Abstract
Immune checkpoint inhibitors are a class of antineoplastic therapies that unleash immune cells to kill malignant cells. These medications commonly cause immune-related adverse effects due to activated adaptive and innate immune cells, autoantibody production, and/or cytokine dysregulation. Hematologic toxicities are rare and of uncertain mechanism, and therefore management is often based on experiences with familiar conditions involving these perturbed immune responses. Management is challenging because one must attend to the hematologic toxicity while simultaneously attending to the malignancy, with the imperative that therapeutic effects be maintained or minimally interrupted when possible.
Learning Objectives
Recognize ICI toxicity within a complex clinical milieu
Manage hematologic toxicity without threatening the management of malignancy
CLINICAL CASE
A 39-year-old woman was diagnosed with stage IV M1d melanoma metastatic to the liver, bone, soft tissues, lymph nodes, and brain. She was started on dual checkpoint inhibitor therapy with ipilimumab + nivolumab and completed 4 cycles followed by maintenance nivolumab. Four months into maintenance therapy, PET-CT revealed a good overall response but a single new metastasis in the left ilium. She continued nivolumab and received radiation therapy to the bone lesion. Two months after completing radiation therapy, PET-CT showed a new fluorodeoxyglucose-avid left common iliac node. Single-agent nivolumab was stopped and dual checkpoint inhibitor therapy reinitiated. She received ipilimumab + nivolumab for 3 cycles, at which time it was stopped because of continued disease progression. While red cell and platelet counts remained near normal during the second round of dual checkpoint therapy, the absolute neutrophil count (ANC) fell from 2990/µL before it was resumed to 1250/µL after 2 cycles to 10/µL after 3 cycles. At no time were there fevers, mouth sores, or any other symptoms or signs of infection.
What are immune checkpoint inhibitors?
Ipilimumab is a human IgG4 monoclonal antibody that binds and inhibits cytotoxic T lymphocyte associated protein-4 (CTLA-4). CTLA-4 is a protein receptor expressed on activated CD4+ and CD8+ lymphocytes that inhibits these immune cells. It does this by directly transducing an inhibitory signal and by subverting a stimulatory pathway mediated by lymphocyte CD28 binding to CD80/86 present on antigen-presenting dendritic cells (APCs) and macrophages. It subverts CD28-mediated lymphocyte activation because its higher affinity for CD80/86 results in competitive inhibition of CD28 binding to CD80/86 (Figure 1).
How the CTLA-4 inhibitor works. Anti-CTLA-4 therapy blocks inhibitory signals to cytotoxic (CD8+) and helper (Th1 and Th2) T lymphocytes and suppresses the activation of regulatory T cells (CD4/25+). (Reprinted from Kroll et al1 with permission.)
How the CTLA-4 inhibitor works. Anti-CTLA-4 therapy blocks inhibitory signals to cytotoxic (CD8+) and helper (Th1 and Th2) T lymphocytes and suppresses the activation of regulatory T cells (CD4/25+). (Reprinted from Kroll et al1 with permission.)
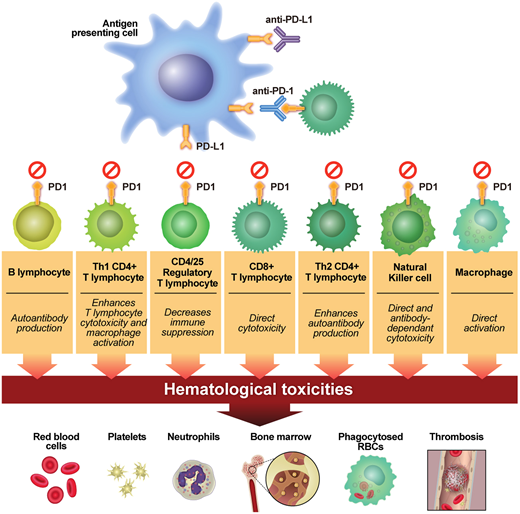
Nivolumab is a human IgG4 monoclonal antibody that binds to and inhibits programmed cell death protein-1 (PD-1). PD-1 is a protein receptor found on activated CD4+ and CD8+ T lymphocytes, as well as on B lymphocytes, macrophages, natural killer cells, and myeloid-derived suppressor cells. It binds to programmed death-ligand 1 (PD-L1) expressed on antigen-presenting dendritic cells and macrophages and tumor cells, and this binding transduces inhibitory signals (Figure 2).
