hemophilia B . | Hemophilia A . | Hemophilia B . |
| Preop peak factor activity goal | 50%-80% | 50%-80% | 80%-100% | 60%-80% |
| Postop peak factor activity goal | 30%-80% for 1-5 days | 30%-80% for 1-5 days | 60%-80% days 1-3 40%-60% days 4-6 30%-50% days 7-14 | 40%-60% days 1-3 30%-50% days 4-6 20%-40% days 7-14 |
| . | Minor procedures or surgeries . | Major surgeries . | ||
|---|---|---|---|---|
| . | Examples include tooth extraction, invasive biopsies . | Examples include orthopedic, cardiovascular, intra-abdominal, intracanal surgeries . | ||
| . | Hemophilia A . | Hemophilia B . | Hemophilia A . | Hemophilia B . |
| Preop peak factor activity goal | 50%-80% | 50%-80% | 80%-100% | 60%-80% |
| Postop peak factor activity goal | 30%-80% for 1-5 days | 30%-80% for 1-5 days | 60%-80% days 1-3 40%-60% days 4-6 30%-50% days 7-14 | 40%-60% days 1-3 30%-50% days 4-6 20%-40% days 7-14 |
Data reproduced with permission of Srivastava.1
National Hemophilia Foundation Medical and Scientific Advisory Council Recommendation for length of postoperative replacement therapy
| Minor surgical procedures (eg, tooth extraction, invasive biopsies) | 3 days |
| Major surgical procedures (eg, orthopedic, cardiovascular, intra-abdominal, intracanal surgeries) | 7-10 days |
| Minor surgical procedures (eg, tooth extraction, invasive biopsies) | 3 days |
| Major surgical procedures (eg, orthopedic, cardiovascular, intra-abdominal, intracanal surgeries) | 7-10 days |
Clotting factor concentrates
Multiple CFC products are available for hemophilia A and B that can be used in the perioperative setting. The CFC product should be readily available at the site of surgery and postoperatively if needed, depending on the severity of the patient's hemophilia and anticipated surgical risk. Most patients with hemophilia require CFC support in the perioperative setting.
A preoperative bolus of CFC should be administered 30 to 60 minutes prior to the procedure. For patients without a history of an inhibitor, CFC dosing should be based on the patient's weight, baseline factor levels, target factor level, and volume of distribution. Postoperative factor levels should be obtained depending on the half-life of the CFC: 8 to 12 hours after the last dose of standard half-life FVIII CFCs and 12 to 24 hours after the last dose of standard half-life FIX CFCs. If atypical or unexpected bleeding occurs, a stat factor level should be obtained to confirm sufficient levels, and alternative causes of bleeding should be considered, including anatomical drivers.
Desmopressin
Desmopressin (1-deamino-8-D-arginine vasopressin; DDAVP) monotherapy can be used for some patients with mild hemophilia A undergoing minor procedures. Desmopressin increases the release of endogenous FVIII with varying effectiveness between individuals. A preoperative trial of desmopressin with pre-FVIII and post-FVIII monitoring to confirm an adequate response is recommended. Repeated dosing with desmopressin can cause tachyphylaxis (a rapidly diminishing response), limiting its use to 1 to 2 doses; thus it should not be used as monotherapy if patients require adequate hemostatic support for 3 or more days. Additionally, desmopressin has been associated with intravascular water retention and hyponatremia.10 It should be used with caution in certain populations, including patients under age 2 or those with renal or cardiovascular disease, advanced age, or a baseline higher risk of seizures.11
Bypassing agents for inhibitor patients
Patients with FVIII or FIX inhibitors are typically treated with bypassing agents. The WFH recommends against the use of activated prothrombin complex concentrates (aPCCs; FVIII inhibitor bypass activity; FEIBA) for patients with congenital hemophilia B and inhibitors due to the risk of accumulation of clotting factors II, VII, and X, which can be associated with a higher risk of thrombotic complications. Rarely, patients with inhibitors may receive FVIII or FIX CFC when the inhibitor titer is negligible or low (<5 Bethesda units; BU), and CFC support is anticipated for a short duration with the understanding that reexposure to FVIII or FIX CFC results in an anamnestic response with a rise in the inhibitor titer and a loss of response to the CFC product (discussed more below).
