Key Points
HIT diagnosis needs to be rapid and accurate.
AcuStar IgG anti-PF4/H allows detection of anti-PF4/H antibodies in <1 hour with a high diagnostic accuracy.
Visual Abstract
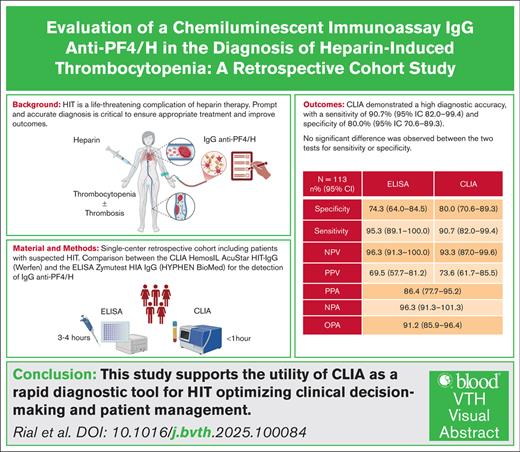
Heparin-induced thrombocytopenia (HIT) is a life-threatening complication of heparin therapy, mediated by immunoglobulin G (IgG) antibodies targeting platelet factor 4/heparin (PF4/H) complexes. Prompt and accurate diagnosis is critical to ensure appropriate treatment and improve outcomes. We aimed to evaluate the performance of the rapid chemiluminescent immunoassay (CLIA) HemosIL AcuStar HIT-IgG (Werfen) for detecting IgG anti-PF4/H antibodies for HIT diagnosis compared with the Zymutest HIA IgG (Hyphen BioMed) enzyme-linked immunosorbent assay (ELISA). This single-center retrospective cohort included all patients with suspected HIT (4Ts score >3). CLIA and ELISA were performed and compared, and results were evaluated against the serotonin-release assay. We included 113 patients with suspected HIT, and HIT was confirmed in 43 (38.1%). Discordant results occurred in 5 patients (4.4%) with confirmed HIT: 3 patients had positive ELISA and negative CLIA, one had both negative ELISA and CLIA, and one had negative ELISA and positive CLIA. CLIA demonstrated a high diagnostic accuracy, with a sensitivity of 90.7% (95% confidence interval [CI], 82.0-99.4) and specificity of 80.0% (95% CI, 70.6-89.3). ELISA showed a sensitivity of 95.3% (95% CI, 89.1-100.0) and negative predictive value of 96.3% (95% CI, 91.3-100.0) compared with 93.3% (95% CI, 87.0-99.6) for CLIA. No significant difference was observed between the 2 tests for sensitivity or specificity. Positive and negative percent agreements were 86.4% (95% CI, 77.7-95.2) and 96.3% (95% CI, 91.3-101.3), respectively. Overall percent agreement was 91.2% (95% CI, 85.9-96.4). This study supports the utility of CLIA as a rapid diagnostic tool for HIT optimizing clinical decision-making and patient management.
Introduction
Heparin-induced thrombocytopenia (HIT) is an uncommon complication of heparin therapy that results from an immune mechanism mediated by immunoglobulin G (IgG) directed against platelet 4 factor/heparin (PF4/H) complexes.1-3 HIT represents a life-threatening condition because of its high thrombotic risk under heparin therapy. Indeed, because anti-PF4/H antibodies activate monocytes, neutrophils, endothelial cells, platelets, and therefore coagulation, they are responsible for arterial and venous thromboembolism (VTE).1 Usually, thrombocytopenia occurs after 5 days from exposure to heparin; however, the onset can be earlier if reexposure to heparin occurs in the presence of circulating antibodies. The diagnosis of HIT requires multiple tools, and experienced team and laboratory. First, HIT pretest probability is estimated by the 4Ts score, which considers various biological and clinical settings, and stratifies HIT risk as low (score 1-3), intermediate (score 4-5), or high (6-8).4-6 Heparin discontinuation, its immediate replacement with a nonheparin anticoagulant, and detection of anti-PF4/H antibodies are recommended when HIT probability is intermediate or high.5,7 The risk of thrombosis in patients with acute HIT has been as high as 50% with a mortality rate of 10% to 30%.8-11 Current recommendations from the American Society of Hematology for laboratory diagnosis of patients with HIT suspicion consist of an anti-PF4/H assay and, if the clinical probability of HIT is intermediate or high with significant titer of anti-PF4 antibodies detected, a functional test should be performed to confirm HIT.7 To conclude on the pathogenic nature of the anti-PF4/H antibodies, platelet functional tests are carried out, such as the 14C-serotonin release assay (SRA),12 heparin-induced platelet activation assay,13 or platelet activation detected by flow cytometry.14 Testing for IgG specifically increases the specificity and the positive predictive value (PPV) of enzyme-linked immunosorbent assay (ELISA).15 ELISA testing usually is batched, as testing is lengthy (∼3 hours), and manual steps; accordingly, test results are often not received in a timely fashion. Adopting an automated assay with equivalent sensitivity would reduce costs and save time for the laboratory, while also increasing daily testing capacity (eg, during night shifts or weekends), thereby enabling faster result delivery to clinicians for real-time evaluation of a patient’s thrombocytopenia. More recently, the AcuStar IgG anti-PF4/H (Werfen, Bedford, MA), a rapid chemiluminescent immunoassay (CLIA), which allows the detection of IgG anti-PF4/H antibodies in <1 hour, has been developed. The aim of our study was to evaluate this new test for the diagnosis of HIT in suspected patients with a 4Ts score >3.
Materials and methods
Study design and inclusion process
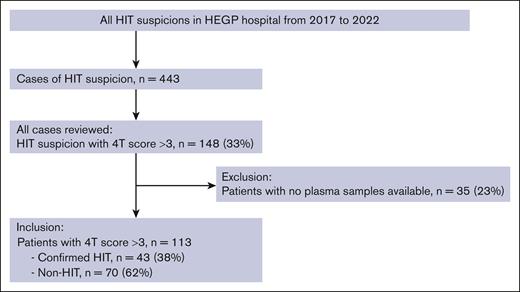
In this single-center, retrospective cohort, we enrolled all consecutive patients with HIT suspicion referred to the HIT Team at the Hôpital européen Georges Pompidou (HEGP, Assistance Publique-Hôpitaux de Paris, APHP, Paris, France) teaching hospital from 2017 to 2022. The HIT team is composed of physicians from the pharmacovigilance center and from the hematology department, and provides recommendations on the diagnosis and treatment of suspected and/or diagnosed HIT for patients from HEGP and other hospitals located in the greater Paris area, as previously described.16 Only patients with suspected HIT with a 4Ts score >3 were included in the present study, in accordance with the international recommendations of no further investigation in patients with low pretest probability. Patients with no plasma available were excluded from this study.
