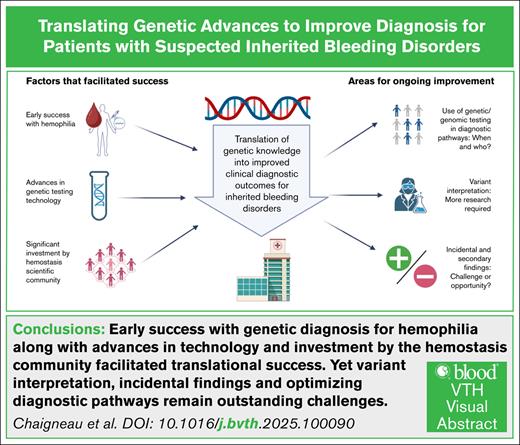
Visual Abstract
Inherited bleeding disorders are a heterogenous group of conditions characterized by the presence of abnormal bleeding. Currently, the diagnostic odyssey for patients with a suspected bleeding disorder is lengthy, costly, resource intensive, emotionally draining, and often futile, because up to half of patients will remain without a clear diagnosis. Genetic testing has been suggested as a possible remedy for these diagnostic challenges and is increasingly incorporated into clinical care. In this review, we outline 3 factors that contributed to the translation of genetic advances into improved diagnostic outcomes. These include the early success experienced with hemophilia, advances in genetic sequencing technology, and significant investment by the hemostasis scientific community. We also identify 3 areas for improvement to facilitate ongoing translation, highlighting the need to optimize integration of genetic and genomic testing into diagnostic pathways and improve variant classification through ongoing research and curation efforts, and reconsider whether incidental and secondary findings represent a disadvantage or an opportunity.
Introduction
Inherited bleeding disorders are conditions characterized by defects along the hemostatic pathway that result in abnormal bleeding symptoms, ranging from mild nuisance bleeding to life-threatening hemorrhage. This heterogenous group of diseases can be roughly categorized into defects of primary hemostasis (ie, von Willebrand disease [VWD] and platelet function disorders), secondary hemostasis (ie, factor deficiencies), and abnormalities of connective tissues/collagen (ie, vascular Ehlers-Danlos syndrome).1 Type 1 VWD is the most common of the inherited bleeding disorders, with a prevalence of 1 per 1000 in the general population, whereas the other conditions are in the rare-disease category, with a prevalence of <50 per 100 000.2-4 The challenges of diagnosing these rare disorders include issues of symptom dismissal as well as difficulties inherent to current specialized coagulation tests.5,6
The current diagnostic pathway begins with a physical examination and both a comprehensive bleeding and family history.1 Bleeding histories are taken with use of a standardized bleeding assessment tool (BAT), which results in a numeric bleeding score, classifiable as normal or abnormal with score magnitude reflecting bleeding severity.1 The most commonly used expert-administered BAT is the International Society on Thrombosis and Haemostasis BAT (ISTH-BAT), which has been modified for self-administration with the Self-BAT.7-9 These BATs have been validated as screening tools for inherited bleeding disorders in both adult and pediatric populations across many conditions, and an abnormal bleeding score is an indication to proceed with coagulation testing.7,10
First-line coagulation testing effectively diagnoses ∼30% of new referrals, skewed toward identification of VWD and hemophilia A and B (factor VIII [FVIII] and FIX deficiency, respectively).11 For the remaining 70% of patients, subsequent rounds of testing aim to identify platelet function disorders, other rare factor deficiencies, and fibrinolytic disorders, however tests are nonspecific, have low sensitivity, and low overall yield.1 All coagulation testing must be done in specialized coagulation laboratories, only found in large urban areas and not easily accessible by much of the population.3 Moreover, coagulation tests are highly affected by preanalytical variables (eg, transport time, maintenance of the cold chain, physiologic stress, and hormones), necessitating repetitive testing for validation of results.12,13 Some patients are unable to proceed to further testing because of factors such as medication use or pregnancy, both of which can interfere with diagnostic accuracy and validity.13 For example, patients on medication for anxiety or depression are unable to complete the full workup because most selective serotonin-reuptake inhibitors interfere with platelet function testing.