How PD-1 and PD-L1 inhibitors work. PD-1/PD-L1 therapies have block inhibitory signals to cytotoxic and helper T lymphocytes, B lymphocytes, natural killer cells, and macrophages and suppress the activation of regulatory T cells. RBCs, red blood cells. (Reprinted from Kroll et al1 with permission.)
How PD-1 and PD-L1 inhibitors work. PD-1/PD-L1 therapies have block inhibitory signals to cytotoxic and helper T lymphocytes, B lymphocytes, natural killer cells, and macrophages and suppress the activation of regulatory T cells. RBCs, red blood cells. (Reprinted from Kroll et al1 with permission.)
Most FDA-approved immune checkpoint inhibitors (ICIs) are directed against PD-1 or PD-L1; ipilimumab is the only available CTLA-4 inhibitor.1 Many clinical trials are underway evaluating checkpoint inhibitors of B lymphocytes,2 as well as checkpoint inhibitors of innate immunity mediated by natural killer cells, neutrophils, macrophages, and myeloid-derived suppressor cells.3-5
Is this patient's severe neutropenia caused by ipilimumab + nivolumab?
The parsimonious explanation is that this is ICI toxicity. While rare, ICI-associated neutropenia and agranulocytosis have been catalogued.6 One analysis of 47 clinical trials encompassing over 9000 patients calculated the incidence of common terminology criteria for adverse events grade 3 (<1000/µL), 4 (<500/µL), and 5 (lethal) neutropenia as ranging from 0.4 to 1.7%.7 Another catalogue of 5923 patients from 19 clinical trials identified grade 2 (<1500/µL) or worse neutropenia in 0.61%.8 Dual checkpoint blockade, as was used in this case, may double the risk of immune-mediated toxicity.9 Radiotherapy, as was also used in this case, appears to not increase the rate of ICI toxicities, but the combination of ipilimumab + radiotherapy is associated with higher-grade toxicities.10 Further evidence that this is an ICI effect is that the time of onset of our patient's grade 4 (also referred to as severe) neutropenia occurred sometime between week 8 and 12 after reinitiating dual ICI therapy, similar to the typical median onset of severe neutropenia as described in several reports.11-14 Finally, the absence of fever in our patient is not unexpected, as fewer than half of those with severe neutropenia suffer febrile episodes.13
How can one be sure that there isn't another cause of this patient's neutropenia?
The first step is to review medications to determine if a recently added nonchemotherapy drug caused the severe neutropenia.15 In this case no drug culprit emerged. The next step, here and in all cases of ICI-associated cytopenia, is to rule out myelophthisis from metastatic cancer. This can often be identified on the blood smear, but in the absence of leukoerythroblastic features and uncertainty about metastatic marrow involvement, it is reasonable to examine the bone marrow (Figure 3). It was not done in this case because other blood counts were preserved and the blood smear was unremarkable other than the absence of myeloid elements. In one case series of ICI-induced neutropenia, bone marrow examination done on 24 patients suggested pleiotropic pathologic mechanisms: ~45% were normal, ~10% were hyperplastic, and ~45% demonstrated either myeloid hypoplasia or maturation arrest.13 These results provide insight into the mechanism and may direct therapy (see below). Antineutrophil antibodies were not measured in this case, as this same case series showed that they are unlikely to be useful: only 4 of 32 patients were tested, 2 of whom tested positive and none of whom were managed differently.13
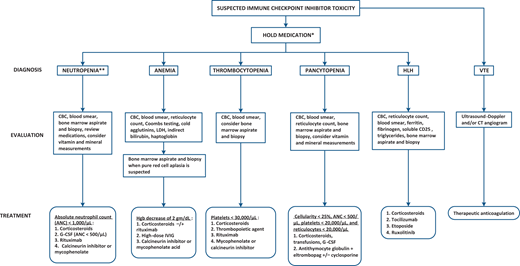
Management of immune checkpoint inhibitor hematologic toxicity. *Recommended thresholds for holding therapy are 1500/µL neutrophils, 75 000/µL platelets, and 8 g/dL serum hemoglobin concentration. **Please be aware of the possibility of Duffy null associated neutrophil count (previously designated “benign ethnic neutropenia”) among patients of African or Middle Eastern ancestry. CBC, complete blood cell count.
