TO THE EDITOR:
The cornerstone of diabetes management focuses on addressing cardiovascular disease (CVD) risks and achieving normoglycemia, as guided by glycated hemoglobin (HbA1c).1 Studies indicate improving HbA1c may reduce microvascular complications, however, the impact on macrovascular events is unclear.2 The existing clinical prediction scores lack individualisation3 and crucially, overlook the coagulation system, the final step of the complex multifactorial milieu resulting in atherothrombosis.
Conventional coagulation markers measure only time to clot formation, rendering them inadequate to assess hypercoagulable states. Global coagulation assays (GCAs), however, provide a more comprehensive assessment of clot formation and breakdown. We have demonstrated GCAs were able to discriminate healthy and hypercoagulable populations including chronic kidney disease4 and in predicting preexisting diabetic complications.5 The incorporation of GCAs into a multimodal cardiovascular risk prediction model for patients on dialysis outperformed traditional approaches including the Framingham Risk Score.4,6 As proof of concept, we evaluated the use of a multimodal model combining traditional risks and GCAs to predict prospective vascular events in patients with diabetes.
This prospective observational study was performed at Northern Health in Australia. Adult patients (aged ≥18 years) with diabetes as defined by the American Diabetes Association7 were recruited between February 2017 and August 2020. Exclusion criteria included use of anticoagulation, malignancy, or end-stage kidney failure (estimated glomerular filtration rate of <15 mL/min per 1.73 m2), with 93.5% of these patients being dialysis dependent and this subgroup is known to have significantly increased risk of thrombosis compared to patients without a dialysis requirement.8 Written informed consent was obtained from all participants, with ethics approval obtained via the Austin Health Human Research Ethics Committee (HREC/Austin/16/459).
Baseline demographics and blood samples were collected at time of patient recruitment. Three GCAs were performed; (1) thromboelastography using citrated whole blood, (2) thrombin generation using a calibrated automated thrombogram, and (3) fibrin generation using overall hemostatic potential using platelet-poor plasma (supplemental Figure 1). The primary outcome was arterial vascular events defined as myocardial infarction, stroke, transient ischemic attack or critical limb ischemia. Medical records were reviewed every 12 months, with the date of follow-up censored at the first of time-to-first event, mortality, or last point of contact with the health service for the time-to-event analysis. The median follow-up time was 3.81 years (interquartile range, 2.64-4.27). Statistical analysis was performed using Stata version 18.0 (StataCorp, College Station, TX). Detailed statistical methods are outlined in under "Statistical Analysis" in the data supplement.
A total of 154 patients (median age, 63 years; 55.8% male) were recruited, including 127 with type 2 diabetes mellitus (82.5%) and 27 with autoimmune diabetes (17.5%; Table 1). Fifteen (9.7%) vascular events were captured (3.17 per 100 person-years) including 7 non–ST-elevation myocardial infarctions, 2 ST-elevation myocardial infarctions, 3 strokes, and 3 transient ischemic attack, with no critical limb ischemia. Median time to event was 1.84 years. The overall mortality was 13.0% (n = 20) including 4 deaths in patients who had vascular events, although no deaths were directly attributed to vascular complications. Two venous thrombotic events were captured (1.3%), further analysis of venous events was not performed because of low event numbers.