Ultimately, after a diagnostic journey that is long, costly, resource intensive, and emotionally draining for patients, 50% of referrals end up labeled as “bleeding disorder of unknown cause” (BDUC).13 BDUC is a broad diagnostic category defined as a patient with a significant bleeding phenotype and normal hemostatic investigations.14 Managing bleeding complications in patients with BDUC is challenging because the etiology is unknown and these patients experience major bleeding symptoms such as postpartum hemorrhage despite attempts at nonspecific hemostatic prophylaxis (eg, tranexamic acid).15
From the patient’s perspective, the burden of these diagnostic challenges is significant because it includes multiple hospital visits, repeated blood draws, travel costs, worry, and uncertainty.5 The negative consequences of uncontrolled bleeding symptoms include reduced health-related quality of life, work/school absenteeism, and social isolation.16-19 The current reported time from symptom onset to diagnosis of an inherited bleeding disorder ranges from 7 to 12 years for the 30% of patients that achieve a first-line diagnosis and even longer for patients that need second- and third-line testing.5 This of course excludes the 50% of patients who end up labeled with BDUC and will live indefinitely with this diagnostic uncertainty.
A key characteristic of the inherited bleeding disorders lies in the name, they are all inherited conditions and, as such, genetic testing has been proposed as an area with the potential to improve these diagnostic challenges.6,20 Genomic analysis for the diagnosis of inherited bleeding disorders has been increasingly incorporated into clinical diagnostic pathways, increasing diagnostic yield with positive effects for patients.21 In this narrative review, we identify 3 key factors that contributed to the successful translation of genetic knowledge into clinical practice, and then identify 3 key areas for improvement to facilitate ongoing translation.
From the gene to the clinic: 3 factors that facilitated knowledge translation
Early success with hemophilia
Understanding the molecular basis of a disease can be a catalyst for improvements in both diagnosis and treatment. This was case for hemophilia, which has seen incredible advances in disease outcomes since the discovery and sequencing of the responsible genes more than 40 years ago.22 Hemophilia A (FVIII deficiency) and hemophilia B (FIX deficiency) are monogenic disorders that arise from pathogenic variants in either the F8 gene or F9 gene. The genes for these clotting factor proteins were some of the earliest to be characterized with the cloning of the F9 gene in 1983 and the F8 gene in 1984.23-26 Genetic testing to identify hemophilia carriers quickly followed in 1984, and prenatal genetic testing for male fetuses shortly afterward in 1985.27-29 Over the years the molecular basis of both types of hemophilia have become very well characterized in several thousand patients because of the high uptake of genetic testing in well-resourced settings. There are now >4000 unique variants recorded in the leading Coagulation Factor Variant Database maintained by the European Association for Haemophilia and Allied Disorders.30
Genetic characterization paved the way for the development of new recombinant treatment therapies, a much safer alternative to plasma-derived therapies, which saw infection of patients with hemophilia with HIV and hepatitis C.31 It also showed hemophilia to be one of the few diseases with a strong correlation between genotype and phenotype, meaning genetic testing can not only provide a diagnosis of hemophilia but can also indicate disease severity and predict plasma factor level.32 Genetic testing is also the only means of accurately identifying all female carriers of hemophilia. Historically, genetic testing for potential female hemophilia carriers was focused on the identification of affected male offspring and offered only for reproductive planning purposes.33 Knowledge of carrier status was not seen as relevant to the health of the female herself, although there was evidence from as early as the 1950s suggesting that carriers may experience abnormal bleeding symptoms.34 Because of focused research efforts in the last few decades, it has now been clearly shown that all females affected by hemophilia are at increased risk of bleeding, even those with factor levels in the normal range.35-37 This has raised important questions regarding the timing of genetic testing, with a recent consensus publication now recommending genetic testing to all potentially affected females when they are competent to consent and understand results, with testing in early childhood when clinically relevant.38