Management of immune checkpoint inhibitor hematologic toxicity. *Recommended thresholds for holding therapy are 1500/µL neutrophils, 75 000/µL platelets, and 8 g/dL serum hemoglobin concentration. **Please be aware of the possibility of Duffy null associated neutrophil count (previously designated “benign ethnic neutropenia”) among patients of African or Middle Eastern ancestry. CBC, complete blood cell count.
CLINICAL CASE (continued)
Nivolumab and pembrolizumab were stopped because of progressive melanoma coincident with the development of severe neutropenia (ANC 10/µL). She was started on prednisone. After 2 weeks of prednisone 40 mg twice daily her ANC rose to 2900/µL. Prednisone was continued at 30 mg twice daily for 2 weeks followed by 20 mg twice daily for 2 weeks, at which time the ANC was 2300/µL. Prednisone tapering continued.
Was this patient treated appropriately?
It is recommended that ICIs be held when neutrophils fall below 1500/µL (Figure 3). Our case supports this recommendation, as the additional cycle of dual checkpoint inhibitors given at the time her ANC was below 1500/µL culminated in severe neutropenia, permitting speculation that this could have been mitigated had the ICIs been stopped immediately after neutropenia was identified. As done in this case, active therapy begins when the ANC falls below 1000/µL using corticosteroids without or with granulocyte colony-stimulating factor (G-CSF). G-CSF was not needed in this case because the patient was asymptomatic and the neutrophil count began rising within days of beginning prednisone 40 mg twice daily. Rituximab is sometimes used; it has been given during induction, during maintenance (to support corticosteroid taper), or following relapse when steroids are resumed.
When there is no early response, high-dose intravenous immunoglobulin (IVIG) can be administered to those with normal or hypercellular bone marrow (considered to suffer splenic autoantibody-mediated immune destruction) and a calcineurin inhibitor (cyclosporine or tacrolimus) or mycophenolate for those with hypoplastic or maturation-arrested bone marrow (considered to suffer CD8+ T lymphocyte–mediated neutrophil precursor destruction or suppression). When there are no bone marrow biopsy results, a calcineurin inhibitor or mycophenolate is preferred as second-line therapy, as clinical responses to IVIG are infrequent.6,13 Treatment generally works: in 1 systematic review of 27 patients, 70% responded to immune suppression, with the median time to response of less than 1 month.12 Two patients died, but death was attributed to cancer progression rather than intractable neutropenia.
Are there other hematologic complications from checkpoint inhibitors?
The previously acknowledged systematic review identified hematologic toxicities in 3.6% of all patients with cancer treated with ICIs.7 In 2023 over 1 900 000 Americans will be diagnosed with cancer (excluding nonmelanoma skin cancer), and it is estimated that 12% or ~228 000 will receive and benefit from ICIs, leading to ~8000 new cases of ICI-associated hematologic toxicity.16,17 In addition to neutropenia, which develops in at least 0.4% of patients (perhaps 50 patients this year), the systematic review identified immune thrombocytopenia (ITP) (1%), aplastic anemia/pancytopenia (0.6%), hemolytic anemia (0.6%), hemophagocytic lymphohistiocytosis (HLH, 0.4%), and pure red cell aplasia (0.3%).7 Venous thromboembolism may also be associated with ICIs, although risk data are ambiguous due to complex comorbid prothrombotic factors affecting these individuals.18,19 Finally, the American Society of Clinical Oncology management guidelines list thrombotic thrombocytopenic purpura, atypical hemolytic uremic syndrome, lymphopenia, and acquired hemophilia A as ICI toxicities.20 Except for HLH (see below), PD-1, PD-L1, and CTLA-4 inhibitors appear to be associated with hematologic toxicities of similar rates, types, magnitudes, and clinical courses.
