Learning Objectives
Describe the burden of bone disease and bone pain in SCD
Summarize the available evidence, including the efficacy and safety of bisphosphonates, in SCD
CLINICAL CASE
J.D., a 12-year-old boy with hemoglobin sickle cell disease (Hb SCD) and infrequent vaso-occlusive episodes (VOEs) in childhood, developed excruciating, atypical pain in his thighs after resolution of an uncomplicated VOE. Opioids, nonsteroidal anti-inflammatory drugs, gabapentin, antidepressants, and muscle relaxants did not alleviate his lower-extremity pain. X-rays, magnetic resonance imaging, and computed tomographic scans of his lower extremities were unrevealing. Physical, occupational, and alternative therapies such as acupuncture offered minimal relief. Over the next 5 years, J.D. became increasingly withdrawn as his chronic pain and debility negatively impacted his school attendance and peer relationships. At age 17 years (weight, 51.4 kg; height, 1.7 m; body mass index, 17.1 kg/m2), he underwent his first dual- energy X-ray absorptiometry (DXA) scan, which showed height-adjusted bone mineral density (BMD) Z-scores of −2.25 (lumbar spine) and −3.30 (total body less head) relative to age-, sex-, and race-matched references, consistent with low bone density for age in validated non-SCD populations. He received intravenous (IV) zoledronic acid (ZA) in April 2024 and reported acute-onset fatigue and diffuse body pain that improved within 2 weeks without medical intervention. His severe chronic thigh pain also completely resolved, and he was able to attend his high school graduation. J.D.'s lower-extremity pain unfortunately recurred; he received a second dose of ZA in July 2024 with eventual pain resolution. His pediatric endocrinologist plans to administer ZA every 3 months until growth completion and to repeat his DXA scan in 1 to 2 years. Given the remarkable recovery in this and other reported cases, should we consider bisphosphonates as treatment for SCD-related bone disease?
Sickle cell bone disease
SCD is a monogenic hemoglobin disorder that affects over 100 000 people in the United States and millions more worldwide.1 VOEs—the hallmark complication of SCD— occur when dense aggregates of sickled erythrocytes, activated white blood cells, and platelets adhere to the vascular endothelium and impair blood flow downstream of the obstruction, causing severe pain and ischemia/reperfusion tissue injury.2 Hydroxyurea, chronic red blood cell transfusions, and other SCD-modifying drugs, along with public health interventions like universal newborn screening, penicillin prophylaxis, and vaccinations against encapsulated organisms, have transformed SCD from a genetic disorder with high childhood mortality to a chronic illness characterized by progressive pain and end-organ damage.3 Skeletal injury in SCD arises when recurrent hypoxia-reperfusion stress induces the release of cytokines that accelerate bone resorption,4 while nutritional deficits, low muscle mass, delayed puberty, and physical inactivity hinder bone formation (Figure 1).5,6 Skeletal complications of SCD, also known as sickle bone disease (SBD),7 include acute (eg, bone infarcts, osteomyelitis) and chronic (eg, osteonecrosis, osteoporosis, and vertebral compression fractures) abnormalities that are radiographically evident from childhood and progressively worsen with age.8-10 Bone pain may persist after curative hematopoietic stem cell transplant,11 and it remains to be seen whether recently approved SCD gene therapies can impact SBD.12,13 This unmet clinical need highlights the importance of identifying safe and effective therapies for skeletal morbidity, including bone pain, in people living with SCD.