On the day of HIT suspicion, ELISA were systematically performed. SRA was performed later on to confirm or exclude the diagnosis of HIT. Samples were frozen and later unfrozen to perform HemosIL AcuStar HIT-IgG (CLIA, Werfen) for the purpose of this study.
Data collection
For all included patients, baseline characteristics (age, sex, body mass index, medical, and surgical history), clinical, biological, HIT treatment-related data were retrieved from the medical records. Creatinine clearance was calculated using the Cockcroft and Gault formula. D-dimer levels were measured using the Vidas D-dimers (bioMérieux, Marcy-Etoile, France). Complete blood count was performed on a Sysmex XN hematology analyzer. All data were retrospectively compiled on an electronic case report form hosted on the REDCap platform of our center.17 Patient confidentiality was ensured with anonymization of their clinical record.
HIT diagnosis strategy
For all patients with suspected HIT included in the present study, HIT diagnosis was supported by clinical and laboratory data. Criteria for HIT suspicion used by the HIT team were those of the 4Ts score.4,7,18 An intermediate or high probability for HIT (4Ts score >3)4 led to IgG anti-PF4/H antibodies testing by ELISA. As only a subset of anti-PF4/H antibodies is able to activate platelets and cause clinical HIT, a functional assay using SRA was required to confirm HIT diagnosis when antibody testing was positive,5 or in case of high probability for HIT whatever the result of ELISA (4Ts score ≥6). HIT diagnosis was confirmed based on the SRA results, and a discussion in a multidisciplinary team meeting.
Blood samples
Blood samples were routinely handled according to the current recommendations for the preanalytical phase.19 Plasma was obtained by centrifugation at 2000g to 2500g at +18°C for 15 minutes, within 2 hours after sampling. Routine coagulation tests were immediately performed, and the remaining plasma was aliquoted and stored at −40°C until testing, then thawed (5 minutes at 37°C) for ELISA and CLIA.
ELISA
All plasma samples were investigated using an ELISA (Zymutest HIA IgG Hyphen BioMed, Neuville sur Oise, France) that specifically detects IgG against PF4/H complexes. This assay was performed according to the manufacturer’s instructions, and an optical density (OD) of ≥0.50 was considered positive; weakly positive when 0.30 ≤ OD < 0.50; or negative if OD < 0.30.
CLIA
Plasma was thawed, and CLIA was performed for all patients included with available plasma. The HemosIL AcuStar HIT-IgG (Werfen) assay is a fully automated CLIA for the detection of anti-PF4/H IgG antibodies, commonly associated with HIT. The reagent mechanism consists of magnetic particles coated with a PF4 and polyvinyl sulfonate (PVS, a compound similar to heparin) complex, which has the ability to capture anti-PF4/H IgG antibodies commonly associated with HIT. The antigen-coated magnetic particles capture the anti-PF4/H antibodies, if present in the sample. An isoluminol-labeled secondary antibody in the tracer binds to the captured antibodies, resulting in an emission of light proportional to the concentration of anti-PF4/H antibodies. The signal proportional to the anti-PF4/H antibody concentration was provided within 30 minutes, and a result ≥1 U/mL was considered as positive. The AcuStar analyzer and reagent were made available free of charge by Werfen to carry out this study.
SRA
The “gold-standard” SRA was performed. This test investigates the capability of the antibodies to activate platelets in the presence of heparin. Washed platelets of selected healthy donors free from aspirin and nonsteroidal anti-inflammatory drugs for at least 10 days and known to react well in the SRA were used for the assays, which were performed according to the recommendations of the International Society on Thrombosis and Haemostasis.20 Before performing the SRA, plasma samples were heated at 56°C for 30 minutes to inactivate traces of thrombin, as recommended.21 Heat-inactivated patient plasma and increasing concentrations of unfractionated heparin (UFH) were then added to these healthy donor platelets previously incubated with radioactive 14C-serotonin. 14C-Serotonin released by activated platelets in response to the presence of anti-PF4/H antibodies was measured. A positive SRA test was defined by significant serotonin release (>30%) from donor platelets when mixed with patient plasma, and low heparin dose (0.1 and/or 0.5 U/mL) associated with inhibition of platelet activation by at least 50% from maximal activation values when high-dose heparin (100.0 U/mL) was added.22 However, if the release was over 30% in the presence of high UFH concentration, the result was considered as indeterminate. The result was considered negative if serotonin release was <20%, and indeterminate if it was between 20% and 30% using low UFH doses with at least 3 healthy platelet donors. Positive and negative control plasma were tested in parallel in each series.
Statistical analysis
Continuous variables were summarized by medians and the interquartile range (IQR, 25th-75th percentile). Categorical variables were described by counts and percentages. Associations between categorical groups were calculated using the Pearson χ2 test or Fisher exact test when appropriate. Continuous variables were compared with a Wilcoxon rank sum test and exact test, depending on distribution. Linear regression models were used to examine associations between outcomes and predictors. Receiver operating characteristic curve analysis was performed to assess the diagnostic potential of CLIA and ELISA in detecting HIT. The area under the curve (AUC) was computed, and optimal cut-off values were estimated using the Youden index. We compared the sensitivity and specificity of ELISA and CLIA using McNemar’s test to assess potential differences in diagnostic performance. In addition, we calculated the positive percentage agreement (PPA), negative percentage agreement (NPA), and overall percentage agreement (OPA) between both assays. All pertinent assumptions for the tests that were applied were checked stringently and met. All statistical analyses were performed using R version 4.1.1 revised 2021-08-10 in RStudio Software version 2021, R Core Team, version 4.0.4.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local medical ethics committee (CERAPHP.5, institutional review board registration: 00011928, no. 2022-03-15, RESTI-HOP study).
Results
Study population
Between 2017 and 2022, a total of 443 consecutive patients with HIT suspicion were referred to the HIT team of our center. Among them, 148 (33%) with a 4Ts score >3 were enrolled. Overall, 113 patients with available plasma were included in the study (Figure 1). SRA was positive for 42 patients, negative for 18 patients, and doubtful for 4 patients. After discussion in a multidisciplinary meeting, 1 HIT case with a doubtful SRA result was considered confirmed, and 3 were considered non-HIT.
