Key Points
Andexanet alfa reverses DOAC anticoagulation, but can overcorrect, leading to increased clotting risk.
Andexanet alfa promotes clotting by suppressing natural anticoagulant defenses, TFPI and heparan sulfate–bound antithrombin.
Visual Abstract
Patients with hemorrhage after treatment with direct oral anticoagulants (DOACs) are saved from hematoma expansion by administration of andexanet alfa, a modified factor Xa analog. A recent clinical trial points to incidental prothrombotic effects after andexanet alfa treatment. We investigated mechanisms explaining the thrombogenic side effect. Calibrated automated thrombin generation was employed to assess the effect of andexanet alfa on the coagulant potential of DOAC-treated plasma and whole blood. The study examined anticoagulant inhibition pathways involving tissue factor pathway inhibitor (TFPI), antithrombin, and endothelial cell components. Treatment of control plasmas with rivaroxaban, apixaban, or edoxaban resulted in an impaired thrombin generation, exemplified by a prolonged lag time and reduced thrombin peak height. Andexanet alfa over-converted this anticoagulant activity into a procoagulant effect. Plasma treatment with andexanet alfa similarly increased thrombin generation in the absence of DOACs, even at 8 nM far below the therapeutic dose. The enhancement was abrogated in the absence or by blocking of TFPI. The andexanet alfa effect was enhanced in the presence of heparin, thereby reversing the antithrombin protection. In the presence of endothelial cells, andexanet alfa stimulated coagulation via TFPI and heparin inhibition. Andexanet alfa overconverts the anticoagulant effect of rivaroxaban, apixaban, and edoxaban to achieve a hypercoagulable plasma state. The action mechanism involves TFPI inhibition and neutralization of antithrombin-heparin complexes. This may account for the incidence of thrombosis observed in patients treated with andexanet alfa.
Introduction
Andexanet alfa is currently the only reversing agents for factor Xa inhibition that is approved by regulatory authorities.1 Clinically, the reversal of factor Xa inhibition is used as a lifesaving intervention for patients receiving direct oral anticoagulants (DOACs) directed against factor Xa, when they develop hemorrhages.2 A recent randomized clinical trial confirmed that andexanet alfa treatment significantly reverses hematoma expansions, but at the expense of incidental thrombotic complications.2 Aim of the current study was to find mechanistic evidence for hypercoagulant side effects of andexanet alfa in the physiology of coagulation regulation.
The drug andexanet alfa is a recombinant, catalytically inactive variant of factor Xa which lacks the phosphatidylserine-binding Gla domain but contains a binding site to capture DOACs from the circulation.3 Prescription of andexanet alfa has been approved by the US Food and Drug Administration and the European Medicines Agency for patients treated with rivaroxaban or apixaban. The approval was obtained after targeted clinical studies with adult patients taking rivaroxaban or apixaban who experienced intracranial internal hemorrhage prior to study admission.4-6 In addition, a single-arm trial which quantified prothrombotic complications after andexanet alfa treatment was conducted.2 A recent randomized clinical trial involving patients on either rivaroxaban or apixaban revealed that patients administered andexanet alfa experience a 10.3% incidence of new-onset thrombosis, in comparison to a 5.6% incidence in patients who received regular treatment.2 In earlier toxicology experiments, it was found that andexanet alfa led to elevated plasma levels of F1+2 and D-dimers, suggesting a procoagulant off-target effect.7
One possible explanation for thrombogenicity of patients receiving andexanet alfa is an interference in the natural anticoagulant systems, such as provided by tissue factor (TF) pathway inhibitor (TFPI) and antithrombin.8 Another possibility lies in the reversal of heparin-dependent anticoagulant mechanisms.9-11 Accordingly, it has been suggested that andexanet alfa competes with factor Xa or thrombin for the binding of heparinized antithrombin, thus reversing the action of heparins.12 Such an effect may provide a thrombosis risk in patient receiving the drug.2,13
Using thrombin generation measurement as a recognized test of the integrated plasma coagulant profile, we investigated in detail the how andexanet alfa altered the coagulation system. We observed a consistent procoagulant effect even in the absence of DOACs that relied on TFPI inhibition and heparin antagonism.