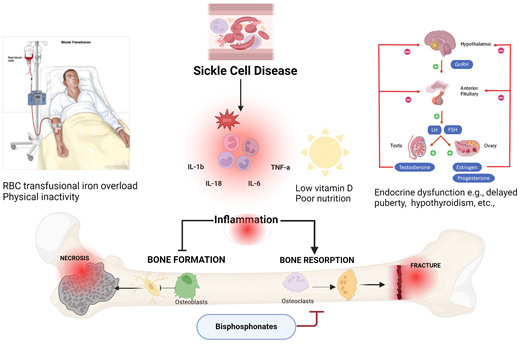
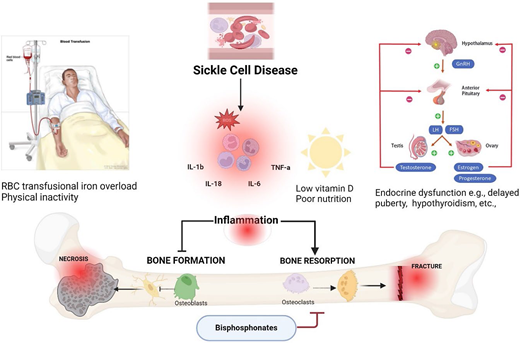
Contributing factors such as inflammation, low vitamin D, iron overload, delayed puberty, and bouts of inactivity in SCD result in decreased bone formation by osteoblasts and increased bone resorption by osteoclasts, leading to avascular necrosis, fractures, and pain. Osteoclast mediated resorption can be limited by bisphosphonates. FSH, follicle-stimulating hormone; GnRH, gonadotropin hormone-releasing hormone; IL, interleukin; LH, luteinizing hormone; ROS, reactive oxygen species. Created with Biorender.com
Contributing factors such as inflammation, low vitamin D, iron overload, delayed puberty, and bouts of inactivity in SCD result in decreased bone formation by osteoblasts and increased bone resorption by osteoclasts, leading to avascular necrosis, fractures, and pain. Osteoclast mediated resorption can be limited by bisphosphonates. FSH, follicle-stimulating hormone; GnRH, gonadotropin hormone-releasing hormone; IL, interleukin; LH, luteinizing hormone; ROS, reactive oxygen species. Created with Biorender.com
Skeletal morbidity and chronic pain in SCD
Osteonecrosis, a highly prevalent form of SBD, is strongly associated with chronic SCD pain.14 While opioid analgesics and nonsteroidal anti-inflammatory drugs effectively treat acute VOEs, the increased risk of hyperalgesia, opioid dependence,15 and acute kidney injury limit their long-term use in chronic SCD pain management.16 The inflammatory bone microenvironment in SCD exacerbates bone resorption, impairs bone formation, and may contribute to bone pain.4,17-19 We therefore hypothesize that medications that target bone turnover may alleviate pain from SBD (Figure 1). Herein, we summarize emerging evidence on the role of bisphosphonates in treating bone pain and other skeletal complications in this vulnerable patient population.
Bisphosphonates in SCD
Bisphosphonates are synthetic analogues of pyrophosphate that increase BMD by binding hydroxyapatite in bone and inhibiting osteoclast-driven bone resorption.20 Bisphosphonates are indicated for the treatment of osteoporosis, Paget's disease of the bone, and thalassemia-related bone loss and to prevent pathologic fractures in multiple myeloma.21-23 Despite these well-established therapeutic benefits of bisphosphonates on bone health, few studies have looked at their potential role in treating SBD. Grimbly et al reported clinical outcomes in a retrospective cohort of 46 children and adolescents with SBD (43% females; 87% HbSS genotype; mean age 11.8 ± 3.9 years) recruited from 3 tertiary pediatric centers in Canada.9 Twenty-three subjects (50%) received IV bisphosphonates as either weight-based pamidronate or ZA every 4 or 6 months, respectively. Ten of the 11 children (91%) who completed pain assessments before and after bisphosphonates reported improvement or resolution of their pain. The investigators reported histological evidence of improved trabecular microarchitecture and cortical wall thickness on transiliac bone biopsy (Figure 2). They also noted remodeling of some necrotic joints on imaging, though these changes were not statistically significant. Four children experienced acute phase reactions, and 1 child developed symptomatic hypocalcemia after administration of IV bisphosphonates. There were no serious bisphosphonate-related complications like osteonecrosis of the jaw, atypical femoral fractures, or premature closure of growth plates. More importantly, no one developed VOEs, acute chest syndrome, strokes, or other acute SCD complications. These promising results are limited by the retrospective study design, small number of participants, and sole inclusion of pediatric patients with a low baseline prevalence of chronic SCD pain. The investigators did not report any opioid or nonopioid analgesic use in the children or adolescents before or after IV bisphosphonate treatment. Others have reported spontaneous regression of osteonecrosis in children with SCD,24 so some of the clinical improvements observed in this retrospective pediatric cohort may be attributed, in part, to optimization of vitamin D or calcium levels, pubertal onset, or longer-term exposure to SCD-modifying therapies.