The baseline characteristics of the patients are described in Table 1. Briefly, the mean age of the patients was 65 years (54-72 years), and 67 (59%) were men, with no difference between patients with confirmed HIT and non-HIT. Only body mass index was significantly higher in patients with confirmed HIT compared with non-HIT (28 vs 25 kg/m2, P = .021). Relevant medical history included valvular disease in 20 patients (18%), atrial fibrillation in 19 patients (17%), chronic kidney disease in 15 patients (13%), and pulmonary embolism or deep vein thrombosis in 11 patients (10%) and 10 patients (9%), respectively, with no difference between patients with HIT or non-HIT. At admission, antiplatelet agents were taken by 25 patients (22%) and anticoagulants by 31 patients (27%), with no difference between the 2 groups. During hospitalization, low-molecular-weight heparin (LMWH) was administered in 70 patients (62%) and UFH in 84 (74%), with UFH being more often administered in patients with confirmed HIT (66% vs 88%, P = .007).
Demographics and clinical characteristics of patients with suspected HIT
| Variable . | Total patients with HIT suspicions N = 113 . | Patients with non-HIT n = 70 . | Patients with HIT n = 43 . | P value . |
|---|---|---|---|---|
| Age, median [IQR], y | 65 [54-72] | 66 [53-72] | 65 [56-72] | .8 |
| Male sex, n (%) | 67 (59) | 41 (59) | 26 (60) | .8 |
| BMI, kg/m2 [IQR] | 26 [22-30] | 25 [22-28] | 28 [23-31] | .021 |
| Cardiovascular risk factors, n (%) | ||||
| Valvular heart disease | 20 (18) | 10 (14) | 10 (23) | .2 |
| Atrial fibrillation | 19 (17) | 13 (19) | 6 (14) | .5 |
| Chronic kidney disease | 15 (13) | 9 (13) | 6 (14) | .9 |
| Pulmonary embolism | 11 (10) | 8 (11) | 3 (7) | .5 |
| Deep venous thrombosis | 10 (9) | 7 (10) | 3 (7) | .7 |
| Acute coronary syndrome | 9 (8) | 7 (10) | 2 (5) | .5 |
| Heart failure | 6 (5) | 2 (3) | 4 (9) | .2 |
| Stroke | 7 (6) | 5 (7) | 2 (5) | .7 |
| Antithrombotic therapy at admission, n (%) | ||||
| Antiplatelet agents | .5 | |||
| Aspirin | 25 (22) | 15 (21) | 10 (23) | |
| Clopidogrel | 1 (1) | 1 (1) | 0 (0) | |
| Aspirin + clopidogrel | 4 (4) | 4 (6) | 0 (0) | |
| Therapeutic anticoagulation, n (%) | .1 | |||
| Apixaban | 9 (8) | 5 (7) | 4 (9) | |
| Rivaroxaban | 3 (3) | 2 (3) | 1 (2) | |
| Dabigatran | 1 (1) | 1 (1) | 0 (0) | |
| VKA | 5 (4) | 4 (6) | 1 (2) | |
| LMWH | 8 (7) | 8 (11) | 0 (0) | |
| UFH | 4 (4) | 4 (6) | 0 (0) | |
| Fondaparinux | 1 (1) | 1 (1) | 0 (0) | |
| Heparin therapy during hospitalization, n (%) | ||||
| LMWH | 70 (62) | 47 (67) | 23 (53) | .2 |
| UFH | 84 (74) | 46 (66) | 38 (88) | .007 |
| Variable . | Total patients with HIT suspicions N = 113 . | Patients with non-HIT n = 70 . | Patients with HIT n = 43 . | P value . |
|---|---|---|---|---|
| Age, median [IQR], y | 65 [54-72] | 66 [53-72] | 65 [56-72] | .8 |
| Male sex, n (%) | 67 (59) | 41 (59) | 26 (60) | .8 |
| BMI, kg/m2 [IQR] | 26 [22-30] | 25 [22-28] | 28 [23-31] | .021 |
| Cardiovascular risk factors, n (%) | ||||
| Valvular heart disease | 20 (18) | 10 (14) | 10 (23) | .2 |
| Atrial fibrillation | 19 (17) | 13 (19) | 6 (14) | .5 |
| Chronic kidney disease | 15 (13) | 9 (13) | 6 (14) | .9 |
| Pulmonary embolism | 11 (10) | 8 (11) | 3 (7) | .5 |
| Deep venous thrombosis | 10 (9) | 7 (10) | 3 (7) | .7 |
| Acute coronary syndrome | 9 (8) | 7 (10) | 2 (5) | .5 |
| Heart failure | 6 (5) | 2 (3) | 4 (9) | .2 |
| Stroke | 7 (6) | 5 (7) | 2 (5) | .7 |
| Antithrombotic therapy at admission, n (%) | ||||
| Antiplatelet agents | .5 | |||
| Aspirin | 25 (22) | 15 (21) | 10 (23) | |
| Clopidogrel | 1 (1) | 1 (1) | 0 (0) | |
| Aspirin + clopidogrel | 4 (4) | 4 (6) | 0 (0) | |
| Therapeutic anticoagulation, n (%) | .1 | |||
| Apixaban | 9 (8) | 5 (7) | 4 (9) | |
| Rivaroxaban | 3 (3) | 2 (3) | 1 (2) | |
| Dabigatran | 1 (1) | 1 (1) | 0 (0) | |
| VKA | 5 (4) | 4 (6) | 1 (2) | |
| LMWH | 8 (7) | 8 (11) | 0 (0) | |
| UFH | 4 (4) | 4 (6) | 0 (0) | |
| Fondaparinux | 1 (1) | 1 (1) | 0 (0) | |
| Heparin therapy during hospitalization, n (%) | ||||
| LMWH | 70 (62) | 47 (67) | 23 (53) | .2 |
| UFH | 84 (74) | 46 (66) | 38 (88) | .007 |
BMI, body mass index; LMWH: low-molecular-weight heparin; VKA, vitamin K antagonist.