Thus, the clinical utility of genetic testing is well evidenced in hemophilia. It provides essential clinical information related to disease severity and is the only way to accurately identify female carriers who are universally at higher risk of bleeding symptoms compared with the normal population.32,36 Extensive genetic characterization facilitated the development of important novel treatments such as the recombinant factor products and the highly anticipated new gene therapies.32 The success experienced with hemophilia laid important groundwork for the investigation of additional hemostatic genes. Although many researchers were eager to apply similar techniques to the other inherited bleeding disorders, comparable advances were initially hindered by the limitations of early sequencing techniques.22
Advances in genetic sequencing technology
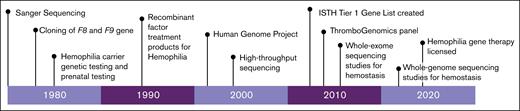
The first method for the detection of genetic variants was by Sanger sequencing, a method that synthesized the nucleic acid sequence 1 nucleotide at a time.39 The development of Sanger sequencing in 1977 was a major scientific achievement that paved the way for many advances in genetics (Figure 1).40 The technique is still used today in limited circumstances, yet there are major technical challenges in the amount and extent of DNA that can be sequenced, which prevented characterization of many of the hemostatic genes.39 When it comes to large-scale genomic sequencing, the Sanger method becomes costly, inefficient, and work intensive. Thus, the advent of high-throughput sequencing (HTS), made possible by the Human Genome Project in the early 2000s, was a major technological development that allowed for the sequencing of a large number of genes in parallel, greatly reducing workload, turnaround time, and associated costs.22,41
Using HTS technology, the first targeted sequencing panel specific to hemostasis was created in 2016, the ThromboGenomics (TG) platform.42 This panel is specific to rare bleeding, thrombotic, and platelet disorders with the initial test evaluating 63 genes and the latest version expanded to cover 96 genes.42,43 The intended goal for the TG platform was to provide a sensitive genetic test to obtain a molecular diagnosis in patients with bleeding and thrombosis.44 Real-world studies found overall diagnostic yield of ∼50%, with slightly higher yields of 63.6% seen in the coagulation-only group (excludes thrombosis).43 The main advantage of HTS panels such as the TG platform is that they are a convenient and accessible way of performing genetic analysis, yet the main criticism is that gene lists are often too restrictive, miss unanticipated findings, and become rapidly outdated.39 HTS panels provide data for a limited portion of the gene, leaving variants outside of these regions undetectable, affecting a significant number of patients who never get a conclusive molecular diagnosis with these more limited methods.45,46
Recently, broader genomic testing strategies have been increasingly suggested with the main advantages and disadvantages of genetic vs genomic analysis techniques outlined in Table 1. Whole-exome sequencing (WES) allows for the identification of 85% of disease-causing variants, whereas whole-genome sequencing (WGS) covers the entire genome, including noncoding regions that were previously considered nonfunctional but are now recognized as playing an important role in regulation.47 The main advantage of WES is that it has a lower cost while still covering the regions of the genome most likely to be implicated in disease. WES is increasingly considered redundant in light of research showing WGS to have more comprehensive coverage, the ability to detect intronic variants, and thus have higher diagnostic yields, without higher costs.39,47 The major disadvantage of WGS lies in the enormous amount of data produced and the complicated analysis required. A mitigation strategy for this limitation is to run a disease-specific panel on a whole-genome or whole-exome backbone as a first pass.48 This strategy limits the amount of analysis, yet allows data to be easily reanalyzed as new variants are discovered without the need for repeat sequencing.22 HTS approaches are not perfect and come with their own limitations. The detection of copy number variants, especially small ones, can be hindered by poor read depth and reduced sensitivity.49 Thus, additional methodologies such as multiplex ligation-dependent probe amplification may be required to identify copy number variants. In addition, complex rearrangements such as inversions (ie, F8 intron 22 inversion) are not detectable using HTS and require other sequencing approaches.