Antifibrinolytics
The antifibrinolytics, tranexamic acid (TXA) and epsilon-aminocaproic acid, can be used as an adjuvant for hemostatic support; these are particularly effective for mucosal bleeding and have indications for use in trauma and orthopedic surgeries in the general patient population. The use of antifibrinolytics intraoperatively for major orthopedic surgeries has been shown to decreased perioperative and postoperative bleeding in hemophilia.12,13 Overall, the use of TXA during major surgeries has not shown an increased risk for thromboembolism.14
Neuraxial analgesia
Before proceeding with neuraxial analgesia, the factor level should be increased to at least 50% (Table 4).1
Types of hemostatic agents for hemophilia
| Hemophilia A without high titer inhibitor . | Hemophilia A with high titer inhibitor (>5 BU) . | Hemophilia B–specific agents . | Hemophilia B with inhibitor . | Adjuvant agents . |
|---|---|---|---|---|
| Standard half-life FVIII CFCs | aPCCs, ie, FVIII bypassing agents (FEIBA)c | Standard half-life FIX CFCs | Activated rFVIIad | Antifibrinolytice |
| Extended half-life FVIII CFCs | Activated rFVIIad | Extended half-life FIX CFCs | Topical fibrin glue | |
| Desmopressina | Emicizumabb | Transfusion support | ||
| Emcizumabb |
| Hemophilia A without high titer inhibitor . | Hemophilia A with high titer inhibitor (>5 BU) . | Hemophilia B–specific agents . | Hemophilia B with inhibitor . | Adjuvant agents . |
|---|---|---|---|---|
| Standard half-life FVIII CFCs | aPCCs, ie, FVIII bypassing agents (FEIBA)c | Standard half-life FIX CFCs | Activated rFVIIad | Antifibrinolytice |
| Extended half-life FVIII CFCs | Activated rFVIIad | Extended half-life FIX CFCs | Topical fibrin glue | |
| Desmopressina | Emicizumabb | Transfusion support | ||
| Emcizumabb |
Desmopressin may be used only for minor bleeding for some patients with mild hemophilia A; desmopressin has an expected increase in FVIII of 2-4-fold and can be used 30 to 60 minutes prior to the procedure. Due to the risk of tachyphylaxis after 1 to 2 doses, desmopressin should not be used if patients need increased FVIII levels for 2 to 3 days postop.11
Emicizumab is used for hemostatic prophylaxis. Please see details in the section on emicizumab for additional information.
The WFH recommends against the use of aPCC/FEIBA for patients with congenital hemophilia B and inhibitors due to the risk of accumulation of clotting factors II, VII, and X, which can be associated with a higher risk of thrombotic complications.
rFVIIa is preferred for patients with hemophilia A with inhibitors on emicizumab, as well as patients with hemophilia B with a history of anaphylaxis to FIX products, which can be indicative of an underlying inhibitor to FIX.
TXA and epsilon-aminocaproic acid should be avoided in patients with a history of an acute thrombosis, disseminated intravascular coagulation, or thrombotic microangiopathy.
Postoperative considerations
Monitoring for a new inhibitor
All patients who are intermittently or periodically exposed to FVIII or FIX CFCs should be monitored for the development of an inhibitor.2 Patients who are not on baseline CFC prophylaxis should be monitored for a new inhibitor after exposure to FVIII or FIX CFC in the perioperative period. The WFH recommends assessing for an inhibitor 4 to 12 weeks after the procedure.1
Thromboembolic prophylaxis
In general, the WFH guidelines recommend against pharmacologic prophylaxis for patients with hemophilia in the postoperative setting. In our practice we consider each patient's risk factors for thrombosis (such as morbid obesity) and the risk for thrombosis with each surgery. Pharmacologic prophylaxis can be considered following joint replacement surgery given the high risk for thrombosis with these procedures. Compared to the general population, asymptomatic deep vein thrombosis after joint replacement surgery in hemophilia is less likely,15 but thromboembolic events can occur. The use of pharmacologic prophylactic anticoagulation has not been shown to increase postoperative bleeding in patients with hemophilia.13
Physical therapy
Physical therapy should be offered to patients with hemophilia and in some cases considered prior to orthopedic surgery to improve postoperative recovery.1,16,17 Postoperative rehabilitation itself carries an increased bleeding risk for which adequate hemostatic coverage has to be scheduled.18 For patients who require ongoing postoperative hemostatic support, especially those with severe hemophilia, CFC infusions should ideally be timed for the same day and prior to physical therapy to minimize bleeding risk. In our clinical practice, we consider infusing CFCs on the day of physical therapy for postoperative days 7 to 14 in patients who would otherwise receive under 7 days of hemostatic support.