Immune thrombocytopenia
The Registre des Effets Indésirables Sévères des Anticorps Monoclonaux Immunomodulateurs en Cancérologie (REISAMIC) is a prospective multicenter registry of patients treated with anti-PD-1 or anti-PD-L1 therapy. Nine patients in the REISAMIC registry presented with thrombocytopenia.11 Seven of nine patients had laboratory evaluations and bone marrow biopsies consistent with ITP. Thrombocytopenia was severe with a median nadir platelet count of 5000/µL. An antibody to platelet glycoprotein IIb/IIIa was identified in one patient's serum. Severe bleeding developed in 2 of 9 patients, but there was no fatal bleeding.
Management derives from routine therapies for de novo ITP (Figure 3). One would hold the ICI when the platelet count falls below 75 000/µL and begin immune suppressive therapy when the platelet count is below 30 000/µL. Treatment begins with corticosteroids for all—either dexamethasone 40 mg per day for 4 days or prednisone 1-2 mg/kg per day. High-dose IVIG should be added for patients who are bleeding, and we recommend rapidly introducing a thrombopoietic agent when corticosteroids and/or IVIG do not work. We prefer a thrombopoietic agent to rituximab because it decreases immunosuppression, which may have an adverse effect on tumor progression.21 In one survey, recovery occurred in 21 out of 36 patients (58%).12 Drugs that target activated cytotoxic CD8+ T lymphocytes, such as a calcineurin inhibitor or mycophenolate, may be particularly useful for refractory ITP, as cytotoxic T lymphocytes frequently mediate steroid-refractory ITP, and drugs targeting them can lead to platelet recovery.22,23
Cytopenias and bone marrow failure
The REISAMIC registry reported 4 of 5 patients with pancytopenia whose bone marrow showed severe trilineage hypoplasia; 1 patient's bone marrow was “near-normal”; 1 of 5 died of neutropenic sepsis; and 1 of 5 recovered over 8 months.11 A summary of reported cases describes a broad range in the time of onset of bone marrow failure, but the majority occurred within 2–3 months of beginning the ICI.12
One should hold the ICI while providing transfusion and G-CSF support for patients with nonsevere aplasia and begin immunosuppression when aplasia is severe (marrow cellularity <25% with ANC <500/µL, platelets <20 000/µL and reticulocytes <20 000/µL) (Figure 3). Treatment includes corticosteroids + antithymocyte globulin + eltrombopag, with cyclosporine added for those with severe aplasia and active cancer demonstrating a poor or uncertain response to the ICI.24 Similar parameters for treatment, based on the bone marrow cellularity and the myeloid:erythroid ratio, have been used for patients with pure red cell aplasia or bicytopenia. One case of amegakaryocytic thrombocytopenia was treated effectively with prednisone and eltrombopag.25 In contrast to other hematologic toxicities, responses are infrequent (~30%) and mortality is high (~30%).12
Hemolytic anemia
REISAMIC included 9 cases of hemolytic anemia, identified by any CTCAE grade 2 (Hgb <10 g/dL) or worse, a US multicenter retrospective cohort analysis included 14 cases, and the FDA Adverse Events Reporting System identified 68 cases.11,26,27 In REISAMIC all 9 cases had a positive direct antiglobulin test: 3 for IgG and 6 for complement factor 3d; 3 of the latter 6 patients also had IgM autoantibodies (cold agglutinins) in their serum. Among 13 US patients tested in the US multicenter analysis, a positive direct antiglobulin test was identified in 8 (62%); the median number of ICI cycles was 3 (range of 1-12), all events were CTCAE grade 3 or 4 (Hgb <8 g/dL), the median nadir hemoglobin was 6.3 g/dL, and red blood cell transfusion support was required in 11 of 14, including the transfusion of 4 or more units of packed red blood cells in 7 of 14 patients.26 No serologic testing was available in the FDA database. It therefore appears that many cases are due to autoantibody production, possibly due to decreased regulatory T lymphocyte–mediated immune suppression and/or B-cell activation (Figures 1 and 2).
