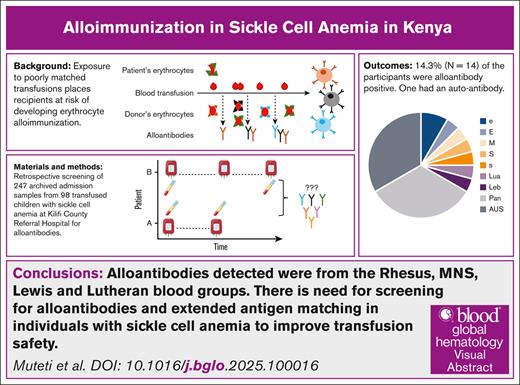
Key Points
Erythrocyte alloantibodies from Rhesus, MNS, Lewis, and Lutheran blood groups occur in children with SCA who received multiple transfusions.
Screening for alloantibodies and extended antigen matching in individuals with SCA will improve transfusion safety.
Visual Abstract
Sickle cell anemia (SCA) management in sub-Saharan Africa relies on transfusion, whose safety is compromised by a lack of extended blood group matching beyond ABO and Rhesus D antigens, and the absence of routine alloantibody screening. To determine the incidence of erythrocyte alloimmunization in children with SCA who received multiple transfusions in Kilifi, Kenya, we retrospectively studied 98 children with SCA admitted to Kilifi County Referral Hospital from 2003 to 2023. Plasma samples collected over this period through a routine ward surveillance study were screened for alloantibodies. Alloantibodies were detected in 14 of 98 (14.3%) participants, and an autoantibody was detected in 1 participant (1.0%). Anti-e was found in 2 children, whereas anti-E, anti-M, anti-S, anti-s, anti-Lua, and anti-Leb were each found in single individuals. Five children had pan-reactive alloantibodies, and 3 had antibodies of unidentified specificity. Older age was significantly associated with the development of alloantibodies (P = .027). Our alloimmunization rate of 14.3% is higher than previously reported from East Africa (2.9%-8%). Given that most alloantibodies were specific to Rhesus and MNS blood groups, and age was significantly associated with alloimmunization, these findings underscore the importance of routine alloantibody screening in children who received multiple transfusions and suggest the need for extended antigen matching in patients with SCA to improve transfusion safety.
Introduction
The burden of sickle cell anemia (SCA) is highest in sub-Saharan Africa, where >300 000 affected children are born each year.1 Recently, we estimated the prevalence of SCA at 0.8% among children in Kilifi County, among whom mortality before age 5 was significantly higher than in those without SCA.2
Blood transfusion is frequently required in the management of individuals with SCA. More than 50% receive a transfusion during childhood, rising to ∼90% during their lifetime.3 The proportion of patients with SCA in Africa who have received a transfusion has been estimated at 30% to 90%.4
The risk of erythrocyte alloimmunization increases if the donor erythrocyte antigens are not well matched to the recipient. Complications include acute or delayed hemolytic transfusion reactions, and future hemolytic disease of the fetus and newborn.5,6 Due to poor hemovigilance, transfusion reactions may be underdiagnosed and managed as sickle cell crises. However, neither pretransfusion alloantibody screening nor extended matching beyond ABO and Rhesus (Rh) D antigens is routinely undertaken in Africa.7 This is likely due to multiple factors, including lack of reagents, inadequate training, and cost implications of additional laboratory testing.
We have investigated the development of erythrocyte alloantibodies in children with SCA living in Kilifi, Kenya.
Study design
This retrospective study was conducted at Kilifi County Referral Hospital (KCRH), which is the sole referral center for children and adults with SCA within Kilifi County, Kenya. The Kenya Medical Research Institute (KEMRI)-Wellcome Trust Research Programme has maintained a surveillance system at KCRH since 1989,8 through which data and blood samples are collected routinely from hospital-admitted children and those attending the routine SCA clinic. Although most children with SCA within the county received their medical care, including transfusions, at KCRH, other smaller facilities also exist where transfusions are administered occasionally. In most facilities in Kenya, blood transfusions are only matched for ABO and RhD antigens.
In 2023, we reviewed the KEMRI-Wellcome Trust Research Programme surveillance database for hospitalization at KCRH during the period from May 2003 to June 2023. Children <14 years of age who were confirmed or suspected to have SCA and had received a blood transfusion during their admission to KCRH were identified. In addition to the index admissions, during which transfusions were administered, we also checked the database for subsequent admissions for the same children and whether or not they received further transfusions. We then checked whether the children had plasma and whole blood samples collected and archived before any interventions were administered during the admissions of interest, or during routine SCA clinic visits. The first transfusion event at KCRH was marked as the start of the duration of follow-up, which ended with the last admission event or SCA clinic visit during which a sample was collected and archived. The samples were then retrieved from −80°C storage for erythrocyte alloantibody screening and sickle genotyping.