HIT suspicion and diagnosis
At the time of HIT suspicion, 69 patients (61%) were treated with UFH and 44 (39%) with low-molecular-weight heparin. Among patients treated with heparin, 75 (71%) had a therapeutic dose and 30 (29%) had a prophylactic dose, with no difference between the 2 groups (Table 2). Heparin was started a median of 8 days before HIT suspicion, with a longer delay for patients with confirmed HIT (9 vs 7 days, P = .01), and 15 patients (13%) had heparin in the last 3 months before HIT suspicion, with no difference between the 2 groups. At the time of HIT suspicion, coronary artery bypass graft surgery had been performed in 30 patients (27%), extracorporeal membrane oxygenation in 9 (8%), and dialysis in 24 (21%), with coronary artery bypass graft being more frequent in patients with confirmed HIT (37% vs 20%, P = .04). Clinical manifestations of HIT suspicion including arterial thrombosis or extension of preexisting arterial thrombosis were present in 13 (12%) and 6 patients (5%), respectively, and VTE or extension of preexisting thrombosis in 29 (26%) and 7 patients (6%), respectively, with no difference between groups. Also, 7 patients (6%) had cutaneous effects with no difference between both groups.
HIT diagnosis
| Variable . | Total patients with HIT suspicions N = 113 . | Patients with non-HIT n = 70 . | Patients with HIT n = 43 . | P value . |
|---|---|---|---|---|
| Heparin therapy at HIT suspicion, n (%) | .5 | |||
| UFH | 69 (61) | 41 (59) | 28 (65) | |
| LMWH | 44 (39) | 29 (41) | 15 (35) | |
| Therapeutic anticoagulation | 75 (71) | 44 (67) | 31 (79) | .2 |
| Prophylactic anticoagulation | 30 (29) | 22 (33) | 8 (21) | |
| Delay after heparin initiation, median [IQR], d | 8.0 [6.0-12.0] | 7.0 [5.2-12.0] | 9.0 [8.0-12.0] | .01 |
| Heparin treatment in the last 3 months | 15 (13) | 12 (17) | 3 (7) | .3 |
| Clinical setting at the time of HIT suspicion, n (%) | ||||
| CABG | 30 (27) | 14 (20) | 16 (37) | .04 |
| ECMO | 9 (8) | 6 (9) | 3 (7) | 1.0 |
| ECMO thrombosis | 1 (1) | 1 (1) | 0 (0) | 1.0 |
| Dialysis | 24 (21) | 17 (24) | 7 (16) | .3 |
| Citrate | 12 (52) | 8 (50) | 4 (57) | |
| Heparin | 11 (48) | 8 (50) | 3 (43) | |
| Dialysis thrombosis | 9 (29) | 5 (21) | 4 (57) | .2 |
| Clinical manifestations at the time of HIT suspicion, n (%) | ||||
| Arterial thrombosis | 13 (12) | 7 (10) | 6 (14) | .6 |
| Extension of arterial thrombosis | 6 (5) | 3 (4) | 3 (7) | .7 |
| Venous thrombosis | 29 (26) | 18 (26) | 11 (26) | 1.0 |
| DVT | 10 (9) | 8 (11) | 2 (5) | .3 |
| PE | 10 (9) | 6 (9) | 4 (9) | 1.0 |
| Extension of preexisting thrombosis | 7 (6) | 4 (6) | 3 (7) | 1.0 |
| Cutaneous effect | 7 (6) | 3 (4) | 4 (9) | .4 |
| Biological examination at the time of HIT suspicion, median [IQR] | ||||
| Platelets (×109/L) | 83 [49-115] | 77 [53-108] | 87 [48-122] | .9 |
| Leukocytes (×109/L) | 11 [8-16] | 10 [7-15] | 13 [10-16] | .02 |
| Hemoglobin (g/L) | 94 [85-109] | 95 [85-109] | 93 [84-106] | .5 |
| PT (%) | 76 [68-85] | 76 [66-85] | 79 [68-86] | .4 |
| Hemoglobin nadir (g/L) | 82 [72-96] | 81 [72-98] | 83 [72-94] | 1.0 |
| Platelet nadir (×109/L) | 55 [34, 88] | 57 [32, 87] | 52 [36, 88] | 1.0 |
| Platelets return to normal, n (%) | 77 (73) | 43 (66) | 34 (85) | .03 |
| Time to normalize platelets (d) | 6 [3-9] | 5 [4-10] | 6 [3-8] | .3 |
| 4Ts score, n (%) | .001 | |||
| 4 and 5 | 89 (79) | 62 (89) | 27 (63) | |
| 6-8 | 24 (21) | 8 (11) | 16 (37) | |
| IgG anti-PF4/H, median [IQR], UI/mL | ||||
| ELISA | 0.60 [0.06-1.74] | 0.07 [0.04-0.49] | 1.88 [1.44-2.39] | <.001 |
| CLIA | 0.74 [0.10-15.72] | 0.13 [0.03-0.71] | 17.6 [4.18-36.9] | <.001 |
| SRA, n (%) | <.001 | |||
| Positive | 42 (66) | 0 (0) | 42 (66) | |
| Negative | 18 (28) | 18 (86) | 0 (0) | |
| Indeterminate | 4 (6) | 3 (14) | 1 (2) | |
| Variable . | Total patients with HIT suspicions N = 113 . | Patients with non-HIT n = 70 . | Patients with HIT n = 43 . | P value . |
|---|---|---|---|---|
| Heparin therapy at HIT suspicion, n (%) | .5 | |||
| UFH | 69 (61) | 41 (59) | 28 (65) | |
| LMWH | 44 (39) | 29 (41) | 15 (35) | |
| Therapeutic anticoagulation | 75 (71) | 44 (67) | 31 (79) | .2 |
| Prophylactic anticoagulation | 30 (29) | 22 (33) | 8 (21) | |
| Delay after heparin initiation, median [IQR], d | 8.0 [6.0-12.0] | 7.0 [5.2-12.0] | 9.0 [8.0-12.0] | .01 |
| Heparin treatment in the last 3 months | 15 (13) | 12 (17) | 3 (7) | .3 |
| Clinical setting at the time of HIT suspicion, n (%) | ||||
| CABG | 30 (27) | 14 (20) | 16 (37) | .04 |
| ECMO | 9 (8) | 6 (9) | 3 (7) | 1.0 |
| ECMO thrombosis | 1 (1) | 1 (1) | 0 (0) | 1.0 |
| Dialysis | 24 (21) | 17 (24) | 7 (16) | .3 |
| Citrate | 12 (52) | 8 (50) | 4 (57) | |
| Heparin | 11 (48) | 8 (50) | 3 (43) | |
| Dialysis thrombosis | 9 (29) | 5 (21) | 4 (57) | .2 |
| Clinical manifestations at the time of HIT suspicion, n (%) | ||||
| Arterial thrombosis | 13 (12) | 7 (10) | 6 (14) | .6 |