Summary of all patients and comparison between those who experienced a vascular event and those who did not
| . | Laboratory reference interval . | All patients (N = 154) . | No vascular event (n = 139) . | Vascular event (n = 15) . | P value (vascular event vs no vascular event) . |
|---|---|---|---|---|---|
| Clinical demographics | |||||
| Age, y | — | 63.0 (50.0-72.0) | 63.0 (50.0-72.0) | 59.0 (54.0-66.0) | .49 |
| Male sex, n (%) | — | 86 (55.8) | 75 (54.0) | 11 (73.3) | .15 |
| Type 2 diabetes, n (%) | — | 127 (82.5) | 114 (82.0) | 13 (86.7) | .65 |
| Autoimmune diabetes, n (%) | — | 27 (17.5) | 25 (18.0) | 2 (13.3) | |
| Duration of diabetes, y | — | 10.0 (5.0-17.0) | 10 (4.5-17.0) | 15.0 (6.0-17.0) | .23 |
| No. of cardiovascular risk factors, mean (SD) | — | 4.2 (1.4) | 4.1 (1.4) | 4.8 (0.9) | .077 |
| Framingham Risk Score, n (%) | — | .71 | |||
| Low-risk score | 24 (15.7) | 23 (16.5) | 1 (7.1) | ||
| Moderate-risk score | 28 (18.3) | 26 (18.7) | 2 (14.3) | ||
| High-risk score | 101 (66.0) | 90 (64.7) | 11 (78.6) | ||
| Hypertension, n (%) | — | 114 (74.0) | 99 (71.2) | 15 (100.0) | .016 |
| Hypercholesterolemia, n (%) | — | 113 (73.4) | 98 (70.5) | 15 (100.0) | .014 |
| History of microvascular disease, n (%) | — | 35 (22.7) | 30 (21.6) | 5 (33.3) | .3 |
| Family history of CVD, n (%) | — | 58 (37.7) | 52 (37.4) | 6 (40.0) | .84 |
| History of stroke/TIA, n (%) | — | 11 (7.1) | 11 (7.9) | 0 (0.0) | .26 |
| History of peripheral artery disease, n (%) | — | 11 (7.1) | 9 (6.5) | 2 (13.3) | .33 |
| History of coronary artery disease, n (%) | — | 35 (22.7) | 30 (21.6) | 5 (33.3) | .3 |
| Body mass index, kg/m2 | — | 30.8 (25.8-36.8) | 30.8 (25.7-36.2) | 31.7 (27.9-37.4) | .38 |
| Obesity, n (%) | — | 87 (56.5) | 78 (56.1) | 9 (60.0) | .77 |
| Current smoker, n (%) | — | 30 (19.5) | 26 (18.7) | 4 (26.7) | .46 |
| Antiplatelet therapy, n (%) | — | 65 (42.2) | 56 (40.3) | 9 (60.0) | .89 |
| Standard laboratory parameters | |||||
| HbA1c, % | <6.5 | 7.5 (6.6-8.5) | 7.3 (6.6-8.5) | 8.4 (7.5-9.7) | .065 |
| Hemoglobin, mean (SD), g/L | Male, 128-175; female, 115-155 | 137.8 (16.3) | 138.1 (15.5) | 134.8 (22.4) | .46 |
| PT, s | 11.0-17.0 | 11.7 (10.9-12.6) | 11.9 (10.8-12.6) | 11.3 (10.9-11.5) | .045 |
| APTT, s | 25.0-38.0 | 27.3 (26.0-29.0) | 27.4 (26.1-29.3) | 25.7 (23.7-26.9) | .002 |
| Fibrinogen, g/L | 2.0-4.0 | 3.8 (3.2-4.5) | 3.8 (3.2-4.5) | 3.9 (3.3-4.5) | .41 |