Materials and methods
Blood and plasma donors
The study was conducted as guided by the Declaration of Helsinki and was approved by the medical ethics committee of the Maastricht University Medical Center. Whole blood was collected after fully informed consent from healthy donors aged ≥18 and <65 years by venipuncture into 9 mL 3.2% sodium citrate Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ). Excluded were donors with a coagulation related pathology, receiving anticoagulant treatment, or with pregnancy/postpartum (3 months). Platelet-poor plasma (PPP) was prepared by centrifuging citrated blood samples at 2580g for 10 min. Platelet-rich plasma (PRP) was made by centrifugation of blood at 220g for 15 minutes. The blood and plasma samples were used within 1 to 4 hours of blood collection. Aliquots of PPP were snap-frozen and stored at −80°C. As a common reference, normal pool plasma (NPP) was prepared from PPP obtained from 46 healthy individuals, which were collected, mixed and frozen in 1 session.
Materials
TF was provided by Innovin (Dade Behring, Marburg, Germany). Procoagulant phospholipid mixture was composed of 20 mol% phosphatidylserine, 60 mol% phosphatidylcholine, and 20 mol% phosphatidylethanolamine (Avanti, Alabaster, AL). The thrombin substrate Z-Gly-Gly-Arg-aminomethyl coumarin (ZGGR-AMC) came from Bachem (Basel, Switzerland). A calibrating agent (α2-macroglobulin–bound thrombin) and NPP were prepared in house by Synapse Research Institute. Plasma depleted from TFPI was provided by BioMedica Diagnostics (Windsor, NS, Canada).
Blocking antibody against human TFPI, a biosimilar of concizumab, came from MedChem Express (Princeton, NJ). Andexanet alfa was obtained from Alexion, AstraZeneca Rare Disease (Munich, Germany); rat tail collagen from Millipore (Darmstadt, Germany). Human umbilical vein endothelial cells (HUVEC, C-12203) were provided by Promocell (Heidelberg, Germany), whereas endothelial cell growth medium came from Gibco (Waltham, MA). Recombinant luciferase was purified in Synapse Research Institute (Maastricht, The Netherlands); d-luciferin potassium salt from Synchem UG (Felsberg-Altenburg, Germany). Rivaroxaban was purchased from Bayer (Leverkusen, Germany), apixaban from Pfizer (New York, NY), edoxaban from Daiichi Sankyo (Tokyo, Japan), and pentasaccharide anticoagulant from Mylan (Canonsburg, PA). Unfractionated heparin (UFH) was bought from Sigma-Aldrich (St. Louis, MO).
Buffer medium BSA5 consisted of 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 140 mM NaCl, 0.5% BSA, 0.02% NaN3 (pH 7.35); medium BSA60 consisted of 20 mM HEPES, 6% BSA, 0.02% NaN3 (pH 7.35). Buffer A was composed of 10 mM HEPES, 150 mM NaCl, 1 mM MgSO4, 5 mM KCl, 1 mM dithiothreitol, 0.49 mM MgCl2, and 5 mg/mL BSA, pH 7.45.
Preparation of blood and plasma samples
As required, blood or plasma samples were supplemented with indicated DOAC (0-600 nM), andexanet alfa (0-500 nM), anti-TFPI antibodies (0-250 nM), and/or heparin (ie, 0-0.05 U/mL UFH and 0-250 nM pentasaccharide). Plasma and blood incubations were for 10 minutes at 37°C. For thrombin generation testing, predilutions of supplements were made in BSA5 medium. For platelet secretion testing, predilutions were in buffer A. Control samples (no supplements) were diluted with BSA5 medium for thrombin generation, or with buffer A for platelet secretion testing, as to match the dilution factor of the highest supplementation condition.
Endothelial cell culture
The HUVEC were cultured in growth medium with supplements from Promocell, 1% penicillin/streptomycin and 1% l-glutamine under CO2 atmosphere, as described before.14 Cells were utilized at passages 4 to 6. Before measurements, the endothelial cells from precultures in flasks were seeded in either clear U-bottom 96-well plates (whole-blood assay) or clear flat bottom 96-well plates (platelet granule secretion assay) at a density of 3000 cells per well. The 96-well plates were precoated with rat-tail collagen. After 24 hours, the HUVEC-containing wells were checked for formation of a cobblestone monolayer with viable cells using bright-field microscopy. Prior to experimentation, the cells in wells were washed twice with saline.