| Extension of arterial thrombosis | 6 (5) | 3 (4) | 3 (7) | .7 |
| Venous thrombosis | 29 (26) | 18 (26) | 11 (26) | 1.0 |
| DVT | 10 (9) | 8 (11) | 2 (5) | .3 |
| PE | 10 (9) | 6 (9) | 4 (9) | 1.0 |
| Extension of preexisting thrombosis | 7 (6) | 4 (6) | 3 (7) | 1.0 |
| Cutaneous effect | 7 (6) | 3 (4) | 4 (9) | .4 |
| Biological examination at the time of HIT suspicion, median [IQR] | ||||
| Platelets (×109/L) | 83 [49-115] | 77 [53-108] | 87 [48-122] | .9 |
| Leukocytes (×109/L) | 11 [8-16] | 10 [7-15] | 13 [10-16] | .02 |
| Hemoglobin (g/L) | 94 [85-109] | 95 [85-109] | 93 [84-106] | .5 |
| PT (%) | 76 [68-85] | 76 [66-85] | 79 [68-86] | .4 |
| Hemoglobin nadir (g/L) | 82 [72-96] | 81 [72-98] | 83 [72-94] | 1.0 |
| Platelet nadir (×109/L) | 55 [34, 88] | 57 [32, 87] | 52 [36, 88] | 1.0 |
| Platelets return to normal, n (%) | 77 (73) | 43 (66) | 34 (85) | .03 |
| Time to normalize platelets (d) | 6 [3-9] | 5 [4-10] | 6 [3-8] | .3 |
| 4Ts score, n (%) | .001 | |||
| 4 and 5 | 89 (79) | 62 (89) | 27 (63) | |
| 6-8 | 24 (21) | 8 (11) | 16 (37) | |
| IgG anti-PF4/H, median [IQR], UI/mL | ||||
| ELISA | 0.60 [0.06-1.74] | 0.07 [0.04-0.49] | 1.88 [1.44-2.39] | <.001 |
| CLIA | 0.74 [0.10-15.72] | 0.13 [0.03-0.71] | 17.6 [4.18-36.9] | <.001 |
| SRA, n (%) | <.001 | |||
| Positive | 42 (66) | 0 (0) | 42 (66) | |
| Negative | 18 (28) | 18 (86) | 0 (0) | |
| Indeterminate | 4 (6) | 3 (14) | 1 (2) | |
CABG, coronary artery bypass graft; DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; LMWH, low-molecular-weight heparin; PE, pulmonary embolism; PT, prothrombin time.
Patients with HIT and non-HIT were compared (P value α = 5%).
Concerning biological criteria at the time of HIT suspicion, the median platelet count was 83 × 109/L (IQR, 49-115), and the hemoglobin level was 94 g/L (IQR, 85-109), with no significant difference between the 2 groups. The median leukocyte count was 11 × 109/L (IQR, 8-16), and was significantly higher in the HIT-confirmed group (13 [IQR, 10-16] vs 10 [IQR, 7-15]; P = .02) compared with the non-HIT group. The platelet nadir was 55 × 109/L (IQR, 34-88) and the hemoglobin nadir was 82 g/L (IQR, 72-96), with no difference between the 2 groups. Platelets returned to normal in 77 patients (73%) and more often in the HIT-confirmed group (85% vs 66%, P = .03). The median time to normalize platelet count was 6 days (IQR, 3-9), with no difference between the 2 groups. The 4Ts score was more often of intermediate probability (4-5) in the non-HIT group (89% vs 63%, P = .001), and was more often high-risk (6-8) in the HIT-confirmed group (37% vs 11%, P = .001).
Comparison of ELISA and CLIA results
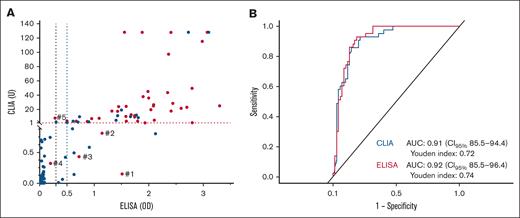
Median anti-PF4/H levels were 0.6 (IQR, 0.06-1.74) for ELISA and 0.74 (IQR, 0.10-15.72) for CLIA, and both tests had higher levels in the HIT group (P < .001). Results of the detection and quantification of IgG anti-PF4/H with both techniques for all patients are represented in Figure 2A. There were discordant results between ELISA and/or CLIA for 5 patients (4.4%) with confirmed HIT. Among them, 3 patients had positive ELISA and negative CLIA (patients 1, 2, and 3), 1 patient had both negative ELISA and CLIA (patient 4), and 1 patient had negative ELISA and positive CLIA (patient 5). Characteristics of these discordant patients are described in Table 3. All 5 of these patients were treated by UFH at the time of HIT suspicion, and no patients had clinical complications of HIT. Only 1 patient had renal insufficiency and dialysis, and 3 patients were anticoagulated for heart valve surgery.
Detection and quantification of IgG anti-PF4/H using CLIA and ELISA in patients with suspected and confirmed HIT. (A) Optical density (OD) results of both IgG anti-PF4/H assays according to HIT diagnosis (red dot) or no HIT diagnosis (blue dot). The red dashed line represents the positive threshold (OD > 1 U) for CLIA, and the black dashed line represents the positive threshold (OD > 0.5) for ELISA. There were discordant results between ELISA and/or CLIA for 5 patients with confirmed HIT (patients 1, 2, 3, 4, and 5). Characteristics of these discordant patients are described in Table 3. (B) AUC representing the performance of both tests in diagnosing HIT.
Detection and quantification of IgG anti-PF4/H using CLIA and ELISA in patients with suspected and confirmed HIT. (A) Optical density (OD) results of both IgG anti-PF4/H assays according to HIT diagnosis (red dot) or no HIT diagnosis (blue dot). The red dashed line represents the positive threshold (OD > 1 U) for CLIA, and the black dashed line represents the positive threshold (OD > 0.5) for ELISA. There were discordant results between ELISA and/or CLIA for 5 patients with confirmed HIT (patients 1, 2, 3, 4, and 5). Characteristics of these discordant patients are described in Table 3. (B) AUC representing the performance of both tests in diagnosing HIT.