| Factor VIII, % | 50-150 | 161.0 (126.0-200.0) | 159.0 (125.0-194.0) | 181.0 (156.5-274.5) | .045 |
| VWF antigen, % | 50-150 | 156.5 (116.0-191.5) | 154.0 (107.5-186.0) | 173.5 (147.0-240.5) | .042 |
| Antithrombin III, mean (SD), % | 70-140 | 100.2 (12.3) | 100.0 (12.2) | 102.3 (13.7) | .51 |
| Protein C, mean (SD), % | 60-110 | 124.4 (27.9) | 124.5 (28.8) | 123.8 (17.3) | .93 |
| Protein S, mean (SD), % | 50-120 | 110.6 (24.2) | 110.1 (23.8) | 115.6 (27.9) | .42 |
| Creatinine, μmol/L | 60-110 | 83.0 (65.0-114.0) | 81.0 (64.0-114.0) | 105.0 (65.0-143.0) | .13 |
| Creatinine clearance, median (IQR), mL/min | >70 | 88.6 (59.7-124.0) | 90.7 (59.7-124.0) | 83.4 (58.1-125.2) | .58 |
| CRP, median (IQR), mg/L | <6 | 2.7 (1.1-6.2) | 2.8 (1.1-6.1) | 1.5 (0.7-7.5) | .56 |
| Total cholesterol, median (IQR), mmol/L | <5.6 | 4.2 (3.6-5.1) | 4.2 (3.6-5.1) | 4.0 (3.3-5.3) | .92 |
| HDL, median (IQR), mmol/L | >0.9 | 1.1 (1.0-1.4) | 1.1 (1.0-1.4) | 1.1 (0.8-1.5) | .8 |
| LDL, median (IQR), mmol/L | <3.1 | 2.1 (1.6-2.9) | 2.1 (1.6-2.9) | 2.1 (1.5-3.0) | .96 |
| Triglycerides, median (IQR), mmol/L | <2.1 | 1.8 (1.2-2.5) | 1.8 (1.2-2.6) | 1.8 (1.6-2.4) | .9 |
| GCAs | |||||
| Thromboelastography | |||||
| R-time, min | 6.5 (5.6-7.8) | 6.5 (5.5-7.7) | 6.6 (6.2-7.9) | .26 | |
| K-time, min | 1.9 (1.5-2.2) | 1.8 (1.5-2.2) | 1.9 (1.4-2.3) | 1.00 | |
| α-Angle, ° | 52.9 (45.6-63.6) | 53.2 (46.0-65.5) | 50.8 (43.7-55.9) | .099 | |
| Maximum amplitude, mm | 68.1 (65.4-72.2) | 68.0 (65.0-71.5) | 70.5 (66.8-75.0) | .048 | |
| Lysis 30, % | 0.0 (0.0-0.5) | 0.0 (0.0-0.5) | 0.2 (0.0-0.4) | .95 | |
| Calibrated automated thrombogram | |||||
| Lag time, min | 3.9 (3.3-4.6) | 3.9 (3.3-4.6) | 3.8 (3.3-4.2) | .71 | |
| ETP, nM.min | 1258.6 (1073.8-1463.0) | 1256.4 (1068.4-1460.1) | 1385.1 (1146.0-1499.0) | .46 | |
| Peak, nM | 211.1 (165.7-256.0) | 209.7 (162.4-257.4) | 226.1 (185.2-248.5) | .38 | |
| Velocity index, nM/min | 63.2 (41.3-86.1) | 60.3 (40.3-86.7) | 66.5 (56.0-76.8) | .40 | |
| Overall hemostatic potential | |||||
| OCP, units | 40.2 (33.2-47.6) | 40.1 (32.9-46.9) | 44.6 (34.9-54.2) | .094 | |
| OHP, units | 9.3 (6.5-13.7) | 9.0 (6.5-13.1) | 14.8 (6.6-20.1) | .032 | |
| OFP, % | 75.3 (69.5-82.2) | 75.8 (70.3-82.2) | 69.2 (55.9-81.8) | .045 | |
| . | Laboratory reference interval . | All patients (N = 154) . | No vascular event (n = 139) . | Vascular event (n = 15) . | P value (vascular event vs no vascular event) . |