Thrombin generation
Calibrated automated thrombogram measurements were performed for real-time measurements of the thrombin generation process in PPP, PRP, or whole blood. As a calibrating agent, thrombin enveloped by α2-macroglobulin was used, which ensured stable thrombin activity during the measurement time. Obtained thrombin generation curves were quantified according to the conventional parameters lag time to initial thrombin (minutes), thrombin peak value (nM), time to peak (minutes), and velocity index (nM per minute), as described.15,16
Unless indicated otherwise, coagulation was initiated with BSA5 medium, containing 6 or 30 pM TF and 24 mM procoagulant phospholipids. Wells in 96-well plates (Immulon 2 HB; ThermoFisher Scientific) were incubated with 80 μL of PPP or PRP, plus 20 μL of TF/phospholipid mixture. Calibrator wells contained 20 μL of 621 nM α2-macroglobulin–bound thrombin plus 80 μL of supplemented PPP or PRP.
Plasma-containing well plates were preincubated for 10 minutes at 37°C in a Fluoroskan Ascent microplate fluorometer (Thermo Labsystems, Helsinki, Finland). Per well, 20 μL of substrate solution was added. This in-house prepared solution contained 1750 μL BSA60 medium, 200 μL CaCl2 (1 M), and 50 μL fluorogenic ZGGR-AMC (100 mM). Final concentrations in the reaction system were 1 or 5 pM TF, 4 μM phospholipids, and 16.7 mM CaCl2. Fluorescence was recorded during a 60-minute period at 20-second intervals. Prior to each measurement, plates were shaken for 10 seconds. Obtained curves were analyzed using Thrombinoscope software, and evaluated in GraphPad 8.3.4. Conditions were measured in triplicates, unless specified otherwise.
Whole-blood thrombin-generation was measured, as described previously.16 In brief, 120 μL of whole blood samples were thoroughly mixed with substrate solution 40 μL (417 μM ZGGR-AMC in BSA60 medium) and 80 μL trigger solution (0.1 pM TF, 6 mM CaCl2 plus 3 mM MgCl2 in BSA5 medium). Fluorescence signals were measured at wavelengths of λex 355 nm and λem 460 nm. A modified Excel-based calculation template was employed to obtain first derivative thrombin generation curves from the raw fluorescence data, and evaluated in GraphPad 8.3.4. Independent measurements were performed 3 times, with each measurement conducted in triplicate.
Platelet granule secretion assay
To measure platelet granule secretion, the kinetics of adenosine triphosphate release were assessed, based on a previously developed procedure.17 Citrated PRP samples were triggered with TF to allow thrombin generation and ensuing platelet activation with granule secretion. The latter was measured in situ by common luciferase measurements. Using 96-well plates, whether or not grown with HUVEC, 25 μL supplemented PRP was mixed with 75 μL reaction mixture at final concentrations of 1 pM TF, 8 μg/mL luciferase, and 1 mM luciferin (prepared in buffer A). Luminescent signals were measured with a Fluoroskan FL reader (ThermoFisher Scientific) reader every 5 seconds for 30 minutes, and quantified as relative light units. The platelet activation time was defined as the time point, at which reached half of the maximum value (LT50). Independent measurements were performed 3 times, with each measurement conducted in duplicate.
Statistical analyses
Statistical analyses were performed with GraphPad Prism 8.3.4 software (San Diego, CA). Data are presented as means ± standard deviation. Significance was determined using a 1-way analysis of variance. A value of P <.05 was considered as statistically significant.
