TO THE EDITOR:
Diabetes mellitus (DM) is strongly linked to the development and progression of cardiovascular disease (CVD).1 Prior studies demonstrated increased platelet activity among those with DM as a potential contributor to the increased risk of CVD.2,3 Given important limitations in measuring platelet activity including cost, time, and variability in the measurement, our group developed a Platelet Reactivity ExpreSsion Score (PRESS), integrating platelet aggregation responses and the platelet transcriptome. PRESS was able to discriminate individuals with a hyperreactive platelet phenotype and those at heightened risk of CV events.4 The current study was designed to investigate the association between PRESS and CV events in patients with DM.
Platelet activity and CV events in peripheral artery disease (PAD) study was a clinical study (ClinicalTrials.gov identifier: NCT02106429) of individuals with symptomatic PAD undergoing lower extremity revascularization and followed for a median of 18 months. Platelets were isolated and collected prior to lower extremity revascularization. Platelet RNA was sequenced in 135 patients. As described previously,4 this cohort was used to derive PRESS and was subsequently validated in an independent cohort of healthy individuals. Briefly, we identified 451 platelet transcripts differentially expressed in patients with (vs without) platelet hyperreactivity and significantly correlated with percent aggregation to epinephrine (0.4 μM). As noted previously, weighted expression values from these 451 genes were used to generate a PRESS for each participant.4 The study was approved by the New York University Langone Health and VA New York Harbor Healthcare System Institutional Review Boards, as well as the Office of Research Administration at Bellevue Hospital with all participants providing informed consent.
The current study included 129 individuals with PAD from the platelet activity and cardiovascular events study with and without DM. DM severity was stratified by insulin use. PRESS was calculated and individuals were categorized as “high” vs “normal” defined as non-high PRESS as described previously.4 Briefly, the PRESS model assigns each participant a prediction probability for platelet hyperreactivity, which is then z-scored according to the derivation mean and standard deviation. PRESS was compared among individuals with vs without DM via linear regression and across DM severity via analysis of variance, adjusted for age and sex. An analysis of variance by likelihood ratio test was used to compare PRESS hyperreactivity by DM status adjusted for age and sex. To determine the association between PRESS and hemoglobin A1c (HbA1c) we calculated a Spearman correlation coefficient.
To determine the association between PRESS and 30-day CV events, we used a composite of major adverse CV and limb events (MACLE), including death, myocardial infarction, stroke, or major amputation.4,5 Cox proportional hazard regression analysis was performed to determine the relationship between PRESS and MACLE. Survival curves were plotted using the Kaplan-Meier method with differences determined via log-rank tests. Statistical analyses were performed with R, version 4.4.2. A 2-sided value of P <.05 was considered statistically significant.
A total of 129 individuals were included in the analysis (mean age, 75.6 ± 10.5 years; 65% male, 64% non-Hispanic White). Overall, 70 (54%) individuals had DM of which 41 (59%) reported insulin use. Baseline characteristics are described in Table 1.
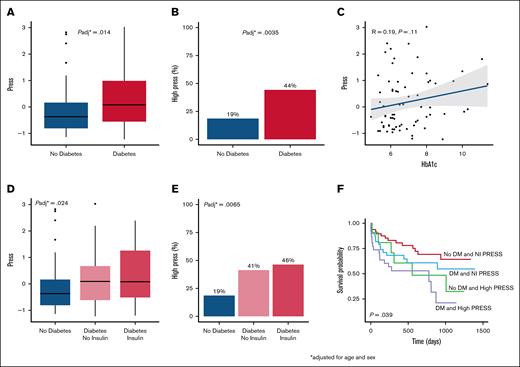
In the overall cohort, patients with DM had a higher PRESS than patients without DM (P = .014; Figure 1A). Among individuals with high PRESS, 44% had DM and 19% did not have DM (P = .004; Figure 1B). We did not find a significant correlation between PRESS and HbA1c overall (R = 0.19, P = .11) and after excluding participants without DM (R = 0.14, P = .33; Figure 1C). When analyzing DM severity, the difference was most notable in PRESS among individuals with DM on insulin compared to those without DM (Figure 1D). Among individuals with high PRESS, 46% had DM on insulin, 41% had DM not on insulin, and 19% did not have DM (P = .007; Figure 1E).
Association between DM and PRESS. (A) Distribution of PRESS by DM status, adjusted for age, sex, and race/ethnicity following z-score normalization. (B) Stratification of PRESS by DM status, adjusted for age, sex, and race/ethnicity. (C) Correlation between PRESS and HbA1c following z-score normalization. (D) Distribution of PRESS by DM severity, adjusted for age and sex. (E) Distribution of PRESS by DM severity, adjusted for age and sex following z-score normalization. (F) Kaplan-Meier curve for categorization of PRESS and DM status. Probability of remaining free of major adverse cardiovascular event or major amputation by high PRESS and DM status. Nl, normal.