|---|---|---|---|---|---|
| Clinical demographics | |||||
| Age, y | — | 63.0 (50.0-72.0) | 63.0 (50.0-72.0) | 59.0 (54.0-66.0) | .49 |
| Male sex, n (%) | — | 86 (55.8) | 75 (54.0) | 11 (73.3) | .15 |
| Type 2 diabetes, n (%) | — | 127 (82.5) | 114 (82.0) | 13 (86.7) | .65 |
| Autoimmune diabetes, n (%) | — | 27 (17.5) | 25 (18.0) | 2 (13.3) | |
| Duration of diabetes, y | — | 10.0 (5.0-17.0) | 10 (4.5-17.0) | 15.0 (6.0-17.0) | .23 |
| No. of cardiovascular risk factors, mean (SD) | — | 4.2 (1.4) | 4.1 (1.4) | 4.8 (0.9) | .077 |
| Framingham Risk Score, n (%) | — | .71 | |||
| Low-risk score | 24 (15.7) | 23 (16.5) | 1 (7.1) | ||
| Moderate-risk score | 28 (18.3) | 26 (18.7) | 2 (14.3) | ||
| High-risk score | 101 (66.0) | 90 (64.7) | 11 (78.6) | ||
| Hypertension, n (%) | — | 114 (74.0) | 99 (71.2) | 15 (100.0) | .016 |
| Hypercholesterolemia, n (%) | — | 113 (73.4) | 98 (70.5) | 15 (100.0) | .014 |
| History of microvascular disease, n (%) | — | 35 (22.7) | 30 (21.6) | 5 (33.3) | .3 |
| Family history of CVD, n (%) | — | 58 (37.7) | 52 (37.4) | 6 (40.0) | .84 |
| History of stroke/TIA, n (%) | — | 11 (7.1) | 11 (7.9) | 0 (0.0) | .26 |
| History of peripheral artery disease, n (%) | — | 11 (7.1) | 9 (6.5) | 2 (13.3) | .33 |
| History of coronary artery disease, n (%) | — | 35 (22.7) | 30 (21.6) | 5 (33.3) | .3 |
| Body mass index, kg/m2 | — | 30.8 (25.8-36.8) | 30.8 (25.7-36.2) | 31.7 (27.9-37.4) | .38 |
| Obesity, n (%) | — | 87 (56.5) | 78 (56.1) | 9 (60.0) | .77 |
| Current smoker, n (%) | — | 30 (19.5) | 26 (18.7) | 4 (26.7) | .46 |
| Antiplatelet therapy, n (%) | — | 65 (42.2) | 56 (40.3) | 9 (60.0) | .89 |
| Standard laboratory parameters | |||||
| HbA1c, % | <6.5 | 7.5 (6.6-8.5) | 7.3 (6.6-8.5) | 8.4 (7.5-9.7) | .065 |
| Hemoglobin, mean (SD), g/L | Male, 128-175; female, 115-155 | 137.8 (16.3) | 138.1 (15.5) | 134.8 (22.4) | .46 |
| PT, s | 11.0-17.0 | 11.7 (10.9-12.6) | 11.9 (10.8-12.6) | 11.3 (10.9-11.5) | .045 |
| APTT, s | 25.0-38.0 | 27.3 (26.0-29.0) | 27.4 (26.1-29.3) | 25.7 (23.7-26.9) | .002 |
| Fibrinogen, g/L | 2.0-4.0 | 3.8 (3.2-4.5) | 3.8 (3.2-4.5) | 3.9 (3.3-4.5) | .41 |
| Factor VIII, % | 50-150 | 161.0 (126.0-200.0) | 159.0 (125.0-194.0) | 181.0 (156.5-274.5) | .045 |
| VWF antigen, % | 50-150 | 156.5 (116.0-191.5) | 154.0 (107.5-186.0) | 173.5 (147.0-240.5) | .042 |
| Antithrombin III, mean (SD), % | 70-140 | 100.2 (12.3) | 100.0 (12.2) | 102.3 (13.7) | .51 |
