Key Points
Outcomes of patients with MM in Jordan remain inferior to those in Western populations, due to limited access to advanced therapeutics.
Auto-HSCT remains a cornerstone of management of MM in developing countries.
Visual Abstract
Multiple myeloma (MM) is a plasma cell malignancy with significant global disparities in outcomes owing to variable access to effective therapies. This study evaluates the clinical outcomes of patients with newly diagnosed MM (NDMM) undergoing autologous hematopoietic stem cell transplantation (auto-HSCT) at King Hussein Cancer Center (KHCC) in Jordan. We included all adult patients with NDMM who underwent auto-HSCT after first-line induction at KHCC between 2009 and 2023. Data on frontline regimens, before and after HSCT responses, and survival outcomes were collected. Responses were assessed using the International Myeloma Working Group criteria. Kaplan-Meier estimates were used for overall survival (OS) and progression-free survival (PFS), with log-rank tests for comparisons. A total of 221 patients were identified. The median age at diagnosis was 54 years (range, 32-67). The most common frontline regimen was bortezomib, thalidomide, and dexamethasone (42%), whereas only 2% received lenalidomide. The overall response rate to frontline therapy was 95%, with 2% achieving stringent complete response (sCR), 13% CR, and 50% very good partial response. After auto-HSCT, the sCR and CR rates increased to 13% and 35%, respectively. With a median follow-up of 48 months (range, 6-201), the median PFS was 41.1 months (95% confidence interval [CI], 37.9-47.2), and the median OS was 92 months (95% CI, 72-119). In conclusion, auto-HSCT significantly deepens responses in patients with NDMM, particularly in resource-limited settings. However, limited access to maintenance therapies and novel treatments underscores the need for broader therapeutic options to optimize MM care in such settings.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy characterized by the clonal proliferation of malignant plasma cells in the bone marrow, accounting for ∼1% of all diagnosed cancers and nearly 10% of hematologic malignancies.1-3 Since its introduction in the 1990s, high-dose chemotherapy with autologous hematopoietic stem cell transplantation (auto-HSCT) has significantly improved the outcomes of patients with MM,4-6 with some studies reporting an overall survival (OS) of >10 years.6-9 In transplant-eligible patients with newly diagnosed MM (NDMM), auto-HSCT remains the standard of care recommended by international guidelines.10,11 During the past 2 decades, new therapeutic agents have greatly improved the treatment landscape in MM,12,13 treatments such as proteasome inhibitors, immunomodulatory agents, and anti-CD38 monoclonal antibody therapy have resulted in progressively improved outcomes. Despite these advances, global disparities in access to novel therapies continue to drive outcome disparities.14 In many regions, older treatment options, such as traditionally active agents and auto-HSCT, remain the most viable options for patients with NDMM.15
In Jordan, MM represents ∼1.5% of all newly diagnosed cancers annually and accounts for 13% of hematologic malignancies, with ∼100 cases diagnosed annually.16 Previous reports from surrounding countries have shown a median OS of ∼3 years and a remarkable increase in the number of young patients diagnosed with MM in comparison with the Western population.17,18
Our study aimed to evaluate the outcomes of patients with NDMM undergoing auto-HSCT at King Hussein Cancer Center (KHCC), Jordan’s leading cancer center, and the sole provider of comprehensive hematopoietic transplant services in the country.
Methods
Study design and data collection
We performed a retrospective analysis of all adult patients who underwent auto-HSCT for a diagnosis of MM at KHCC over a 14-year period between 2009 and 2023. The initial data set was obtained from KHCC’s local HSCT registry and supplemented with data from medical record data regarding demographics, disease characteristics, treatment regimens, and clinical outcomes. Patients with other plasma cell dyscrasias or plasma cell leukemia were excluded from the analysis. Analysis was restricted to patients with NDMM who received a single line of treatment before auto-HSCT. This study was reviewed and approved by KHCC’s institutional review board.