Results
Reversal of andexanet alfa of DOAC effects in normal plasma
The ability of andexanet alfa as a reversal agent against the DOACs rivaroxaban, edoxaban, and apixaban was confirmed by thrombin generation measurements of normal pool plasma. The DOAC concentrations used in vitro matched the reported peak levels in human blood. Strikingly, these completely suppressed the thrombin generation curves triggered by 5 pM TF (Figure 1A). Furthermore, from the lowest dose used (300-600 nM), we found that andexanet alfa over-completely reversed the DOAC-induced curve inhibition. In particular, andexanet alfa reduced the thrombin-generation lag time, and increased the thrombin peak height, even in combination with rivaroxaban (Figure 1B-C) or apixaban (Figure 1H-I). These effects were observed, starting from a 1:1 molecular ratio with DOAC. In the presence of edoxaban, similar effects were seen from a 1:2 ratio (Figure 1E-F). Remarkably, the lag times of thrombin generation were shortened in all plasma samples containing andexanet alfa. Similarly, the peak heights were higher in the andexanet alfa containing plasmas, when compared to the control samples. These results proved the high sensitivity of thrombin generation measurements for factor Xa inhibition and reversal.
Consistent reversal of DOAC effect by andexanet alfa on plasma thrombin generation. (A-C) Assessed thrombin generation in NPP with 600 nM rivaroxaban and 0 to 19 200 nM andexanet alfa (trigger 5 pM TF). Shown are representative traces (A) and the parameters lag time (B) and thrombin peak level (C). Further, assessed TG in NPP with 300 nM edoxaban (D-F) or 400 nM apixaban (G-I) and at indicated concentration of andexanet alfa (nM). Shown are representative traces (D,G), lag time (E,H), and thrombin peak level (G,I). Control condition represents absence of DOAC or andexanet alfa. Means ± standard deviation (SD) (n = 3). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 (1-way analysis of variance [ANOVA]). N.S, not significant.
Consistent reversal of DOAC effect by andexanet alfa on plasma thrombin generation. (A-C) Assessed thrombin generation in NPP with 600 nM rivaroxaban and 0 to 19 200 nM andexanet alfa (trigger 5 pM TF). Shown are representative traces (A) and the parameters lag time (B) and thrombin peak level (C). Further, assessed TG in NPP with 300 nM edoxaban (D-F) or 400 nM apixaban (G-I) and at indicated concentration of andexanet alfa (nM). Shown are representative traces (D,G), lag time (E,H), and thrombin peak level (G,I). Control condition represents absence of DOAC or andexanet alfa. Means ± standard deviation (SD) (n = 3). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 (1-way analysis of variance [ANOVA]). N.S, not significant.
Consistent procoagulant effect of andexanet alfa assessed by thrombin generation
To investigate effects in the absence of DOACs, we titrated andexanet alfa into normal pool plasma and measured the coagulant potential. Even at low andexanet alfa concentrations, we observed a dose-dependent shortening in thrombin-generation lag time, that is, from 5.2 to 3.8 minutes at 2 to 8 nM andexanet alfa (Figure 2A-B). At higher andexanet alfa concentrations, the lag time did not further decline and remained short at 3.8 minutes, likely because maximal kinetics of the coagulation process were reached. Simultaneously, we observed a substantial, dose-dependent increase in thrombin peak from 180 to 320 nM at the low concentrations of andexanet alfa, again in the range of 2 to 8 nM (Figure 2C). Furthermore, the time to peak decreased from 11 to 6 minutes (Figure 2D), whereas the curve velocity index exhibited a significant fourfold increase from 25 to 110 nM per minute, again at a threshold of 2 to 8 nM andexanet alfa. Overall, this assessment indicated a remarkable procoagulant effect of andexanet alfa, which appears optimal at low concentrations of 2 to 8 nM.
Residual effect of andexanet alfa on thrombin generation in the absence of DOAC. Assessment of TG was performed in NPP in the presence of 1 to 128 nM andexanet alfa (trigger 1 pM TF). Shown are representative traces (A) and the parameters lag time (B), thrombin peak level (C), time to peak (D), and velocity index (E). Means ± SD (n = 3). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 (ANOVA).
Residual effect of andexanet alfa on thrombin generation in the absence of DOAC. Assessment of TG was performed in NPP in the presence of 1 to 128 nM andexanet alfa (trigger 1 pM TF). Shown are representative traces (A) and the parameters lag time (B), thrombin peak level (C), time to peak (D), and velocity index (E). Means ± SD (n = 3). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 (ANOVA).