| Protein C, mean (SD), % | 60-110 | 124.4 (27.9) | 124.5 (28.8) | 123.8 (17.3) | .93 |
| Protein S, mean (SD), % | 50-120 | 110.6 (24.2) | 110.1 (23.8) | 115.6 (27.9) | .42 |
| Creatinine, μmol/L | 60-110 | 83.0 (65.0-114.0) | 81.0 (64.0-114.0) | 105.0 (65.0-143.0) | .13 |
| Creatinine clearance, median (IQR), mL/min | >70 | 88.6 (59.7-124.0) | 90.7 (59.7-124.0) | 83.4 (58.1-125.2) | .58 |
| CRP, median (IQR), mg/L | <6 | 2.7 (1.1-6.2) | 2.8 (1.1-6.1) | 1.5 (0.7-7.5) | .56 |
| Total cholesterol, median (IQR), mmol/L | <5.6 | 4.2 (3.6-5.1) | 4.2 (3.6-5.1) | 4.0 (3.3-5.3) | .92 |
| HDL, median (IQR), mmol/L | >0.9 | 1.1 (1.0-1.4) | 1.1 (1.0-1.4) | 1.1 (0.8-1.5) | .8 |
| LDL, median (IQR), mmol/L | <3.1 | 2.1 (1.6-2.9) | 2.1 (1.6-2.9) | 2.1 (1.5-3.0) | .96 |
| Triglycerides, median (IQR), mmol/L | <2.1 | 1.8 (1.2-2.5) | 1.8 (1.2-2.6) | 1.8 (1.6-2.4) | .9 |
| GCAs | |||||
| Thromboelastography | |||||
| R-time, min | 6.5 (5.6-7.8) | 6.5 (5.5-7.7) | 6.6 (6.2-7.9) | .26 | |
| K-time, min | 1.9 (1.5-2.2) | 1.8 (1.5-2.2) | 1.9 (1.4-2.3) | 1.00 | |
| α-Angle, ° | 52.9 (45.6-63.6) | 53.2 (46.0-65.5) | 50.8 (43.7-55.9) | .099 | |
| Maximum amplitude, mm | 68.1 (65.4-72.2) | 68.0 (65.0-71.5) | 70.5 (66.8-75.0) | .048 | |
| Lysis 30, % | 0.0 (0.0-0.5) | 0.0 (0.0-0.5) | 0.2 (0.0-0.4) | .95 | |
| Calibrated automated thrombogram | |||||
| Lag time, min | 3.9 (3.3-4.6) | 3.9 (3.3-4.6) | 3.8 (3.3-4.2) | .71 | |
| ETP, nM.min | 1258.6 (1073.8-1463.0) | 1256.4 (1068.4-1460.1) | 1385.1 (1146.0-1499.0) | .46 | |
| Peak, nM | 211.1 (165.7-256.0) | 209.7 (162.4-257.4) | 226.1 (185.2-248.5) | .38 | |
| Velocity index, nM/min | 63.2 (41.3-86.1) | 60.3 (40.3-86.7) | 66.5 (56.0-76.8) | .40 | |
| Overall hemostatic potential | |||||
| OCP, units | 40.2 (33.2-47.6) | 40.1 (32.9-46.9) | 44.6 (34.9-54.2) | .094 | |
| OHP, units | 9.3 (6.5-13.7) | 9.0 (6.5-13.1) | 14.8 (6.6-20.1) | .032 | |
| OFP, % | 75.3 (69.5-82.2) | 75.8 (70.3-82.2) | 69.2 (55.9-81.8) | .045 | |
Results reported as median (IQR) unless otherwise specified. P < .05 denotes significance (indicated in bold).
APTT, activated partial thromboplastin time; CRP, C-reactive protein; ETP, endogenous thrombin potential; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; OCP, overall coagulation potential; OFP, overall fibrinolytic potential; OHP, overall hemostatic potential; PT, prothrombin time; SD, standard deviation; TIA, transient ischemic attack; VWF, von Willebrand factor.