Definitions
The diagnostic criteria for MM followed the International Myeloma Working Group guidelines.2 The treatment protocols followed adapted versions of international guidelines based on local resources and medication availability. Risk assessment for patients with NDMM was performed using the International Staging System (ISS),19 and the revised ISS (R-ISS)20 for patients with available cytogenetic data. Response and progression were assessed according to the 2016 International Myeloma Working Group guidelines criteria to ensure a standardized and consistent assessment.21 Cytogenetic abnormalities were identified using fluorescence in situ hybridization (FISH) analysis. Between 100 and 200 nuclei from each sample were scored. Our current FISH panel included the following probes: 13q14, (17p), t (4;14), and t(14;16), the latter 2 were tested as a reflex if an immunoglobulin H (IgH) rearrangement was detected. Results were considered positive if ≥4% of nuclei showed the abnormality for del(13q14), ≥7.5% for del(17p), and ≥2% for IgH rearrangement. Of note, these probes were not universally available throughout the study period and were introduced at different periods. OS was defined as the time from MM diagnosis to death or last follow-up visit, and progression-free survival (PFS) was defined as the time from MM diagnosis to MM progression or death.
Statistical analysis
Continuous variables were summarized using the sample median and range. Categorical variables were summarized as numbers and percentages. Survival outcomes were estimated using the Kaplan-Meier method. Associations with outcomes were evaluated using univariable and multivariable Cox proportional hazards regression models, in which the cause-specific hazard of the given outcome was modeled. The hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Multivariable models were adjusted for all variables associated with the given outcome with a P value <.05 in the single-variable analysis. Statistical significance was set at P values <.05, with all statistical tests being 2-sided. Statistical analyses were performed using SPSS (version 28; SPSS Inc, Chicago, IL).
Results
Patient characteristics
A total of 221 patients were identified during the study period. Table 1 summarizes patient characteristics. The median age at diagnosis was 54 years (interquartile range [IQR], 50-59). The cohort was predominantly male (62%). At presentation, 176 (80%) patients had bone lesions and 73 (33%) had bone fractures. Regarding laboratory findings, 66 (30%) patients had anemia (hemoglobin of <10 gm/dL), 11 (4.9%) patients had hypercalcemia (>11 mg/dL), and 20 (9%) patients had elevated creatinine (>2 g/dL).
At diagnosis, low albumin (<3.5 g/dL) was noted in 51 (23%) patients, elevated lactate dehydrogenase (>222 U/L) was noted in 36 (16.2%) patients, and elevated β-2-microglobulin (>5.5 μg/mL) in 47 (21%) patients. The most common myeloma subtype was IgGκ (n = 91 [41%]). Baseline FISH results were available for 100 (45.2%) patients, with 12 (5.4%) patients having high-risk abnormalities; 8 patients with del 17p and 4 patients with t(4;14). The most common ISS stage was ISS stage I in 78 (35%) patients. Frontline regimens included VTD (bortezomib, thalidomide, and dexamethasone) in 93 (42%) patients; VCD (bortezomib, cyclophosphamide, and dexamethasone) in 35 (16%) patients; thalidomide and dexamethasone in 29 (13%) patients; and cyclophosphamide, thalidomide, and dexamethasone in 16 (7%) patients, with 43 (19%) patients receiving other combination-based regimens, consisting mostly of corticosteroids and chemotherapeutic agents. Only 5 patients in our cohort received induction with bortezomib, lenalidomide, and dexamethasone, and no patient received anti-CD38–based therapy in the frontline setting. Overall response rate (ORR) to frontline therapy was 95%, with 5 (2%) patients achieving stringent complete response (sCR), 28 (13%) patients with CR, 111 (50%) with very good partial response (VGPR), and 65 (30%) patients with a PR.
HSCT characteristics
The median age at auto-HSCT was 55 years (IQR, 50-59), with a median time from diagnosis to auto-HSCT of 10 months (IQR, 7-14), and 72 (33%) patients proceeding to auto-HSCT >12 months after diagnosis. A total of 208 patients (94%) received melphalan conditioning at a dose of 200 mg/m2, with a median cell dose of 4.83 × 106 CD34+ cells per kg (IQR, 4.02 × 106 to 5.43 × 106 CD34+ cells per kg). The median time to neutrophil engraftment was 11 days (IQR, 11-12) and to platelet engraftment was 16 days (IQR, 16-17).