Modulating action of andexanet alfa via TFPI
To assess how the procoagulant effect of andexanet alfa relied on TFPI inhibition, we first performed experiments in a TFPI-depleted plasma. The obtained thrombin generation traces indicated full reversal by andexanet alfa of the anticoagulant effect of apixaban, but no longer with a reduced lag time or increase the peak height, when compared to the control condition (supplemental Figure 1A-C).
To take this further, we used a monoclonal antibody blocking the K2 domain of TFPI. Markedly, using normal pool plasma, the anti-TFPI monoclonal antibody showed procoagulant effects on curve lag time, thrombin peak, time to peak, and velocity index comparable to those of andexanet alfa (supplemental Figure 2A-E). Furthermore, with blocked TFPI, andexanet alfa no longer altered the thrombin generation parameters. Jointly, these data suggested that andexanet alfa acted at least partly by antagonizing TFPI as a procoagulant off-target effect.
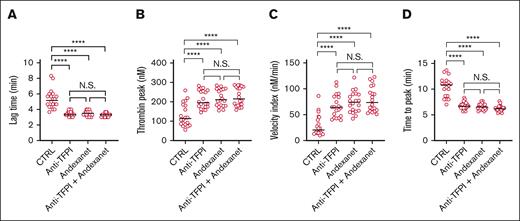
In plasma samples from healthy individuals, coagulation inhibitors vary in efficacy, when compared to normal pool plasma. To reconcile this, we compared how andexanet alfa affected the coagulant activity in plasmas from 20 healthy subjects in the presence or absence of anti-TFPI mAb. The established thrombin generation profiles confirmed, for essentially all subject, that blockage of TFPI resulted in a coagulation stimulation comparable to that of andexanet alfa regarding curve lag time, thrombin peak height, time to peak, and velocity index (Figure 3A-D). Herein, the combination of antibody and andexanet alfa was not more effective than either treatment alone. Notably, between plasmas from the various subjects, the lag times substantially varied, but these consistently shortened to 3.5 minute upon inhibition of TFPI (Figure 3A). Similar effects were observed for time-to-peak values (Figure 3D). Together, this supported the conclusion that andexanet alfa interaction with TFPI contributes to its procoagulant action mechanism.
Effect of anti-TPFI antibodies on subject-dependent thrombin generation with andexanet alfa. Assessment of TG in plasmas from 20 healthy subjects in the presence of 200 nM blocking anti-TFPI mAb and 200 nM andexanet alfa (trigger 1 pM TF). Shown are the trace parameters lag time (A), thrombin peak level (B), velocity index (C), and time to peak (D); medians (n = 20). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
Effect of anti-TPFI antibodies on subject-dependent thrombin generation with andexanet alfa. Assessment of TG in plasmas from 20 healthy subjects in the presence of 200 nM blocking anti-TFPI mAb and 200 nM andexanet alfa (trigger 1 pM TF). Shown are the trace parameters lag time (A), thrombin peak level (B), velocity index (C), and time to peak (D); medians (n = 20). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
Modulating action of andexanet alfa via heparin and antithrombin
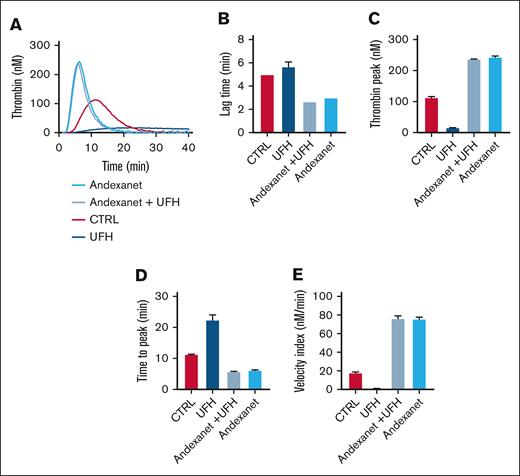
We then examined possible off-target effects of andexanet alfa via antithrombin modulation. For this purpose, thrombin generation was measured in the presence of a commonly used heparin (UFH). In the presence of low UFH (0.05 IU/mL), we confirmed that TF–triggered generation of thrombin was essentially abrogated (Figure 4A). Markedly, with andexanet alfa present, this abrogation was more than completely reversed, in that lag times were shortened and thrombin peaks were increased in comparison to the control condition (Figure 4B-E).