All patients who experienced vascular events had hypertension and hypercholesterolemia, higher than those without vascular events (Table 1). Number of risk factors, history of macrovascular/microvascular complications and Framingham score were not discriminatory. Patients with insulin-dependent type 2 diabetes mellitus were more likely to experience a vascular event (9/13 [69.2%] vs 43/114 [37.7%]; P = .029), with no association seen in other glucose-lowering therapies or antiplatelet use.
There was a trend toward higher HbA1c in the vascular event group (8.4% vs 7.3%; P = .065), with no differences noted in duration of diabetes, renal function, or lipid profile. Conventional coagulation studies remained within reference intervals, although von Willebrand factor antigen and factor VIII levels were higher in the group with vascular events (P = .042 and P = .045, respectively).
Patients who experienced vascular events demonstrated relatively hypercoagulable GCA parameters, including higher maximum amplitude (increased fibrin clot strength) on thromboelastography (70.5 vs 68.0 mm; P = .048), along with higher overall hemostatic potential (14.8 vs 9.0 units; P = .032) and lower overall fibrinolytic potential (69.2% vs 75.8%; P = .045), representing increased fibrin production with reduced fibrinolytic response. On univariate analysis, no significant differences were seen in the calibrated automated thrombogram parameters.
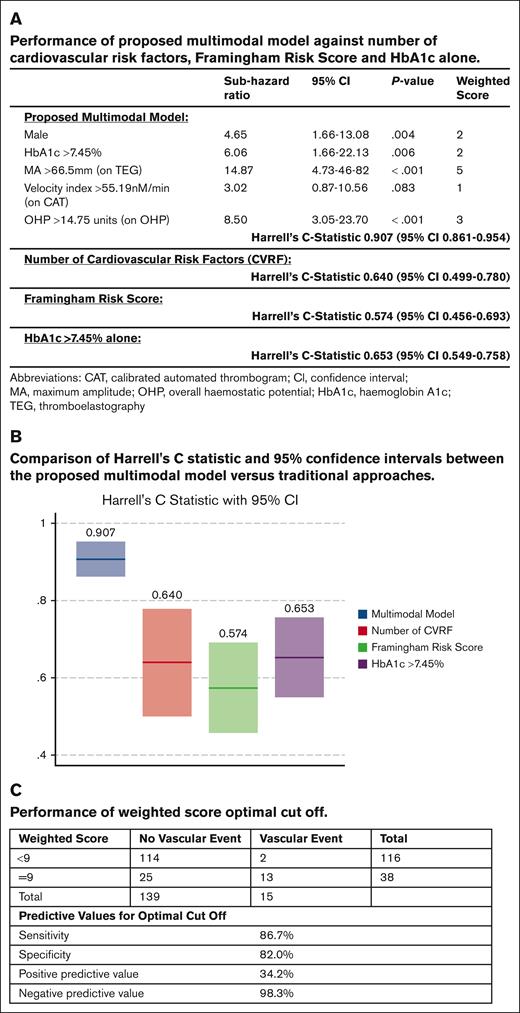
A time-to-event analysis was performed, and the best performing model was selected (Figure 1A). This model had a C-statistic of 0.907 (95% confidence interval [CI], 0.861-0.954), outperforming the Framingham Risk Score (C-statistic, 0.574; 95% CI, 0.456-0.693), total number of cardiovascular risk factors (C-statistic, 0.640; 95% CI, 0.499-0.78), and HbA1c of >7.45% alone (C-statistic, 0.653; 95% CI, 0.549-0.758; Figure 1A-B). The individual parameters were assigned a weighted score with a calculated optimal cutoff score of ≥9 to create a clinically applicable risk prediction tool. Patients above the cutoff point were at high risk for vascular events with a positive predictive value of 34.2% (Figure 1C). Patients below the cutoff were at low risk, with a negative predictive value of 98.3%.
Proposed multimodal risk prediction model. (A-B) Comparison of the proposed multimodal model against traditional surrogate markers and risk scores. (C) Performance of the weight score and optimal cutoffs.