SCA was confirmed by polymerase chain reaction as described previously.9 Plasma samples were screened for alloantibodies using Bio-Rad reagent erythrocytes (ID-DiaCell I-II-III, catalog no. 004310v) and ID-LISS/Coombs gel cards. Samples showing a positive reaction were further analyzed using the ID-DiaPanel of 11 selected erythrocyte antigen phenotypes (Bio-Rad, catalog no. 004114V) to identify the specific alloantibodies.
Categorical variables were expressed as percentages, whereas continuous variables were expressed as medians with interquartile ranges. We tested for associations between erythrocyte alloantibody development and putative predictor variables, including age over the follow-up period, age at admission, sex, and the number of transfusions received.
The study was approved by the Kenya Medical Research Institute’s Scientific and Ethics Review Unit (protocol number KEMRI/SERU/CGMR-C/243/4380) and the Kenya National Commission for Science, Technology and Innovation (protocol number NACOSTI/P/22/17744). The study was conducted in accordance with the Declaration of Helsinki.
Results and discussion
A total of 2690 hospital admissions were observed among 814 children with SCA. Among these admissions, 688 included transfusion events in 213 children. Based on sample availability, 98 children who received 314 transfusions were included in the study. A total of 247 plasma samples from hospital admissions (n = 100) or SCA clinic visits (n = 147) were retrieved for analysis (Figure 1). The 98 children included 50 males and 48 females with a median age of 5.2 years (interquartile range [IQR], 2.4-8.0), and median age at first admission requiring transfusion of 2.1 years (IQR, 2.1-4.5). The number of transfusions per participant ranged from 1 to 13 (median [IQR], 2 [1-3]). Severe anemia was the primary admission diagnosis among children who received transfusions (n = 258); other causes of admission were classified as malaria (n = 24) and nonmalaria syndromes (n = 37).
Twenty-four of the 247 samples screened, (9.7%) among 14 of 98 children (14.3%), were positive for alloantibodies. One participant had an erythrocyte autoantibody that displayed agglutination to all the screening and identification erythrocytes. Seven specific alloantibodies were identified from Rh, MNS, Lutheran, and Lewis blood groups: anti-e (n = 2), anti-E, anti-M, anti-S, anti-s, anti-Lua, and anti-Leb, whereas nonspecific antibodies included 8 pan-reactive alloantibodies and 8 antibodies of unidentified specificity (AUSs).
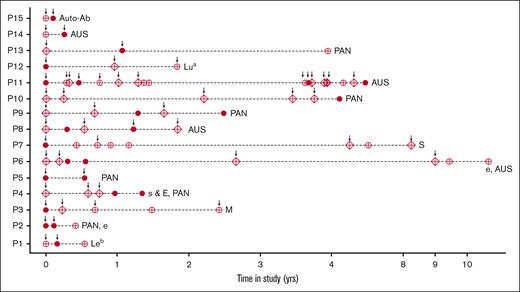
Figure 2 illustrates a time series analysis highlighting the transfusion events and the samples screened among antibody-positive children. The autoantibody (P15) was detected 1 month after the initial transfusion, during a readmission when the individual was sampled before receiving a second transfusion. Participants P10 and P13 had pan-reactive alloantibodies after their fifth and first transfusion events, respectively. Participant P14 was negative on the first screen, but a subsequent sample collected before their second transfusion (4 months later) was alloantibody positive (AUS). Most individuals with multiple alloantibody-positive samples had either pan-reactive antibodies or AUSs. Participant P2 had an anti-e alloantibody identified in the second positive sample, whereas P4 had a combination of 2 alloantibodies (anti-s and anti-E) identified in their first positive sample. Participant P6 was found to be anti-e alloantibody positive 1.5 months after a second transfusion, and was AUS positive 4 months later. Participant P11 had the highest number of transfusion events (n = 13) administered over 4 years, and had 4 AUS-positive samples. The first positive sample was identified at the first admission for transfusion at KCRH at the age of 8 years; the other positive samples were identified at 5 months, 3.8 years, and 4.5 years later. The proportion of alloantibody-positive children increased significantly after the age of 5 years (14.3%; P = .02) compared with those in the younger ages (5.9%). No significant associations were found between alloantibody positivity and age at first transfusion, gender, time since last transfusion, or the number of previous transfusions (supplemental Table 1).