Characteristics of patients with discordant ELISA and CLIA results
| Patient no. . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Age, y | 75 | 66 | 63 | 71 | 56 |
| Sex | M | F | M | F | M |
| BMI, kg/m2 | 37 | 30 | 23 | 25 | 28 |
| ECMO | No | No | No | No | No |
| Renal function | |||||
| CrCl, mL/min | 115 | 44 | 127 | 80 | 111 |
| Dialysis at the time of HIT diagnosis | No | Yes | No | No | No |
| Heparin therapy | |||||
| Indication | Heart valve surgery and AF | Heart valve surgery | Aortic thrombosis | DVT | Heart valve surgery |
| Type | UFH | UFH | UFH | UFH | UFH |
| HIT diagnosis | |||||
| 4Ts score | 5 | 6 | 5 | 7 | 4 |
| Delay since heparin initiation, d | 7 | 10 | 10 | 6 | 15 |
| HITT | None | Extension of preexisting thrombosis (jugular vein) + dialysis filter thrombosis | None | Extension of preexisting thrombosis | None |
| IgG PF4/H (OD)∗ ELISA | 1.513 | 1.145 | 0.722 | 0.199 | 0.283 |
| IgG PF4/H (OD)† CLIA | 0.15 | 0.81 | 0.43 | 0.32 | 7.57 |
| SRA (%) | |||||
| Low-dose UFH | 97 | 31 | 53 | 80 | 74 |
| High-dose UFH | 15 | 1 | 4 | 0 | 0 |
| Platelet count nadir, ×109/L | 121 | 58 | 118 | 52 | 101 |
| Parenteral anticoagulant switch after stopping heparin | |||||
| Type, dose, and duration | IV danaparoid bolus 1000 U; then 300/h, 4 h, 150/h, 2 h; then VKA | IV argatroban 0.5 μg/kg/min, 10 d, then VKA | IV danaparoid bolus 2500 U; then, 300/h, 4 h; 150/h, 4 d, then fondaparinux | IV danaparoid bolus 2500 U; then 400/h, 4 h, 300/h, 4 h; 150/h, 17 d, then fondaparinux | None |
| Anticoagulant therapy | |||||
| Platelet count at anticoagulant introduction, ×109/L | 298 | 174 | 280 | 358 | — |
| Anticoagulant type, dose, duration | VKA, fluindione 10 mg, NA | VKA, warfarin 4 mg, long-term for AF | Fondaparinux, 7.5 mg, 90 d | Fondaparinux, 7.5 mg, 40 d, then factor Xa inhibitor, rivaroxaban 15 mg, long-term for active cancer | — |
| Follow-up after heparin discontinuation | |||||
| Time to platelet recovery, d | 3 | 6 | 2 | No recovery | 11 |
| Outcome events‡ | None | None | None | None | None |
| Time of last follow-up after anticoagulant introduction, mo | 0 | 5 | 2 | — | 14 |
| Patient no. . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Age, y | 75 | 66 | 63 | 71 | 56 |
| Sex | M | F | M | F | M |
| BMI, kg/m2 | 37 | 30 | 23 | 25 | 28 |
| ECMO | No | No | No | No | No |
| Renal function | |||||
| CrCl, mL/min | 115 | 44 | 127 | 80 | 111 |
| Dialysis at the time of HIT diagnosis | No | Yes | No | No | No |
| Heparin therapy | |||||
| Indication | Heart valve surgery and AF | Heart valve surgery | Aortic thrombosis | DVT | Heart valve surgery |
| Type | UFH | UFH | UFH | UFH | UFH |
| HIT diagnosis | |||||
| 4Ts score | 5 | 6 | 5 | 7 | 4 |
| Delay since heparin initiation, d | 7 | 10 | 10 | 6 | 15 |
| HITT | None | Extension of preexisting thrombosis (jugular vein) + dialysis filter thrombosis | None | Extension of preexisting thrombosis | None |
| IgG PF4/H (OD)∗ ELISA | 1.513 | 1.145 | 0.722 | 0.199 | 0.283 |
| IgG PF4/H (OD)† CLIA | 0.15 | 0.81 | 0.43 | 0.32 | 7.57 |
| SRA (%) | |||||
| Low-dose UFH | 97 | 31 | 53 | 80 | 74 |
| High-dose UFH | 15 | 1 | 4 | 0 | 0 |
| Platelet count nadir, ×109/L | 121 | 58 | 118 | 52 | 101 |
| Parenteral anticoagulant switch after stopping heparin | |||||
| Type, dose, and duration | IV danaparoid bolus 1000 U; then 300/h, 4 h, 150/h, 2 h; then VKA | IV argatroban 0.5 μg/kg/min, 10 d, then VKA | IV danaparoid bolus 2500 U; then, 300/h, 4 h; 150/h, 4 d, then fondaparinux | IV danaparoid bolus 2500 U; then 400/h, 4 h, 300/h, 4 h; 150/h, 17 d, then fondaparinux | None |
| Anticoagulant therapy | |||||
| Platelet count at anticoagulant introduction, ×109/L | 298 | 174 | 280 | 358 | — |
| Anticoagulant type, dose, duration | VKA, fluindione 10 mg, NA | VKA, warfarin 4 mg, long-term for AF | Fondaparinux, 7.5 mg, 90 d | Fondaparinux, 7.5 mg, 40 d, then factor Xa inhibitor, rivaroxaban 15 mg, long-term for active cancer | — |
| Follow-up after heparin discontinuation | |||||
| Time to platelet recovery, d | 3 | 6 | 2 | No recovery | 11 |
| Outcome events‡ | None | None | None | None | None |
| Time of last follow-up after anticoagulant introduction, mo | 0 | 5 | 2 | — | 14 |
AF, atrial fibrillation; BMI, body mass index; CrCl, creatinine clearance evaluated with Cockcroft and Gault formula; DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; F, female; HITT, heparin-induced thrombocytopenia-related thrombosis; M, male; NA, not available; VKA, vitamin K antagonist.
Positive threshold OD > 0.5.
Positive threshold OD > 1.
Thrombosis, major bleeding, clinically relevant nonmajor bleeding or death.