Managing antiplatelet and/or therapeutic anticoagulation
Some patients with hemophilia require postoperative antiplatelet and/or therapeutic anticoagulation. Advanced interdisciplinary conversations prior to intervention are critical, and therapeutic approaches should focus on minimizing the use and duration of antiplatelet agents and/or anticoagulation when possible to reduce the risk of severe bleeding in patients with hemophilia. The WFH recommendations stress an individualized approach that accounts for each patient's clinical situation and bleeding risks. FVIII or FIX levels should be maintained above 15% to 30% for dual antiplatelet therapy and above 1% to 5% for single antiplatelet therapy. For patients who require anticoagulation, the WFH recommends maintaining trough factor levels of at least 15% to 30%.1 For patients with hemophilia A on emicizumab prophylaxis, it is unclear if emicizumab monotherapy is sufficient while on antiplatelet and/or anticoagulation, and patients should be closely monitored for breakthrough bleeding. Emicizumab prophylaxis can also be considered to ease outpatient management while on antiplatelet and/or anticoagulation for patients with mild hemophilia A.
Key points
Periprocedural hemostatic treatment plans should include the patient's diagnosis, the type of surgery, the timing and need for lab monitoring, and plans for hemostatic support both preoperatively and postoperatively, including frequency, duration, and location of CFC infusions.
Each patient should be carefully assessed for comorbidities and exposures that may increase bleeding and thrombotic risks.
For minor surgical procedures, patients should have peak factor levels of 50% to 80% prior to the procedure and factors levels of 30% to 80% for the first 1 to 5 postoperative days. For major surgeries, peak factors levels should be 80% to 100% prior to the procedure (with gradual reduction in peak factor level goals over the first 14 postoperative days).
Updates in hemophilia A—impact of emicizumab
CLINICAL CASE 1
A 65-year-old man with moderate hemophilia A (baseline FVIII activity of 5%) and no history of an FVIII inhibitor is undergoing resection of a cancerous lesion on his nose using a skin flap from his forehead. He has a history of hepatitis C that has not been treated and a mildly decreased platelet count of 101 000. He also has a history of colon cancer, for which he underwent resection 6 months earlier. His oncologist started him on emicizumab at a dose of 1.5 mg/kg weekly. Since the preoperative activated partial thromboplastin time (aPTT) was in the normal range and one-stage FVIII activity was 96%, a decision was made not to treat with additional FVIII concentrate. During and after the surgery, the patient experienced excessive bleeding.
The expected hemostatic potential of emicizumab
Emicizumab is a bispecific monoclonal antibody that mimics the function of FVIII.19 Peak thrombin generation correlates linearly, with an emicizumab plasma concentration up to about 80 to 100 mcg/mL, and then tends to level off.20 During maintenance treatment at the currently approved emicizumab dosing (1.5 mg/kg weekly, 3 mg/kg every 2 weeks, or 6 mg/kg every 4 weeks), emicizumab trough levels of approximately 50 mcg/mL are expected,20 which is estimated to translate to an FVIII activity equivalence of at least 15%.21 Therefore, while emicizumab might have sufficient hemostatic potential to perform in minor procedures and surgeries, it should not be presumed to give adequate coverage by itself for major surgeries.4 In the case of major surgery, perioperative management should include replacement with regular CFC as described above.