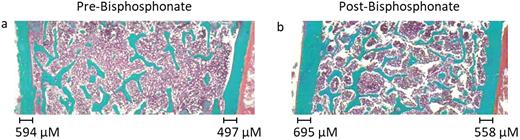
Improvement in bone architecture as well as cortical wall thickness as seen in an individual treated with bisphosphonates for 2 years. © 2022 by Grimbly et al9 and licensed under CC BY-NC 4.0. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc/4.0/.
Improvement in bone architecture as well as cortical wall thickness as seen in an individual treated with bisphosphonates for 2 years. © 2022 by Grimbly et al9 and licensed under CC BY-NC 4.0. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc/4.0/.
Published data on bisphosphonates' effect on bone pain in adults with SCD is less robust. De Franceschi et al conducted a retrospective cohort study in 71 SCD adults (mean age, 39 ± 10 years; 84% HbSS/Sβ0 thalassemia) from 2 academic centers in Italy between 2009 and 2017.10 Fifty-three percent of the study cohort required vitamin D supplementation, and 9 subjects (13%) received vitamin D and ZA. Though not detailed in the paper, Dr. Dalle Carbonare (senior author) confirmed that ZA was given at least annually for 2 doses, primarily for vertebral compression fractures. One of the 9 adults treated with ZA developed an acute phase reaction with joint pain and fever. Similar to the Grimbly et al pediatric study, none of the adults who received ZA developed any acute SCD complications.9 Using receiver operator curve analyses of bone densitometry and spine X-ray results, the investigators generated an SBD treatment algorithm recommending bisphosphonate initiation in SCD adults with vertebral fractures or a BMD T-score equal to or less than −1.4. We recently analyzed clinical data on the long-term use of alendronate (oral bisphosphonate) in a retrospective cohort of 64 Brazilian adults with SCD and low BMD. Four subjects (6%) reported mild, self-limited gastrointestinal symptoms attributed to alendronate, while 1 male patient was incidentally diagnosed with a diaphyseal femoral fracture, considered a serious complication of alendronate, on imaging obtained to monitor resolution of osteomyelitis (manuscript under review).
Future directions
As expected from their primary therapeutic indication, bisphosphonates may preserve or increase BMD in children and adults with SCD. The clinical case and studies summarized above also suggest they alleviate bone pain in children and adolescents with SBD (2B/2C). The small sample sizes, retrospective cohort study design, and multiple uncontrolled confounders preclude our recommending bisphosphonates as a treatment for SBD at this time. The case presentation and reviewed studies highlight that low bone mass (defined as BMD Z-scores ≤ −2 in children, adolescents, or adults <50 years) may contribute to chronic SCD pain. There are currently no bone density–monitoring guidelines in SCD—eg, age at first DXA scan, frequency and interval of imaging studies, adjusting for short stature in children and adolescents with growth stunting or pubertal delay, etc. At this time, we recommend a multidisciplinary team approach with hematology, endocrinology, radiology, and pharmacy to guide DXA screening and bisphosphonate use for low BMD in SCD, especially in pediatric patients. We are currently conducting a prospective feasibility study of alendronate in adults with SCD complicated by osteonecrosis to determine its safety and preliminary efficacy (NCT06016634). Future studies on bisphosphonates, including randomized, placebo-controlled clinical trials, will help us determine treatment indication(s), optimal treatment duration, and clinical effects, such as the resolution of bone pain or the regression of skeletal injury. Additional follow-up can shed light on the safety of long-term bisphosphonate use and whether osteoclast inhibition affects hematopoiesis. We eagerly anticipate results from these studies to better determine whether bisphosphonates can alleviate bone pain and prevent or reverse skeletal complications in people with SCD.
Conflict-of-interest disclosure
Jahnavi Gollamudi: no competing financial interests to declare.
Oyebimpe Adesina: no competing financial interests to declare.
Off-label drug use
Jahnavi Gollamudi: Bisphosphonates that have not been extensively studies in SCD are discussed.
Oyebimpe Adesina: Bisphosphonates that have not been extensively studies in SCD are discussed.