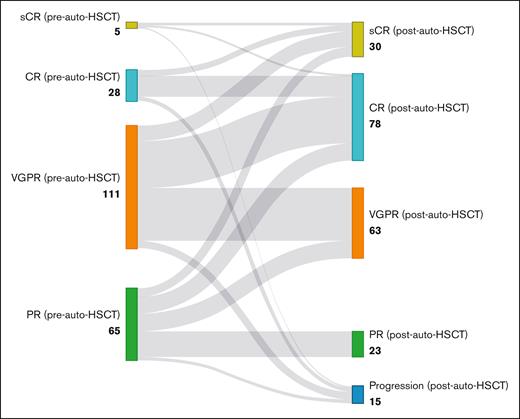
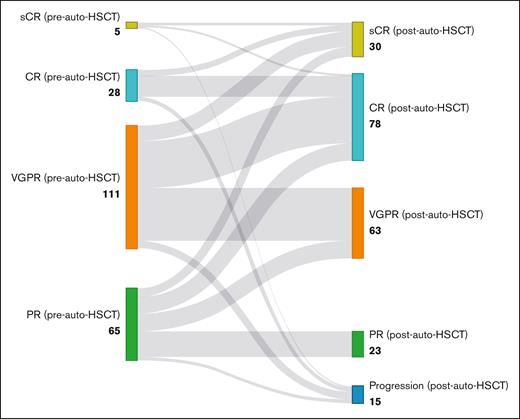
At the 3-month assessment after the auto-HSCT, 33 (14.8%) patients were in sCR and 82 (36.9%) patients were in CR (Figure 1).
Treatment responses before and after auto-HSCT in patients with MM. This Sankey diagram visualizes the transition of patients with MM from their pretransplant response categories to their posttransplant outcomes, demonstrating the deepening of treatment responses after auto-HSCT. PD, progressive disease.
Treatment responses before and after auto-HSCT in patients with MM. This Sankey diagram visualizes the transition of patients with MM from their pretransplant response categories to their posttransplant outcomes, demonstrating the deepening of treatment responses after auto-HSCT. PD, progressive disease.
Of those with sCR before auto-HSCT (n = 5), 2 patients (33.3%) remained in sCR and 2 patients (33.3%) were in CR (bone marrow biopsy was not performed, but no other indications for progression were noted). Of those with CR before auto-HSCT (n = 28), 6 achieved sCR (21.4%), 18 (64.3%) patients remained in CR, and 4 (14.3%) patients had disease progression. Of those with VGPR before auto-HSCT (n = 112), 14 (12.5%) patients achieved sCR, 43 (38.4%) patients achieved CR, 47 (42%) patients remained in VGPR, and 8 (7%) had disease progression. Of those with PR before auto-HSCT (n = 65), 8 (12.3%) patients achieved sCR, 15 (23.1%) patients achieved CR, 16 (24.6%) patients achieved VGPR, 23 (35.4 %) patients remained in PR, and 3 (4.6%) patients had disease progression (Figure 1).The CR rate significantly increased from 15.2% before transplant to 53.1% after transplant (odds ratio, 0.048; 95% CI, 0.018-0.13; P < .001). When considering the combined response of CR or VGPR, the response rate increased from 67% before transplant to 85.2% after transplant (odds ratio, 0.137; 95% CI, 0.058-0.321; P < .001). Twenty (9%) patients received maintenance therapy, with 15 and 5 patients receiving lenalidomide and thalidomide maintenance, respectively.