Impact of emicizumab on laboratory monitoring
The aPTT normalizes even at subtherapeutic emicizumab concentrations.20 Most laboratories in the US utilize the aPTT-based one-stage clotting assay to determine FVIII activity. The immediate shortening of the aPTT as a result of even small doses of emicizumab leads to a gross overestimation of FVIII activity (often >150%, even at subtherapeutic doses).22 The one-stage FVIII clotting assay is not a reliable method of determining hemostatic potential in patients on emicizumab. The FVIII activity measured in the patient above was therefore a substantial overestimation and led to a false assumption of hemostatic risk. To accurately monitor FVIII levels in patients on emicizumab, a chromogenic FVIII activity level must be measured with bovine laboratory reagents, which do not interact with emicizumab (Table 5).
Reliability of laboratory tests in the presence of emicizumab
| Laboratory test . | Reliability of measuring emicizumab activity . | Reliability of measuring activity of extrinsically given FVIII concentrate . |
|---|---|---|
| aPTT | Not reliable | Not reliable |
| FVIII activity—one stage method | Not reliable—grossly overestimates hemostatic potential | Not reliable |
| FVIII activity—chromogenic method with bovine reagents | Does not measure emicizumab | Reliable method of measuring underlying intrinsic and administered FVIII activity. Unaffected by emicizumab. |
| FVIII activity—chromogenic method with human reagents | Linear correlation with emicizumab blood concentration and can be used as a surrogate to an emicizumab level | Measures underlying intrinsic and administered FVIII activity, but emicizumab will interfere |
| Emicizumab concentration | Reliable method of emicizumab concentration but not readily available | Does not measure FVIII activity |
| Laboratory test . | Reliability of measuring emicizumab activity . | Reliability of measuring activity of extrinsically given FVIII concentrate . |
|---|---|---|
| aPTT | Not reliable | Not reliable |
| FVIII activity—one stage method | Not reliable—grossly overestimates hemostatic potential | Not reliable |
| FVIII activity—chromogenic method with bovine reagents | Does not measure emicizumab | Reliable method of measuring underlying intrinsic and administered FVIII activity. Unaffected by emicizumab. |
| FVIII activity—chromogenic method with human reagents | Linear correlation with emicizumab blood concentration and can be used as a surrogate to an emicizumab level | Measures underlying intrinsic and administered FVIII activity, but emicizumab will interfere |
| Emicizumab concentration | Reliable method of emicizumab concentration but not readily available | Does not measure FVIII activity |
Considering the contribution of underlying comorbidities to the overall hemostatic risk
The patient above has a history of hepatitis C, and the bleeding risk based on this should have been taken under consideration prior to surgery. The patient may have undiagnosed cirrhosis with inadequate thrombopoietin and splenomegaly, causing his thrombocytopenia. Due to the loss of both procoagulation and anticoagulation factors, the prothrombin time/international normalized ratio and platelet count are not reliable predictors of bleeding in cirrhosis.23 To avoid overcorrection and excessive transfusion, the recommendations for minor procedures have shifted for patients with cirrhosis without underlying bleeding disorders, with multiple hepatology societies now recommending against evaluation and correction of the international normalized ratio and/or platelet count prior to lower-risk procedures.24-26 There is less guidance for patients with bleeding disorders and cirrhosis.27 Furthermore, the use and safety of emicizumab have not been extensively studied in patients with advanced cirrhosis.
CLINICAL CASE 2
A 28-year-old man with severe hemophilia A with a history of a high titer inhibitor fractured his ankle in a car accident and is now scheduled for pinning of his distal tibial fracture. His inhibitor was detected in childhood at 126 BU; however, he never underwent immune tolerance because neither of his two brothers with hemophilia and inhibitors responded to immune tolerance. The patient has been on emicizumab for 4 years without spontaneous breakthrough bleeding; he has required the use of recombinant factor VIIa (rFVIIa) only for traumatic bleeding events about once a year. His inhibitor titer during his last comprehensive visit 4 months earlier was undetectable, likely due to no recent exposure to FVIII CFC.