Specificity, sensitivity, negative predictive value (NPV), and PPV of ELISA and CLIA were calculated, and are presented in Table 4. With the threshold recommended, 0.5 for ELISA and 1.0 for CLIA, the specificity of ELISA was 74.3% (95% confidence interval [CI], 64.0-84.5), the sensitivity was 95.3% (95% CI, 89.1-100.0), the NPV was 96.3% (95% CI, 91.3-100.0), and the PPV was 69.5% (95% CI, 57.7-81.2). For the CLIA test, the specificity was 80.0% (95% CI, 70.6-89.3), the sensitivity was 90.7% (95% CI, 82.0-99.4), the NPV was 93.3% (95% CI, 87.0-99.6), and the PPV was 73.6% (95% CI, 61.7-85.5).
Test diagnostic performance
| Parameter (N = 113) . | ELISA Positive threshold: 0.5 . | CLIA Positive threshold: 1 . | ELISA Positive threshold: 0.3 . | CLIA Positive threshold: 0.8 . |
|---|---|---|---|---|
| Specificity, TN/(TN + FP), % (95% CI) | 74.3 (64.0-84.5) | 80.0 (70.6-89.3) | 68.6 (57.7-79.4) | 77.1 (67.3-87.0) |
| Sensitivity, TP/(TP + FN), % (95% CI) | 95.3 (89.1-100.0) | 90.7 (82.0-99.4) | 95.3 (89.1-100.0) | 93.0 (85.4-100.0) |
| NPV, % (95% CI) | 96.3 (91.3-100.0) | 93.3 (87.0-99.6) | 96.0 (90.6-100.0) | 94.7 (88.9-100.0) |
| PPV, % (95% CI) | 69.5 (57.7-81.2) | 73.6 (61.7-85.5) | 65.1 (53.3-76.9) | 71.4 (59.6-83.3) |
| TN, n | 52 | 56 | 48 | 54 |
| FP, n | 18 | 14 | 22 | 16 |
| TP, n | 41 | 39 | 41 | 40 |
| FN, n | 2 | 4 | 2 | 3 |
| Parameter (N = 113) . | ELISA Positive threshold: 0.5 . | CLIA Positive threshold: 1 . | ELISA Positive threshold: 0.3 . | CLIA Positive threshold: 0.8 . |
|---|---|---|---|---|
| Specificity, TN/(TN + FP), % (95% CI) | 74.3 (64.0-84.5) | 80.0 (70.6-89.3) | 68.6 (57.7-79.4) | 77.1 (67.3-87.0) |
| Sensitivity, TP/(TP + FN), % (95% CI) | 95.3 (89.1-100.0) | 90.7 (82.0-99.4) | 95.3 (89.1-100.0) | 93.0 (85.4-100.0) |
| NPV, % (95% CI) | 96.3 (91.3-100.0) | 93.3 (87.0-99.6) | 96.0 (90.6-100.0) | 94.7 (88.9-100.0) |
| PPV, % (95% CI) | 69.5 (57.7-81.2) | 73.6 (61.7-85.5) | 65.1 (53.3-76.9) | 71.4 (59.6-83.3) |
| TN, n | 52 | 56 | 48 | 54 |
| FP, n | 18 | 14 | 22 | 16 |
| TP, n | 41 | 39 | 41 | 40 |
| FN, n | 2 | 4 | 2 | 3 |
FN, false negative; FP, false positive; TN, true negative; TP, true positive.
With a lower threshold of 0.3 for ELISA described by the manufacturer as weakly positive, and 0.8 for CLIA, as suggested by Jousselme et al,23 ELISA specificity was 68.6% (95% CI, 57.7-79.4) and CLIA 77.1% (95% CI, 67.3-87.0), and ELISA sensitivity was 95.3% (95% CI, 89.1-100.0) and CLIA 93.0% (95% CI, 85.4-100.0). ELISA NPV was 96.0% (95% CI, 90.6-100.0) and 94.7% (95% CI, 88.9-100.0) for CLIA, and ELISA PPV was 65.1% (95% CI, 53.3-76.9) and 71.4% (95% CI, 59.6-83.3) for CLIA.
Next, we compared the sensitivity and specificity of ELISA and CLIA to assess potential differences in diagnostic performance, using the thresholds recommended by the manufacturers. No statistically significant difference was observed between the 2 tests for either sensitivity (P = .63) or specificity (P = .22). We then calculated the PPA (86.4% [95% CI, 77.7-95.2]), NPA (96.3% [95% CI, 91.3-101.3]), and OPA (91.2% [95% CI, 85.9-96.4]), which showed strong overall agreement between the 2 assays (Table 5). Finally, receiver operating characteristic curves representing the performance of both tests for HIT diagnosis are shown in Figure 2B. Both tests had a good discrimination, as AUC values were >0.9 (0.92 [95% CI, 0.85-0.94] for ELISA vs 0.91 [95% CI, 0.85-0.96] for CLIA). The Youden indexes of both assays were similar, with 0.72 for CLIA and 0.74 for ELISA, indicating a strong balance between sensitivity and specificity.
Agreement between ELISA and CLIA results
| Assay . | ELISA . | CLIA . |
|---|---|---|
| Reference | Zymutest HIA IgG (Hyphen BioMed) | HemosIL AcuStar HIT-IgG (Werfen) |
| PPA, % (95% CI) | 86.4 (77.7-95.2) | |
| NPA, % (95% CI) | 96.3 (91.3-101.3) | |
| OPA, % (95% CI) | 91.2 (85.9-96.4) | |
| Assay . | ELISA . | CLIA . |
|---|---|---|
| Reference | Zymutest HIA IgG (Hyphen BioMed) | HemosIL AcuStar HIT-IgG (Werfen) |
| PPA, % (95% CI) | 86.4 (77.7-95.2) | |
| NPA, % (95% CI) | 96.3 (91.3-101.3) | |
| OPA, % (95% CI) | 91.2 (85.9-96.4) | |
NPA, negative percent agreement; OPA, overall percent agreement; PPA, positive percent agreement.
Discussion
Because HIT is a life- and limb-threatening complication of heparin exposure, it requires a rapid and accurate diagnostic approach24 to guide clinical management of patients with suspected HIT, and accurately identify those who will need to be switched to nonheparin anticoagulants, such as argatroban or danaparoid.25,26 Indeed, false-negative tests lead to an increased risk of thrombosis due to the lack of management of HIT, while false-positive tests result in a hemorrhagic risk related to the use of anticoagulants that are less manageable and less familiar to health care services, as well as an additional economic burden due to the higher cost of these medications.1
PF4/H or PF4/PVS ELISAs are preferentially tailored for testing multiple samples in batches rather than single patient samples. CLIA is an attractive rapid immunoassay that can be performed 24 hours a day, is automated, and delivers rapid results. Additionally, the reagents remain stable for up to 6 weeks, and centrifuged tubes can either be loaded directly into the machine or processed as aliquots.