A key advantage of all types of HTS (panels and WES/WGS) is that they can be performed with low sample volume or nonblood samples and have fewer preanalytical restrictions than current coagulation tests, meaning the samples are easier to transport to centralized locations.21 The ability to mail-in samples is particularly important for patients in nonurban locations or lower-resourced settings.3 For lower-resourced countries, a new diagnostic paradigm has been suggested in which genetic testing techniques are used as up-front diagnostic tools after screening with a BAT; then only patients with confirmed genetic variants would need to travel to a major center for confirmation and further characterization with coagulation testing.6
However, in order to change the diagnostic paradigm for inherited bleeding disorders, not only the sequencing technology but also the expertise to interpret the resulting genetic data is needed. This is a gargantuan task because of the high level of disease complexity, made more complicated by issues of incomplete penetrance and variable expressivity. Yet significant progress in this area has been achieved thanks to investment from the hemostasis scientific community.
Significant investment by the hemostasis scientific community
The ISTH is the leading thrombosis and hemostasis–related professional organization worldwide, and it has a dedicated scientific and standardization committee (SSC) on genomics. A major aim and responsibility of the ISTH Genomics SSC is to maintain and curate evidence-based public databases for confirmed and emerging genes for inherited blood, thrombotic, and platelet disorders.50 Since the implementation of HTS for inherited bleeding disorders, there has been an explosion of genetic information but of varying quality. Thus, in 2014 the ISTH began a project to generate and maintain a tier 1 gene list; an annually updated, well-curated, evidence-based catalog that can be used for diagnostic genetic screening of bleeding, thrombotic, and platelet disorders.50 This is an essential resource for effective knowledge translation, with the presence of an ISTH tier 1 variant in a patient allowing the clinician to make a confident molecular diagnosis. The ISTH Genomics SSC also maintains a tier 2 gene list of more recent gene discoveries that currently lack sufficient evidence to be considered diagnostic, most commonly because they are limited to small pedigrees or lacking confirmation by functional assays/animal models. New genes are added to the lists annually, but they can also be removed if there is insufficient or new, conflicting evidence.51 The commitment of the ISTH in creating, maintaining, and curating the diagnostic-grade tier 1 gene list has greatly facilitated the translation of genetic/genomic testing for inherited bleeding disorders into clinical practice.
Not there yet: 3 areas for improvement to facilitate ongoing translation
When and for whom? Optimizing integration of genetic analysis into diagnostic pathways
Because of, in part, the 3 factors outlined earlier, genetic and/or genomic testing is being used in the diagnostic pathways for inherited bleeding disorders worldwide, yet in very different ways depending on the location. Even in the well-resourced parts of the world, integration of genetic testing into diagnostic pathways is far from uniform. In the Canadian setting, testing is limited to single-gene analysis done at the end of the diagnostic pathway to confirm a laboratory diagnosis or because of relevant family history.52 There is currently no national option for an HTS panel/WES/WGS assessment specific to hemostasis and so, if required, samples must be sent to private corporations in the United States, at the expense of the patient, private-insurance, or through specially secured public funding. Contrastingly, in the United Kingdom, both single-gene analysis and an HTS bleeding and platelet disorder panel (R90 panel) are publicly available through the national health care system and can be ordered by any hematologist as needed.53 The economic impact of incorporating genomic analysis strategies has not been studied for inherited bleeding disorders and is a major gap is optimizing the integration of this technology into the diagnostic pathway.