Inhibitor status at the time of surgery dictates hemostatic coverage options
The patient in the above case currently has an undetectable inhibitor titer due to a lack of reexposure to FVIII CFCs. Most patients with historically high titer inhibitors (>5 BU) whose titers decrease over time due to a lack of reexposure to FVIII CFCs have an anamnestic response upon reexposure. While the inhibitor is low or undetectable, patients may transiently respond to FVIII CFCs until the titer rises, typically 4 to 5 days after reexposure to FVIII.28 Thus, FVIII CFCs may be used for short durations and can be particularly considered for procedures associated with higher thrombotic risk. If a bypassing agent is used, rFVIIa is recommended for patients on emicizumab due to an increased risk of thrombotic microangiopathy with the concurrent use of emicizumab and aPCCs.
Use of bypassing agents in patients on emicizumab
The hemostatic potential of emicizumab is not sufficient for major surgeries. Bypassing agents can successfully and safely be used in patients with high titer inhibitors who are receiving emicizumab.29 Since the concomitant use of emicizumab and aPCC has led to unwanted thrombotic and thrombotic microangiopathic events,30 rFVIIa is the bypassing agent of choice in the surgical setting. The use of rFVIIa should be adjusted to clinical hemostatic response. A proposed regimen for major surgeries is as follows29 :
Preoperatively, give rFVIIa at a dose of 90-120 µg/kg, plus 10 mg/kg TXA intravenously
Perioperatively, give rFVIIa at a dose of 90-120 µg/kg every 2 hours
Postoperatively:
- º
Days 1 to 2 (0-48 hours): a 90-µg/kg dose of rFVIIa every 2 to 3 hours plus 10 mg/kg TXA
- º
Days 3 to 4 (48-96 hours): a 90-µg/kg dose of rFVIIa every 4 hours plus 10 mg/kg TXA
- º
Days 5 to 7 (96-168 hours): a 90-µg/kg dose of rFVIIa every 6 hours plus 10 mg/kg TXA
- º
Monitoring for anamnestic inhibitor response
Regardless of the historical inhibitor titer, all patients with a history of an inhibitor should be carefully monitored for an increased inhibitor titer after reexposure to FVIII CFC. This includes monitoring for sufficient response and rise in FVIII after exposure to FVIII, as well as trending the inhibitor titer. Bypassing agents should be used if the patient has insufficient response to FVIII CFC or if the inhibitor titer is over 5 BU.
Reducing the need for postoperative dosing for hemostatic support in patients on emicizumab
The underlying hemostatic potential of emicizumab likely lessens the need for postoperative CFC or bypassing agents for more than 1 week after surgery. There are no clinical trials to lend guidance. In general, CFCs or a bypassing agent should be given when an FVIII equivalent level greater than 15% to 30% is desired.
Updates in hemophilia B—impact of extended half-life
CLINICAL CASE 3
A 54-year-old man with severe hemophilia B is seen for surgical planning for a total right knee arthroplasty. He is currently on an extended half-life FIX concentrate, which he takes every 7 days. On this regimen he has not experienced breakthrough bleeding in several years. He has normal liver and renal function and is otherwise healthy. Per the WFH guidelines, the following factor goals are desired: a preoperative FIX activity of 80% to 100%, a FIX activity of 40% to 60% on days 1 to 3, a FIX activity of 30% to 50% on days 4 to 6, and a FIX activity of 20% to 40% on days 7 to 14.
Role of extended half-life products in the postoperative setting
Generally, the use of extended half-life products in the surgical setting have been found to be safe,31-33 but the individual product's half-life and the patient's response must be considered, including when to redose the product (Table 6). Extended half-life products may be particularly beneficial for patients with mild hemophilia to limit the need for infusions in the postoperative setting.