Treatment aims at autoantibody-mediated hemolysis: corticosteroids and rituximab, given simultaneously or sequentially, possibly along with IVIG (Figure 3). Failing that, a calcineurin inhibitor or mycophenolate acid is recommended with the caveat that little data are available to support an approach targeting cytotoxic T lymphocytes.28
Hemophagocytic lymphohistiocytosis
Over 200 cases of hemophagocytic lymphohistiocytosis have been reported.11,29,30 Most are associated with anti-PD1, anti-PD-L1, and combination therapies; only 7/190 (5.7%) were associated with single-agent ipilimumab.30 Their onset was anytime between 1 week and over a year after the ICI was begun. The most common presenting symptoms were fever and organomegaly, the mean ferritin level was 27 000 µg/L, and 16 of 18 patients had demonstrable hemophagocytosis in the bone marrow aspirate or blood smear. In over half of the cases alternative potential predispositions or triggers for HLH were identified, such as progressive malignancy, infection, and 1 deleterious perforin mutation.31 Guidelines for managing ICI-related HLH are derived from HLH Society guidelines, which recommends starting corticosteroids and tocilizumab, and adding etoposide if there is no response after 48 hours (Figure 3).32 In 1 case series of 20 patients with ICI-associated HLH, all were treated with ICI withdrawal and corticosteroids, 6 were treated with etoposide, 1 with tocilizumab, and 1 with anakinra: 15 of 20 patients recovered and 3 of 20 patients died.29
Thrombosis
Venous thromboembolism risk from ICIs is uncertain and there appears to be no risk of arterial thrombosis.18,19,33,34 Pharmacologic thromboprophylaxis is not recommended among ambulatory patients with cancer receiving an ICI, and ICI therapy should not be stopped when acute venous thromboembolism is diagnosed.
CLINICAL CASE (continued)
Within 2 weeks of completing an 8-week course of prednisone, the ANC fell to 550/µL. Prednisone was resumed and rituximab started. About 1 month later the ANC was 2800/µL.
Is relapse unusual, and how much do we know about long-term outcomes?
Relapse after medication discontinuation and steroid taper is not uncommon: 1 systematic review reported a 9% relapse rate.6 General conclusions about long-term clinical outcomes are confounded by heterogeneous clinical factors and varying therapies. Nonetheless, except for patients with pancytopenia (who have poorer outcomes) and HLH (who, surprisingly, have better outcomes), about two-thirds of patients with hematologic toxicity will recover within 1 month following the initiation of immune suppression. For patients who recover and whose malignancy was controlled by the ICI, the ICI can sometimes be safely resumed (along with ongoing but minimized corticosteroid dosing).1,6,12,21 For those who don't respond, second-line immune suppression is begun with either rituximab—as was used in this case—or medications that target cytotoxic T lymphocytes such as cyclosporine, tacrolimus, or mycophenolate, the latter of which may be preferred for isolated thrombocytopenia.35 For those whose toxicity resolves and require another treatment with targeted therapy, there appears to be no risk of recurrent or worsening ICI toxicity.36 While persistent organ- specific ICI toxicities are being increasingly recognized, to date persistent hematologic toxicities are not among them; and, while up to 15% of patients suffering from ICI-associated hematologic toxicity die, death is only rarely related directly to the hematologic problem.6,37
CLINICAL CASE (continued)
Following neutrophil recovery and identification of an activating mutation in tumor N-Ras, she was considered for enrollment in the phase 2 NAUTILUS trial of the histone deacetylase inhibitor OKI-179 + the mitogen-extracellular activated protein kinase inhibitor binimetinib. Unfortunately, the ANC fell to 1760/µL during prednisone taper, making her ineligible for OK-179 therapy. She is currently exploring phase 1 studies targeting mutant N-Ras.
Conclusion
ICI-associated hematologic toxicities are rare but easily recognizable. Except for thromboses, management begins with pausing the offending agent. Treatments follow standards developed for patients with related hematologic disorders. ICI discontinuation and therapeutic immunosuppression must be balanced against the need to maintain an effective antitumor immune response.
Acknowledgments
I thank Jordan Pietz for medical illustrations and Mary Lou Warren for guidance on Figure 3.
Conflict-of-interest disclosure
Michael H. Kroll: no competing financial interests to declare.
Off-label drug use
Michael H. Kroll: The use of cyclosporine, tacrolimus, mycophenolate, rituximab, tociluzimab, or anakinra to treat ICI-associated hematological toxicities is off-label.