Outcomes
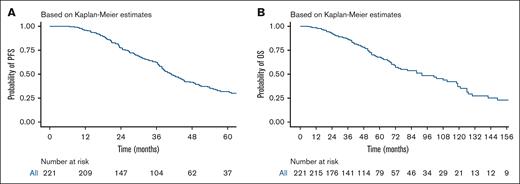
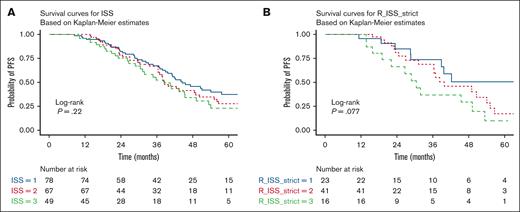
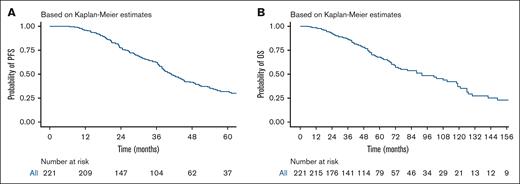
With a median follow-up of 48 months (range, 6-201), the median PFS was 41.1 months (95% CI, 37.9-47.2), with a 5-year PFS of 31.8 % (Figure 2A). Median OS was 92 months (95% CI, 72-119) with a 5-year OS rate of 67.8% (Figure 2B). Nonrelapse mortality at day 100 after auto-HSCT was 1.8%. When stratified by ISS stage, patients with ISS stage I , stage II, and stage II, had a PFS of 44.4, 38.3, and 38.4 months, respectively, and no statistically significant difference was found between the 3 groups (P = .22; Figure 3A). When stratifying by R-ISS, the median PFS for patients with R-ISS stage I, R-ISS stage II, and R-ISS stage III was 78.9, 39.8, and 30.5 months, respectively, and no statistically significant difference was found between the 3 groups (P = .077; Figure 3B). There was no statistically significant difference between patients with R-ISS stage I vs II (HR, 1.52; 95% CI, 0.69-3.37; P = .3), however, patients with R-ISS stage III had a worse PFS (HR, 2.63; 95% CI, 1.10-6.30; P = .03) in comparison with patients with R-ISS stage I. Patients who had responses less than VGPR before auto-HSCT had worse PFS compared with those who achieved VGPR or better before auto-HSCT (HR, 1.7; 95% CI, 1.2-2.41; P =.003). There was no statistically significant difference in outcomes between patients who achieved CR both before and after auto-HSCT and those who achieved CR after auto-HSCT only (HR, 0.8; 95% CI, 0.39-1.65; P = .551).
PFS and OS for the entire cohort. (A) PFS, with a median follow-up of 48 months (range, 6-201). The median PFS was 41.1 months (95% CI, 37.9-47.2). (B) OS; the median OS was 92 months (95% CI, 72-119). The number at risk is shown below each plot.
PFS and OS for the entire cohort. (A) PFS, with a median follow-up of 48 months (range, 6-201). The median PFS was 41.1 months (95% CI, 37.9-47.2). (B) OS; the median OS was 92 months (95% CI, 72-119). The number at risk is shown below each plot.
PFS rates stratified by ISS or R-ISS stage. (A) PFS stratified by ISS stage. Patients with ISS stage I had a median PFS of 44.4 months (95% CI, 39.6-68.5), ISS stage II had a median PFS of 38.3 months (95% CI, 36.4-55.1), and ISS stage III had a median PFS of 38.4 months (95% CI, 31.2-53.3). No statistically significant difference was observed between the 3 groups (log-rank P = .22). (B) PFS stratified by R-ISS stage. The median PFS was 78.9 months for R-ISS stage I, 39.8 months for R-ISS stage II, and 30.5 months for R-ISS stage III. No statistically significant difference was observed between the 3 groups (log-rank P = .077). The number at risk is shown below each plot.
PFS rates stratified by ISS or R-ISS stage. (A) PFS stratified by ISS stage. Patients with ISS stage I had a median PFS of 44.4 months (95% CI, 39.6-68.5), ISS stage II had a median PFS of 38.3 months (95% CI, 36.4-55.1), and ISS stage III had a median PFS of 38.4 months (95% CI, 31.2-53.3). No statistically significant difference was observed between the 3 groups (log-rank P = .22). (B) PFS stratified by R-ISS stage. The median PFS was 78.9 months for R-ISS stage I, 39.8 months for R-ISS stage II, and 30.5 months for R-ISS stage III. No statistically significant difference was observed between the 3 groups (log-rank P = .077). The number at risk is shown below each plot.