Association between DM and PRESS. (A) Distribution of PRESS by DM status, adjusted for age, sex, and race/ethnicity following z-score normalization. (B) Stratification of PRESS by DM status, adjusted for age, sex, and race/ethnicity. (C) Correlation between PRESS and HbA1c following z-score normalization. (D) Distribution of PRESS by DM severity, adjusted for age and sex. (E) Distribution of PRESS by DM severity, adjusted for age and sex following z-score normalization. (F) Kaplan-Meier curve for categorization of PRESS and DM status. Probability of remaining free of major adverse cardiovascular event or major amputation by high PRESS and DM status. Nl, normal.
Finally, we investigated the association between PRESS and cardiovascular events when stratified by PRESS. After a median follow-up of 18 months, patients with DM and high PRESS had the highest incidence of MACLE. (Figure 1F). Compared to individuals without DM and normal PRESS, those with DM and high PRESS had significantly higher risk of MACLE (hazard ratio, 5.27; 95% confidence interval, 1.84-15.13; P = .002) after adjustment for age, sex, race, ethnicity, body mass index, hypertension, coronary artery disease, and antiplatelet therapy, albeit in the context of a small sample size.
The metabolic derangements imparted by DM including obesity, dyslipidemia, insulin resistance, increased release of free fatty acids, and systemic inflammation contribute to endothelial dysfunction, increased adhesive and proinflammatory cytokines, impaired fibrinolysis, and platelet activation.3 Although not fully explored in this study, participants with DM may be more likely to have higher PRESS and increased platelet activity because of increased inflammation, oxidative stress, higher levels of calcium and lower cyclic adenosine monophosphate, impaired nitric oxide production, and increased platelet Fc receptor expression.6,7 These abnormalities contribute to the increased risk of atherosclerosis, thrombosis, and vascular complications. Our findings support these mechanisms, demonstrating higher platelet activity among individuals with PAD and comorbid DM.
Although hyperglycemia is thought to induce vascular damage via accumulation of reactive oxygen species, inducing an imbalance in nitric oxide bioavailability and increasing production of advanced glycation end products,3 it is noteworthy that we did not find a significant association between insulin use or HbA1c and PRESS. Thus, increased platelet activity observed in DM may be independent of glycemic control. Notably, clinical findings are inconsistent for the relationship between glycemic control and micro- or macrovascular outcomes. For example, a study examining the association between glycemic control and microvascular function among individuals with DM found that better glycemic control was associated with improved microvascular function; however, this association did not persist after adjustment for DM duration, suggesting disease duration may play a more important role than glycemic control.8 Additionally, in several trials in patients with DM, intensive glucose control was not associated with a reduction in major macrovascular events or CV death.9,10
Our group previously demonstrated the clinical relevance for PRESS, demonstrating that in women undergoing coronary angiography, PRESS was significantly increased among those with acute myocardial infarction compared to those referred for coronary angiography without myocardial infarction.4 We also found that among patients with PAD followed over time, PRESS was associated with a higher incidence of CV events.4 The current study extends these findings with a focus on individuals with DM demonstrating that those with high PRESS and DM have the highest MACLE event rate.
Our findings have several important clinical implications. Given that individuals with DM demonstrate increased platelet hyperreactivity which in turn was associated with increased risk of CV events, efforts to target platelet hyperreactivity to mitigate atherothrombotic events are paramount.
Our study has several limitations including a small sample size with 70 individuals with DM, which is further limited when stratified by DM severity and PRESS status. The analysis was performed among individuals with prevalent symptomatic PAD on antiplatelet therapy, a population already at increased risk of cardiovascular events. Although a previous study demonstrated that PRESS can discriminate a hyperreactive platelet phenotype in patients with CVD and healthy controls, the utility of PRESS in the setting of DM without PAD is uncertain. Furthermore, although PRESS was previously derived and validated to confer CV risk, its ability to predict CV events specifically in subjects with DM requires additional validation.
In conclusion, we demonstrate that patients with PAD and DM are more likely to have platelet hyperreactivity as measured by a recently developed platelet genetic signature—PRESS. Moreover, a hyperreactive phenotype in the setting of DM is associated with a significantly increased risk of CV events. Future studies are needed to validate these findings, explore potential mechanisms by which DM imparts a more hyperreactive platelet profile, and examine whether targeting PRESS with platelet directed therapies can improve clinical outcomes.
Acknowledgments: Support for this study was provided by National Institutes of Health grants R01HL114978 and R35HL144993 (J.S.B.), R01HL167917 (T.J.B.), and KL2TR001446-09 (C.E.H.).
Contribution: C.E.H. wrote the manuscript; M.A.M. analyzed the data; J.B. and T.J.B. designed the research; and M.A.M., J.D.N., J.B., K.V.R., T.J.B., and J.S.B. reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carine E. Hamo, Department of Medicine, New York University School of Medicine, 530 First Ave, Skirball 9R, New York, NY 10016; email: carine.hamo@nyulangone.org.
References
Author notes
Sequencing data for this study have been deposited in the Gene Expression Omnibus database (accession number GSE232027).
Data are available from the corresponding author, Carine E. Hamo (carine.hamo@nyulangone.org), on request.