Effect of heparin on thrombin generation with andexanet alfa. Assessment of TG in NPP in the presence of 0.05 U/mL heparin (UFH) and 128 nM andexanet alfa (trigger 1 pM TF). Shown are representative traces (A), and the parameters lag time (B), thrombin peak (C), time to peak (D), and velocity index (E). Means ± SD (n = 3). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 (ANOVA).
Effect of heparin on thrombin generation with andexanet alfa. Assessment of TG in NPP in the presence of 0.05 U/mL heparin (UFH) and 128 nM andexanet alfa (trigger 1 pM TF). Shown are representative traces (A), and the parameters lag time (B), thrombin peak (C), time to peak (D), and velocity index (E). Means ± SD (n = 3). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 (ANOVA).
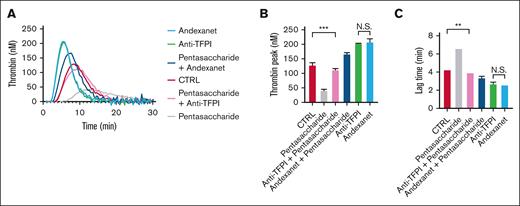
To confirm this heparin effect, we also used pentasaccharide—a specific antithrombin-activating heparin derivative—alone or in combination with anti-TFPI mAb. At a clinically relevant dose of 250 nM, the pentasaccharide suppressed the thrombin generation curves (Figure 5A), in a way completely annulled by the antibody, regarding both lag time and thrombin peak level (Figure 5B-C). Again, the presence of andexanet alfa regulated in an overcompensation, seen as a shortened lag time and increased thrombin peak level. Together, these measurements pointed to an additional heparin-antithrombin antagonizing effect of andexanet alfa.
Effect of pentasaccharide on thrombin generation with andexanet alfa. Assessment of TG in NPP in the presence of 250 nM pentasaccharide, 250 nM anti-TFPI mAb, and/or 500 nM andexanet alfa, as indicated (trigger: 1 pM TF). Shown are representative traces (A) and the parameters lag time (B) and thrombin peak (C). Means ± SD (n = 3). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 (ANOVA).
Effect of pentasaccharide on thrombin generation with andexanet alfa. Assessment of TG in NPP in the presence of 250 nM pentasaccharide, 250 nM anti-TFPI mAb, and/or 500 nM andexanet alfa, as indicated (trigger: 1 pM TF). Shown are representative traces (A) and the parameters lag time (B) and thrombin peak (C). Means ± SD (n = 3). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 (ANOVA).
Modulating action of andexanet alfa by reversing endothelial anticoagulant activity
To approach the anticoagulant activity of the vessel wall, we also studied the effects of andexanet alfa in the presence of a confluent monolayer of HUVEC, that is, an endothelial source that was previously used to establish anticoagulant and antiplatelet phenotypes.14 Using whole-blood thrombin generation performed in control wells, we again observed enhancing effects of anti-TFPI mAb, andexanet alfa and the combination of both, regarding a shortened lag time and an increased thrombin peak level (Figure 6A-C). On the other hand, in wells grown with a HUVEC monolayer, we found complete inhibition of the whole-blood, which effect was partly antagonized by the addition of anti-TFPI mAb (Figure 6D). With endothelial cells present, andexanet alfa alone had a stronger enhancing effect on thrombin generation than anti-TFPI mAb, as appeared from a shorter lag time of 100 vs 18 minutes, respectively (Figure 6E). The combination of andexanet alfa and antibody did not add in effect.
Reversal by andexanet alfa of endothelial suppression of thrombin generation. Assessment of TG in control wells (A-C) or HUVEC-grown wells (D-F) using whole-blood supplemented with 250 nM anti-TFPI mAb and/or 150 nM andexanet alfa (trigger: 0.1 pM TF). Shown are representative traces (A,D) and the parameters lag time (B,E), and thrombin peak level (C,F). Means ± SD (n = 3). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 (ANOVA).