A total of 43 (20%) patients in our cohort underwent a second auto-HSCT, with a median age at the second auto-HSCT of 56 years (IQR, 49-61), of whom, 26 patients had available cytogenetics, with 8 (18.6%) patients having had high-risk cytogenetics. Of 43 patients, 22 (51%) had a planned tandem transplant, which was primarily based on poor response to induction and/or the presence of high-risk cytogenetic features. The remaining 21 patients received a second transplant at the time of relapse.
Salvage regimens before the second transplant included lenalidomide-dexamethasone in 5 patients (23.8%), VCD in 7 patients (33.3%), and VTD in 9 patients (42.9%). At the 3-month posttransplant assessment, 1 patient (4.8%) achieved a sCR, 6 patients (28.6%) achieved a CR, 3 patients (14.3%) achieved a VGPR, 2 patients (9.5%) had a PR, and 1 patient (4.8%) had progressive disease. Thirteen patients (61.9%) experienced a subsequent relapse after the second transplant, with a median time to relapse of 17 months (IQR, 12-21). For the second auto-HSCT, melphalan, given at a dose of 200 mg/m2, was used in 40 (93%) patients. The median cell dose was 5.1 × 106 CD34+ cells per kg (IQR, 4.36 × 106 to 5.61 × 106 CD34+ cells per kg) with a median time to neutrophil engraftment of 11 days (IQR, 10-11) and time to platelet engraftment of 16 days (IQR, 11-13). The ORR to the second auto-HCT was 86%, with 4 patients achieving sCR, 14 (42.6%) patients achieving CR, and 11 patients achieving VGPR at the 3-month assessment.
Univariate and multivariate analysis
When evaluating predictors of PFS, IgAλ MM subtype (HR, 2.06; 95% CI, 1.10-3.85; P = .025), R-ISS stage (HR, 2.63; 95% CI, 1.10-6.30; P = .03), and pre–auto-HSCT response of less than VGPR (HR, 1.7; 95% CI, 1.2-2.41; P = .003) and post-HSCT response of VGPR or less (HR, 2.79; 95% CI, 1.81-4.31; P < .001) was associated with worse PFS (Table 2). On multivariate analysis, only the pre–auto-HSCT response (HR, 2.30; 95% CI, 1.15-4.63; P = .019), and R-ISS stage (HR, 2.54; 95% CI, 1.23-5.23; P = .01) retained their significance (Table 2).
When evaluating predictors of OS, IgAλ subtype (HR, 2.68; 95% CI, 1.15-6.21; P = .02) and IgGλ subtype (HR, 1.79; 95% CI, 1.01-3.16; P = .04) were associated with worse OS; however, only IgGλ subtype was associated with worse OS on multivariate analysis (HR, 3.26; 95% CI, 1.03-10.3; P = .04; Table 3).
Discussion
In our study, we present a comprehensive analysis of outcomes of patients with MM who underwent auto-HSCT over a 14-year period at the KHCC in Jordan, a country in the Middle East in which access to novel therapies and advanced testing remains limited.
The median age at diagnosis in our cohort was 53 years, consistent with regional data suggesting a relatively younger age of diagnosis of MM.22,23 This could be confounded by referral and diagnostic biases against older patients in Jordan, along with the study’s limitation to auto-HSCT recipients. It also reflects the Jordanian population pyramid in which only 3.5% of the population are aged >65.24
The high incidence of skeletal involvement and the substantial proportion of patients presenting with low albumin, elevated lactate dehydrogenase, and β-2 microglobulin levels highlight a higher disease burden at diagnosis than previously reported real-world data.15,25 This may reflect delayed referral of patients, contributing to the late-stage presentation, common in our setting in other diseases.26 Genetic data were only available for 43% of our patients because FISH was incorporated into the backbone of MM risk stratification at the KHCC in 2009; the initial focus was limited to del(17p). More comprehensive testing, including IGH gene rearrangements [eg, t(4;14), t(14;16), t(11;14)], was only incorporated later, and several key markers [eg, t(6;14), t(14;20), del 1p, and gain/amplification 1q] remain not available.