Similarly, another major optimization challenge is the wide variety of diagnostic yields reported in previous work, ranging from 10% to 94% depending on differences in study, inclusion criteria, the sequencing method used (panel vs WES or WGS backbone), and ways of reporting variants (ie, only pathogenic variants vs both pathogenic and likely pathogenic variants). The varying diagnostic yields for 12 clinical genetic analysis studies are reported in Table 2. In a recent review paper, overall diagnostic yields were summarized as 95% for patients with clearly defined bleeding disorders on laboratory testing, between 50% and 70% for patients with less-well-defined disorders on laboratory testing but well-characterized phenotypically, and between 20% and 50% for those with poorly defined disorders.21 This is consistent with diagnostic yields in other conditions, which also show decreasing yields with decreasing disease severity, and overall yields increasing from 25% to 35% with WES to 40% with WGS as a first-tier test.62-65
The observed variation in diagnostic yield raises questions of who should receive genetic analysis, which type should be offered, and at what point in the diagnostic pathway.22 It is generally accepted that for patients with laboratory confirmed diagnoses of hemophilia A/B or some subtypes of VWD (ie, type 2B or type 3 VWD), molecular analysis should be limited to single-gene analysis done at the end of the diagnostic pathway as the most appropriate and cost-effective method.32,66 A notable exception would be type 1 VWD, which has complex genetic determinants leaving ∼30% of patients with no molecular diagnosis despite extensive genetic characterization.66,67 This highlights the limitations of single-gene analysis in understanding the underlying pathophysiology of diseases such as type 1 VWD, which likely requires a polygenic approach that includes analysis of genes beyond the VWF gene, and even beyond the genes currently known to affect hemostasis.67,68 Although the severe bleeding phenotypes are most commonly caused by a single variant as seen with severe hemophilia A/B, a polyvariant pathophysiology with multiple contributing factors may be present with other mild and moderate inherited bleeding disorders.21,32 A study of patients with previously diagnosed rare bleeding disorders found a quarter of participants had co-occurrence of pathogenic/likely pathogenic variants in another gene(s), however the effect on bleeding phenotype was unknown.61 This emphasizes the utility of more universal genomic testing, to further study the possible polyvariant basis of mild/moderate bleeding phenotypes and improve diagnosis for these patients.21
Precise diagnosis of mild bleeding conditions is currently challenging and molecular analysis can contribute to correct treatment decision-making and optimized control of bleeding symptoms.21 The same is true for the BDUC population who have been shown to experience ongoing bleeding despite attempts at general hemostatic prophylaxis with subsequent negative effects on health-related quality of life.14,15,19 Patients with BDUC have exhausted all the coagulation testing options available to them and thus benefit from genomic analysis, despite the lower diagnostic yields reported for this population.53,58,59 Recent efforts of the ISTH to standardize and limit the BDUC diagnoses may lead to increases in genetic diagnostic yields, as previous studies cite as a limitation the high heterogeneity in how their BDUC population was defined.14 Despite the established value of genetic analysis for both BDUC and milder bleeding phenotype populations, it is most commonly performed as the last step in the diagnostic workflow after patients have already been through many rounds of testing and suffered from abnormal bleeding symptoms for years.5,21
Up-front genomic analysis for patients who do not receive a diagnosis with first-line testing may significantly reduce time to diagnosis and will be evaluated in an upcoming multisite randomized controlled trial.69 Real-world clinical studies such as this are required to produce data that will optimize the integration of genomic analysis into clinical diagnostic pathways, including evaluations of cost-effectiveness. Regardless of how these optimization questions are answered, after genetic sequencing comes data analysis and how to interpret specific genetic variants represents another ongoing challenge.
Variant interpretation: more research required
Almost every report on genetic and genomic testing for inherited bleeding disorders cites difficulty interpreting variants as an ongoing limitation.21 At its best, variant identification provides important clinical information such as predicting mode of inheritance (ie, autosomal dominant forms of Glanzmann thrombasthenia), the severity of bleeding (ie, intron 22 inversion in hemophilia A), and likelihood of other different disease-related complications (ie, increased risk of renal disease and deafness with MYH9 platelet disorders).21 For some inherited bleeding disorders, the genetic background has been extensively studied and most variants have been previously described in multinational databases. In these circumstances, variant interpretation can be quite simple and straightforward (ie, F8 and F9).32 However, this is unfortunately not the case for most inherited bleeding disorders and finding a variant of uncertain significance (VUS) is a common occurrence.
VUS usually have some characteristics of being disease causing, but there is either conflicting or insufficient evidence for classification in either the benign or pathogenic categories.70 All variant interpretation must follow the guidelines of the relevant genetics body, such as the American College of Medical Genetics and Genomics (ACMG).70 ACMG guidelines have clearly defined criteria for determining pathogenicity of variants, yet this does not mean that classification is straightforward.71 Certain criteria are considered stronger evidence of pathogenicity than others; for example, a null variant causing loss of function is considered to be very strong. However variants with “weaker” combinations of supporting criteria may also end up classified as pathogenic variants.72 The effect of a given VUS on bleeding phenotype may be hard to elucidate, which poses complications in the real-world setting because this can be difficult to explain to patients and families and for clinicians to use in developing care plans for patients.73 Newer computer-based in silico pathogenicity programs (eg, Variant Effect Predictor) can be a helpful tool. Yet contradictory interpretations are often observed with these programs because of the complexities of genetic mechanisms of incomplete penetrance, mosaicism, epigenetics, etc.21
Ultimately, what will move a VUS into one category or the other is additional research, segregation of the variant within a family with clear phenotype, and the submission of clinical variants to international variant sharing databases so that the body of evidence for each variant can be interpreted by qualified individuals.74 The ISTH Genomics SSC recently published the ClinGen Gene Curation Framework to facilitate molecular diagnosis by evaluating novel genes at yearly intervals.75 Ongoing efforts by the hemostasis scientific community will be needed to minimize the VUS problem and facilitate continued knowledge translation.