Time series of Ab-positive participants showing transfusion events and screened samples. The dark circles are the positive samples, open circles are the negative samples, the diamonds are missed samples, and the arrows mark the transfusion events. The erythrocyte alloantibodies identified are listed at the end of each participant’s time series. Ab, antibody.
Time series of Ab-positive participants showing transfusion events and screened samples. The dark circles are the positive samples, open circles are the negative samples, the diamonds are missed samples, and the arrows mark the transfusion events. The erythrocyte alloantibodies identified are listed at the end of each participant’s time series. Ab, antibody.
In this retrospective analysis of children with SCA in Kilifi, Kenya, we observed an overall rate of erythrocyte alloimmunization of 14.3%, predominantly attributable to antibodies against the Rh, MNS, Lutheran, and Lewis blood groups. Older age was significantly associated with antibody development. Alloimmunization rates of 4% to 26% have previously been reported among children with SCA in sub-Saharan Africa, depending on the location.7 The rate seen in our study was higher than that recorded in previous studies conducted in East Africa (2.9%-6.5%).7 This difference is likely attributable to our prospective research approach, testing participants at multiple time points after transfusion, which increased the chances of detecting alloantibodies.
An important limitation of our study was the use of commercial erythrocytes, which are typically derived from White donors; therefore, we may have missed other population-specific antigens. Notably, a study in Ghana established that the commercial test made using erythrocytes from White donors failed to detect alloantibodies developed to antigens more common in African population such as V, VS, and Jsa,10 highlighting the need for locally relevant erythrocyte screening panels.
The identification of specific alloantibodies on subsequent screening of patients with AUSs by Liu and Grossman11 reflects the varied nature of AUSs, which could represent weak reactions against antigens that are not highly expressed or are in the process of developing. Another challenge regarding AUSs involves the temporal evanescence of some alloantibodies, leading to a limited detection window.12,13 We observed this in participant P6, who had anti-e antibodies and AUS detected at 1.5 and 4 months, respectively, after the second transfusion.
Our study was also limited by the availability of archived plasma samples owing to low blood draws in patients with anemia. The retrospective identification of participants was also done without knowledge of transfusions that may have been administered in other health facilities. The details of the transfusions (whole blood or settled cells, blood volume, and phenotype of donor erythrocyte antigens) administered at KCRH were also unavailable. Finally, the prevalence of alloimmunization found in our study could be an underestimate of the true prevalence, given that only 42% of the tested samples were collected within 3 months after a transfusion. Future prospective studies should consider phenotyping of clinically significant erythrocyte antigens to detect antigen mismatch, and routine screening within 2 to 6 months after transfusion to ensure accurate detection of alloantibodies before evanescence.
To enhance transfusion safety and reduce the risk of alloimmunization in sub-Saharan Africa, there is a need to adopt alloantibody screening in individuals who rely on frequent blood transfusions. The assumption that receiving blood from local African populations that are genetically similar reduces the likelihood of mismatches is debatable. Heterogeneity in terms of partial or no expression of Rh antigens, high and low prevalence of various antigens, and diversity of the RhD and RHCE alleles has been reported in Moroccan, Malian, and Cameroonian populations, respectively.14-16 Understanding the phenotypic and genotypic diversity of clinically significant erythrocyte antigens in African populations will guide the management of individuals with rare antigen phenotypes and inform the development of erythrocyte panels containing antigens of African origin.
Acknowledgments
M.M. is supported by a master’s fellowship from the Katholischer Akademischer Ausländer-Dienst. S.U. is supported by the Wellcome Trust (221998/Z/20/Z) and a Global Research Award 2022 from the American Society of Hematology. T.N.W. is supported by a Senior Fellowship from the Wellcome Trust (202800/Z/16/Z). This article is published with the approval of the director general of the Kenya Medical Research Institute.
Authorship
Contribution: M.M., S.U., L.A.B., G.M., T.N.W., R.E.W., and S.N. designed the study; M.M., J.M., B.T., P.O., C.N., and E.A. conducted the laboratory assays; M.M. and S.U. performed the data analysis; M.M. drafted the manuscript; and all authors revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sophie Uyoga, Epidemiology and Demography Department, Kenya Medical Research Institute-Wellcome Trust Research Programme, PO Box 230, Kilifi 80108, Kenya; email: suyoga@kemri-wellcome.org.
References
Author notes
Data are available on request from the corresponding author, Sophie Uyoga (suyoga@kemri-wellcome.org).
The full-text version of this article contains a data supplement.