Reversal by andexanet alfa of endothelial suppression of thrombin generation. Assessment of TG in control wells (A-C) or HUVEC-grown wells (D-F) using whole-blood supplemented with 250 nM anti-TFPI mAb and/or 150 nM andexanet alfa (trigger: 0.1 pM TF). Shown are representative traces (A,D) and the parameters lag time (B,E), and thrombin peak level (C,F). Means ± SD (n = 3). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 (ANOVA).
To take these findings further, in the same whole-blood conditions, we measured the TF- and thrombin-induced platelet activation using an in-well luciferase assay, assessing the release of platelet-stored ATP.17 We found that the presence of andexanet alfa led to a more shortened lag time of platelet secretion than the anti-TFPI mAb (Figure 7A-B). In wells containing HUVEC, the ATP release was >10 times delayed, such supporting the strong anticoagulant propensity of endothelial cells. Both andexanet alfa and anti-TFPI mAb for a substantial part abrogated the endothelial effect on whole-blood thrombin generation (Figure 7C-D).
Reversal by andexanet alfa on endothelial suppression of platelet activation. Samples of PRP were incubated with andexanet alfa (150 nM) and/or anti-TFPI mAb (250 nM). Triggering in control wells (A,B) or endothelial-covered wells (C,D) was with 1 pM TF. Measured was platelet ATP secretion by luciferin-luciferase interaction. Shown are representative luminescence curves (A,C) and calculated lag times to onset (B,D). Means ± SD (n = 3). ∗P < .05, ∗∗P < .01 (ANOVA).
Reversal by andexanet alfa on endothelial suppression of platelet activation. Samples of PRP were incubated with andexanet alfa (150 nM) and/or anti-TFPI mAb (250 nM). Triggering in control wells (A,B) or endothelial-covered wells (C,D) was with 1 pM TF. Measured was platelet ATP secretion by luciferin-luciferase interaction. Shown are representative luminescence curves (A,C) and calculated lag times to onset (B,D). Means ± SD (n = 3). ∗P < .05, ∗∗P < .01 (ANOVA).
Discussion
At present, andexanet alfa is the only reversal agent for oral direct factor Xa inhibitors (apixaban and rivaroxaban) to prevent hematoma expansion in patients with an acute severe bleeding episode or nonelective major surgery.3 Several indications point to off-target effects, potentially by interfering with the natural anticoagulant mechanisms in plasma, for instance, TFPI18,19 and antithrombin.11 In a clinical trial, some of the patients who received andexanet alfa, were found to experience thrombosis.2 Our present findings show that even low concentrations of andexanet alfa are able to enhance the plasma coagulant activity, as measured in thrombin generation, by the inhibition of TFPI and heparan sulfate–antithrombin complexes.
During treatment, the andexanet alfa concentration can peak to levels of 2000 nM upon instant removal of DOAC.19,20 This raised the suggestion that direct binding of andexanet alfa to TFPI explains the procoagulant phenotype. Given our data, the impact of excessive andexanet alfa concentrations can be substantial, because even low concentrations of around 8 nM accomplished the coagulation-stimulating activity. This is further supported by the low plasma level of TFPI, which is approximately 2 to 3 nM.21 In terms of pharmacokinetics, patients who receive a DOAC reversal bolus of andexanet alfa will need 3 days to reach this low concentration. Such a long exposure time may explain the observed thrombotic events in patients receiving andexanet alfa.2,13 It is important to emphasize that the calculations presented above are estimations based on a simplified assumption, that andexanet alfa clearance and function in vivo can be inferred from, in vitro, observations. This assumption however has not been validated in an in vivo model, allowing to assess the pharmacokinetics, and should therefore be interpreted with caution, also given that TFPI was not measured in our study.