Currently approved extended half-life factor concentrates in the US
| Name . | Mechanism of half-life extension . | Half-life (hours)a . | Surgical recommendations (where available in package inset) . | Reference . |
|---|---|---|---|---|
| Hemophilia A | ||||
| Efraloctocog alfa (rFVIII-Fc; Eloctate) | Fc fusion | 19.7 | 35 | |
| Octocog alfa pegol (BAX855; Adynovate) | Pegylation | 14.7 | 36 | |
| Turoctocog alfa pegol (N8-GP; Esperoct) | Glycopegylation | 21.7 | For minor/major surgery: in adolescents/adults: preoperative dose of 50 IU/kg body weight; in children (<12 years), preoperative dose of 65 IU/kg body weight. Frequency of administration is determined by the treating physician. | 37 |
| Damoctocog alfa pegol (BAY94-9027; Jivi) | Pegylation | 17.4 | 38 | |
| Hemophilia B | ||||
| rFIX-Fc; Alprolix | Fc fusion | 97 | Minor surgery: a single infusion to reach FIX level of 50-80 IU/dL may be sufficient. Repeat as needed after 24-48 hours until bleeding stops and healing is achieved. Major surgery: initial infusion to reach FIX level of 60-100 IU/dL. Consider a repeat dose after 6-10 hours and then every 24 hours for the first 3 days and then every 48 hours until bleeding stops and healing is achieved. | 39 |
| rFIX-FP; Idelvion | Albumin fusion | 104 | 40 | |
| Nonacog beta pegol (N9-GP; Rebinyn) | Pegylation | 83.0 after single dose, 114.9 at steady state | Minor surgery: preop dose of 40 IU/kg Major surgery: preop dose of 80 IU/kg, then, as clinically needed for the perioperative management of bleeding, repeated doses of 40 IU/kg (in 1-3 day intervals) within the first week after major surgery may be administered. Frequency may be extended to once weekly after the first week until bleeding stops and healing is achieved | 41 |
| Name . | Mechanism of half-life extension . | Half-life (hours)a . | Surgical recommendations (where available in package inset) . | Reference . |
|---|---|---|---|---|
| Hemophilia A | ||||
| Efraloctocog alfa (rFVIII-Fc; Eloctate) | Fc fusion | 19.7 | 35 | |
| Octocog alfa pegol (BAX855; Adynovate) | Pegylation | 14.7 | 36 | |
| Turoctocog alfa pegol (N8-GP; Esperoct) | Glycopegylation | 21.7 | For minor/major surgery: in adolescents/adults: preoperative dose of 50 IU/kg body weight; in children (<12 years), preoperative dose of 65 IU/kg body weight. Frequency of administration is determined by the treating physician. | 37 |
| Damoctocog alfa pegol (BAY94-9027; Jivi) | Pegylation | 17.4 | 38 | |
| Hemophilia B | ||||
| rFIX-Fc; Alprolix | Fc fusion | 97 | Minor surgery: a single infusion to reach FIX level of 50-80 IU/dL may be sufficient. Repeat as needed after 24-48 hours until bleeding stops and healing is achieved. Major surgery: initial infusion to reach FIX level of 60-100 IU/dL. Consider a repeat dose after 6-10 hours and then every 24 hours for the first 3 days and then every 48 hours until bleeding stops and healing is achieved. | 39 |
| rFIX-FP; Idelvion | Albumin fusion | 104 | 40 | |
| Nonacog beta pegol (N9-GP; Rebinyn) | Pegylation | 83.0 after single dose, 114.9 at steady state | Minor surgery: preop dose of 40 IU/kg Major surgery: preop dose of 80 IU/kg, then, as clinically needed for the perioperative management of bleeding, repeated doses of 40 IU/kg (in 1-3 day intervals) within the first week after major surgery may be administered. Frequency may be extended to once weekly after the first week until bleeding stops and healing is achieved | 41 |
Based on one-stage clotting assay in adults, half-life tends to be shorter in children.
The half-lives of the different CFC products should not be directly compared. Each patient experiences different pharmacokinetics, and the patient populations were not the same in the individual studies of different CFC products. To date, there has never been a comparison study of pharmacokinetics of different CFC products.
Data reproduced with permission of Ling, Nathwani, and Tuddenham.34
Acknowledgment
This project did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict-of-interest disclosure
Jacqueline N. Poston: consultancy: TeraImmune.
Rebecca Kruse-Jarres: research funding: Genentech; consultancy: Biomarin, Pfizer, Roche; educational speaker: Roche, Takeda.
Off-label drug use
Jacqueline N. Poston: nothing to disclose.
Rebecca Kruse-Jarres: nothing to disclose.