Incidental and secondary findings: a challenge or an opportunity?
Another fairly common occurrence with genetic/genomic analysis is the discovery of incidental findings. Incidental findings are results that are unrelated to the primary indication but are discovered inadvertently during testing (ie, evaluation of platelet genes for primary indication of bleeding symptoms may find variants also associated with myeloid leukemia).71 Incidental findings are more common with broad genomic testing strategies such as WES/WGS but may occur with targeted gene panels as well.21 Many reviews on the use of genetic/genomic testing for inherited bleeding disorders cite incidental findings as a disadvantage, because these findings are not related to the patient’s current phenotype and disclosure also requires arranging appropriate follow-up, which might be out of scope for the treating physician.51,72,76 However, incidental findings also represent an opportunity for patients to learn information that may improve their personal health outcomes and should not necessarily be viewed only in a negative light.
Secondary findings are also unrelated to the primary testing indication, yet they differ from incidental findings because they are intentionally sought after as opposed to inadvertently discovered.77 The current recommendation of the ACMG is that analysis of their list of actionable secondary findings should be offered to patients anytime an individual is receiving clinical WES/WGS, because of the medical actionability of the findings.77 The list is updated annually by the ACMG with careful attention to balancing the benefits to patients and the health care system. The likelihood of incidental or secondary findings must be properly disclosed to patients before any genetic/genomic testing. Pretest counseling should be done by a properly trained health care professional.73 Anytime analysis of secondary findings is being offered, both pretest and posttest counseling should be provided by health care provider who is experienced in setting realistic testing outcome expectations, communicating results, providing necessary follow-up, and organizing cascade testing of family members, as needed.76
With proper counseling and close collaboration between hematology and genetics clinical areas, incidental and secondary findings need not be a disadvantage of genomic testing but instead a way to empower patients who have already experienced a diagnostic odyssey, providing information and, at times, reassurance regarding other common genetic health concerns. Results from studies exploring the impact of these secondary findings on patients and families who underwent WGS shows that they do not experience significant anxiety, distress, or adverse effects.78,79
Conclusions
Significant advances have been made in incorporating genetic testing to improve the diagnostic outcomes for patients with inherited bleeding disorders. The early success seen with hemophilia, advances in genetic sequencing technology, and the significant investment by the hemostasis scientific community collectively laid the foundation for this achievement. However, to maximize clinical utility and patient benefit, more work is needed to determine the optimal timing for integrating genomic testing into the diagnostic pathway, improving variant classification, and reconsidering the benefits and disadvantages of potential incidental and secondary findings. By advancing our knowledge of genomic analysis for inherited bleeding disorders, we have an opportunity to improve the life of patients through improved diagnosis and more accurate treatment.
Authorship
Contribution: M.C. conceived the review topic, conducted the literature search, and drafted the initial manuscript; and M.B., A.G., and P.J. analyzed and interpreted the findings, and critically reviewed the manuscript.
Conflict-of-interest disclosure: P.J. receives research funding from Bayer and consultancy fees from Star/Vega Therapeutics, Band/Guardian Therapeutics, Roche, and BioMarin. The remaining authors declare no competing financial interests.
Correspondence: Megan Chaigneau, Department of Medicine, Queen’s University, 94 Stuart St, Etherington Hall Room 2015, Kingston, ON K7L 3N6, Canada; email: megan.chaigneau@queensu.ca.