Endothelial cells in the vessel wall, mimicked by HUVEC, express TFPI and a heparan sulfate–containing glycocalyx. Our study shows that this antithrombotic defense line can be suppressed by the presence of andexanet alfa. Suppletion of andexanet alfa to plasma-containing UFH or pentasaccharide resulted in an elimination of the anticoagulant effect and hence, contributed to a prothrombotic propensity. Change in the endothelial antithrombotic role might also have pathological consequences. On the basis of our in vitro assays, the procoagulant effect of andexanet alfa appears largely dependent on TFPI inhibition and heparan sulfate–bound antithrombin. This has led to the hypothesis that an engineered variant with lower affinity to TFPI and antithrombin, such as an A404T mutation present in factor X Nottingham,22 could create a TFPI- and antithrombin-independent DOAC reversal agent. However, further studies are needed to confirm whether such a variant would fully abrogate the procoagulant activity of andexanet alfa.
As a limitation of the study, our findings are restricted to coagulation measurements in whole blood and platelet-rich and platelet-poor plasma. Notably, the duration of its procoagulant effect could not be assessed. Accordingly, further work needs to be done to dampen the supposed prothrombotic effect of andexanet alfa in the context of patient treatment. Unfortunately, due to the limited availability of samples from patients treated with andexanet alfa, we were unable to access such material. As a result, we opted to use ex vivo plasma in our study. This approach enables us to more directly observe the effects of andexanet alfa and to better characterize its procoagulant activity. In support, our findings are in line with clinical studies.23
In summary, our study shows that andexanet alfa as an effective DOAC-reversal agent has off-target side effects. The observed hypercoagulation could be explained by inhibition of the physiological anticoagulants, antithrombin, and TFPI. In the clinical use of andexanet alfa, the side effects hence should be taken into consideration and preferably moderated.
Acknowledgments
The authors thank the volunteers who donated blood for this study.
This work was supported by the China Scholarship Council (J.Z., CSC 202308430051; S.S., CSC 201906220218).
Authorship
Contribution: I.M.S.H. and J.Z. performed the experiments, analyzed the results, and drafted the manuscript; S.S., C.S., and H.M. performed the experiments and edited the manuscript; J.W.M.H., M.R., and B.d.L. provided supervision and edited the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosures: B.d.L. and M.R. are employees of the Synapse Research Institute Maastricht, a member of the Stago Diagnostic group. J.W.M.H. is an adviser of the same institute. The remaining authors declare no competing financial interests.
Correspondence: Mark Roest, Synapse Research Institute, Koningin Emmaplein 7, 6217 KD Maastricht, The Netherlands; email: m.roest@thrombin.org.
References
Author notes
The full-text version of this article contains a data supplement.


![Consistent reversal of DOAC effect by andexanet alfa on plasma thrombin generation. (A-C) Assessed thrombin generation in NPP with 600 nM rivaroxaban and 0 to 19 200 nM andexanet alfa (trigger 5 pM TF). Shown are representative traces (A) and the parameters lag time (B) and thrombin peak level (C). Further, assessed TG in NPP with 300 nM edoxaban (D-F) or 400 nM apixaban (G-I) and at indicated concentration of andexanet alfa (nM). Shown are representative traces (D,G), lag time (E,H), and thrombin peak level (G,I). Control condition represents absence of DOAC or andexanet alfa. Means ± standard deviation (SD) (n = 3). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 (1-way analysis of variance [ANOVA]). N.S, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/2/4/10.1016_j.bvth.2025.100100/3/m_bvth_vth-2025-000355-gr1.jpeg?Expires=1767778425&Signature=0YX538HZZguZzvwj-9On97-EBoAHciYIxI3B~f1i0zypl2kcOlDz1o6eWXbX2x8bKdkJCkPLo5k3T1AUgnFQ7NgPfx7BERIMqlWnptjDbiuvB3KBKJx4jjG-AnM9yOhxw6k4H3aOkottMvw-RpN~WrPnkm3ybzpMsJ14clvXg8KzNm1Ps6d0tCsHzIf5MsUqGq0CGsYLt92wheM2Swp0Gebp-P1~4CykGkL6UC4J7KSHITAs-IDZKASGVD3d8qa~DxudzTLaZIfR-qdDway0qn51qVZU83wXdgfBEe1J8gvX3ZuuSipRkbL7BNxbwKI84W7CafGnRsWoby77oHZJgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)