Proposed multimodal risk prediction model. (A-B) Comparison of the proposed multimodal model against traditional surrogate markers and risk scores. (C) Performance of the weight score and optimal cutoffs.
To the best of our knowledge, this pilot study is the first to combine GCAs with traditional risk factors to prospectively predict vascular events in diabetes. Our results highlight the critical role of coagulation system in predicting cardiovascular events. The incorporation of GCA parameters along with sex and HbA1c improved our risk stratification ability. Crucially, the weighted score is simple to use with an easily interpretable total cutoff score. Patients above the cutoff had a 34.2% risk of a vascular event, offering the opportunity to implement preventive strategies in this high-risk group while avoiding unnecessary treatment of low-risk patients. We acknowledge the limitations of a single-center study with relatively small numbers and the potential for overfitting of a risk prediction model designed using a pilot population, hence necessitating external validation. Within these limitations, we were able to demonstrate the importance of hypercoagulability and the superiority of a model incorporating GCA to predict cardiovascular events. As multivariable analysis has been applied, no correction for multiple testing to control for false discovery rate has been applied.
Many studies evaluating CVD have studied the impact of atherosclerosis, endothelial dysfunction, and vascular flow but few have focused on coagulation cascade, which represents the final pathway triggering an event. It has been well established that diabetes is a prothrombotic stat; patients with diabetes with preexisting vascular complications were more likely to have hypercoagulable GCA parameters compared with those who did not.5,9 The association between vascular complications and its influence on GCAs highlights the importance of the often-neglected coagulation system in atherothrombosis.5 Impaired fibrinolysis may play an important role in thrombotic risk in diabetes, even in patients within glycemic control targets.10 Furthermore, the potential confounding impact of the elevated factor VIII and von Willebrand factor antigen, which are procoagulant factors and markers of endothelial dysfunction, should be considered with increased levels linked to inflammation, thrombosis, and atherosclerosis.11,12 Both factors are also raised in patients with diabetes13,14 and may be associated with risk of CVD.
If externally validated, the model will allow for further refinement of cardiovascular risk prediction, with the novel antidiabetes therapies modulating cardiovascular risk independent of HbA1c.2 In addition, it reopens the role of anticoagulation in CVD in the era of direct oral anticoagulants and novel factor XI/XII inhibitors, which may be crucial in preventing the final activation of the coagulation cascade, culminating in the development of arterial events both in diabetes and beyond. If validated, these findings could contribute to personalized cardiovascular care in diabetes and, similarly, in other high cardiovascular risk populations such as chronic kidney disease.
Acknowledgments: The authors acknowledge the contribution of the Northern Health patients and their families for making this research possible, along with the support of the Department of Endocrinology and the Department of General Medicine at Northern Health, Melbourne, Australia.
This work was supported by the Australian Heart Foundation with Health Professional Scholarships (R.B. and H.Y.L.), the National Health and Medical Research Council with a Postgraduate Scholarship (H.Y.L.), Melbourne Postdoctoral Fellow (H.Y.L.), and the Victoria Medical Research Acceleration Fund (with cocontribution by Northern Health).
Contribution: H.N., S.V., P.H., and H.Y.L. contributed to the concept and design of the study; H.Y.L., B.L., and R.B. contributed to patient recruitment and sample collection; R.B., M.S., P.H., H.Y.L., and R.A.A. performed data analysis and interpretation; R.B., P.H., and H.Y.L. prepared the manuscript; and all authors had critically revised the manuscript and provided approval of the final submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hui Yin Lim, Northern Health, 185 Cooper St, Epping, VIC 3076, Australia; email: huiyin.lim@nh.org.au.
References
Author notes
Original data are available on request from the corresponding author, Hui Yin Lim (huiyin.lim@nh.org.au); individual participant data will not be shared.
The full-text version of this article contains a data supplement.