Frontline regimens predominantly included combinations of bortezomib, thalidomide, and dexamethasone, with lenalidomide only used in 5 patients in the frontline setting, reflecting the limited access to newer therapies and at least partially explaining the inferior outcomes in our cohort compared with cohorts in which modern frontline regimens that include lenalidomide,27 carflizomib,28,29 and quadruplet regimens incorporating anti-CD38 antibodies such as daratumumab,30,31 are used. Despite this, patients in our cohort achieved a high ORR, which improved further after auto-HSCT, with more patients achieving deeper responses. Although data from the region are limited, regional reports demonstrate wide variability in OS, ranging from 5.1 to 202 months, with the highest OS reported in Saudi Arabia.22 This disparity is attributed to better access to novel therapies and advanced treatments for relapsed diseases in Saudi Arabia than surrounding countries.15,22,23 The median PFS of 42.5 months and OS of 92 months in our cohort was comparable to that in Egypt,32 while acknowledging the differences between the 2 cohorts, and was slightly lower than that in other countries in the region such as Qatar,33 Saudi Arabia, and Lebanon,23 but remain inferior to that reported in Western cohorts.27,34
Pre–auto-HSCT response and R-ISS stage were significant factors associated with worse PFS. These findings align with other studies that emphasize the prognostic importance of pre–auto-HSCT response and R-ISS stage in MM.
Maintenance therapy was notably underused in our cohort, with only 9% of patients receiving it after auto-HSCT. This underuse reflects a significant gap in care compared with international standards, in which maintenance therapy is considered a cornerstone of modern MM management. When used, maintenance therapy has been associated with improved outcomes, including prolonged PFS, underscoring its potential to enhance patient prognosis in the posttransplant setting.35
Our study has several limitations that should be considered when interpreting the findings. First, the retrospective nature of the analysis may have introduced biases related to data collection and patient selection. Second, the absence of certain cytogenetic markers due to limited testing capabilities has affected the accuracy of risk stratification for some patients and, therefore, our ability to accurately assess the impact of auto-HSCT on different risk categories; however, this lack of comprehensive testing is 1 of the issues this work is attempting to highlight. Similarly, other high-risk features such as extramedullary disease are not reported consistently throughout the study period due to nonuniform and inconsistent use of imaging techniques (ie, initially with skeletal surveys, and later on with magnetic resonance imaging and positron emission tomography). Additionally, the change in practice patterns as a result of variability in availability of medications over the study period have introduced some inconsistencies into the data. Nonetheless, this remains a comprehensive analysis of patients with MM receiving auto-HSCT in the frontline setting in a resource limited country with valuable insights into challenges and practice patterns.
Conclusion
Although the role of auto-HSCT in MM management is evolving globally, it remains a critical and irreplaceable treatment option in countries with limited access to novel therapies. In these settings, auto-HSCT provides substantial clinical benefits and serves as an essential solution with no currently available alternative. Our work highlights the growing disparities in MM management worldwide and emphasizes the urgent need for equitable access to advanced therapies to ensure optimal care for all patients.
Authorship
Contribution: A.Y., M.M., Z.H.A.R., S.A.H., and S.Y. designed the study, performed the research, analyzed the data, and wrote the manuscript; and A.A., A.Z., M.R., M.A., J.A., T.M., R.A., W.D., S.S., Y.T., M.A.-H., H.A.J., K.A.-R., and A.A.-I. performed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: S.A.H. reports having received consulting fees from Jansen, Sanofi, Pfizer, and Galapagos; and reports research funding from International Myeloma Society, Jansen, and Alexion. The remaining authors declare no competing financial interests.
Correspondence: Zaid H. Abdel Rahman, Department of Internal Medicine, King Hussein Cancer Center, Queen Rania St 202, Amman 11941, Jordan; email: za.11040@khcc.jo.
References
Author notes
S.A.H., S.Y., and Z.H.A.R. contributed equally to this study.
Presented, in part, in abstract format at the 66th annual meeting and exposition of the American Society of Hematology, San Diego, CA, 7 to 10 December 2024.
Data are available on request from the corresponding author, Zaid H. Abdel Rahman (za.11040@khcc.jo); access to data will be granted in line with institutional policies and with approval from the King Hussein Cancer Center Institutional Review Board.