This single-center retrospective study showed good reproducibility as CLIA and ELISA results were equivalent for most patients (38/43, 88%), with discordant results for 5 patients with confirmed HIT. Among them, 3 patients had positive ELISA and negative CLIA, 1 patient had both negative ELISA and CLIA, and 1 patient had negative ELISA and positive CLIA. Both assays demonstrated strong sensitivity and specificity. ELISA showed a specificity of 74.3% and a sensitivity of 95.3%, while CLIA showed a specificity of 80.0% and a sensitivity of 90.7%, using the manufacturers’ recommended positive thresholds. No significant difference in sensitivity or specificity was observed between the 2 assays. We observed a strong overall agreement between CLIA and ELISA. The laboratory commercializing CLIA described an excellent agreement (91%) with the SRA. In the literature, several studies described HemosIL AcuStar HIT-IgG CLIA’s performances to diagnose HIT. A study from Marashi-Sabouni et al27 evaluated the diagnostic strategy that combines the 4Ts score with CLIA to confirm the diagnosis of HIT. HIT diagnosis was confirmed with functional assay (SRA or a platelet aggregation assay) or after case review in rare cases. In total, 1300 samples of consecutive patients were enrolled, 94 (7.2%) of which gave positive results in HemosIL AcuStar-IgG. HIT diagnosis was confirmed using functional tests for most patients, with 65 confirmed with HIT. The calculated sensitivity and specificity for patients with a 4Ts score >3, like in our study, were 95.1 (95% CI, 86.3-99.0) and 50.0% (95% CI, 30.6-69.4), respectively. The highest values of the HemosIL AcuStar HIT-IgG were associated with an increased probability of HIT, and diagnostic specificity was significantly increased using the combination of the 4Ts score >3 and a positive titer ≥3.25 U/mL (sensitivity = 78.5% and specificity = 75.9%). In this study, a strong positive titer (>12.40 U/mL) confirmed the diagnosis of HIT (with a specificity of 100%), but patients with negative antibodies (<1.0 U/mL) were not tested with a functional test, apart from 15 who had high probability 4Ts scores, explaining the high sensitivity and NPVs at 100.0%. Another limit is the lack of comparison with a standard ELISA. Jousselme et al23 conducted a prospective study to evaluate the performance of CLIA by comparing it with an ELISA detecting IgG PF4/PVS. A total of 229 patients with suspected HIT were enrolled, excluding 45 patients with a 4Ts score ≤3. Among the remaining 184 patients with suspected HIT tested with CLIA and ELISA, 29 were confirmed by SRA. Although CLIA was negative in 2 patients with confirmed HIT, it demonstrated high sensitivity (93.1% [95% CI, 78.0-98.8]) and specificity (94.2% [95% CI, 89.3-96.0]). ELISA showed higher sensitivity of 100.0% (95% CI, 88.3-100.0) and lower specificity of 89.7% (95% CI, 83.9-93.6). It is important to note that the ELISA used in this study did not recognize the same target antigen as the ELISA used in our study (ie, anti-PF4/PVS vs protamine/heparin/PF4), but both successfully detected antibodies in vaccine-induced immune thrombocytopenia and thrombosis and HIT in a United Kingdom national external quality assessment, but not the CLIA, which detected only HIT antibodies.28
However, considering the need for a rapid and accurate diagnostic approach24 to guide the clinical management of patients with suspected HIT, the STic Expert HIT assay (Diagnostica Stago, Asnières-sur-Seine, France) is a rapid immunoassay commonly used in clinical laboratories for the detection of IgG anti-PF4/H antibodies. The test provides results in ∼30 minutes, making the assay suitable for urgent clinical decisions, similar to CLIA due to its high NPV (99.6%), but positive results should be confirmed by ELISA because of low PPV (44.4%).29 CLIA can be valuable for rapid HIT screening to “rule out” HIT, particularly in scenarios where timely clinical intervention is critical. Furthermore, CLIA can be incorporated into diagnostic algorithms for HIT, such as the Lausanne algorithm.30 This algorithm, based on the Bayesian application of the 4Ts score and 2 sequential rapid anti-PF4/H antibody immunoassays, can predict or exclude HIT in over 95% of patients with a laboratory turnaround time of ≤1 hour.30,31
We acknowledge several limitations in our study. First, the retrospective design may introduce inherent biases. Second, SRA was not performed for all patients, raising the possibility that a case of HIT may have been missed due to negative results from both ELISA and CLIA. Only 1 patient (patient 5) was tested retrospectively by SRA following positive CLIA results. At the time of the suspicion, the patient was managed as non-HIT without any unfavorable outcome. Third, in accordance with French legislation, we acknowledge that the absence of race/ethnicity data is a limitation, as sociocultural factors may influence health outcomes, including thrombotic disorders. This limitation may impact the generalizability of our findings to more diverse populations.
In conclusion, CLIA offers a rapid exclusion of HIT in patients with intermediate or high clinical probability, demonstrating strong overall agreement with ELISA. This study supports the utility of CLIA as a rapid diagnostic tool for HIT optimizing clinical decision-making and patient management.
Acknowledgments
The authors acknowledge all nurses, technicians, and physicians involved in the departments of the Georges Pompidou European Hospital for their help in taking care of patients and including them in the study. The visual abstract was created with BioRender.com (Gendron N. [2025]). The authors thank Werfen for the free loan of the AcuStar analyzer and the reagents.
Authorship
Contribution: C.R., N.G., S.S., and D.H. collected data, analyzed data, and wrote the manuscript; C.R., N.G., A.S., and M.L. contributed to data collection; C.R., N.G., S.S., C.L.B., L.M., R.N., J.-L.D., A.G., A.L.-L.L., D.M.S., and D.H. contributed to data analysis and manuscript revision; and all other authors provided clinical or biological data and reviewed the manuscript.
Conflict-of-interest disclosure: N.G. received consulting fees or travel awards from Stago and Werfen. A.G. received speaker fees from Aguettant, Bristol Myers Squibb–Pfizer, Sanofi, CSL Behring, LFB, Octapharma, Stago, and Viatris. The remaining authors declare no competing financial interests.
Correspondence: Nicolas Gendron, Hematology Department, Hôpital Européen Georges-Pompidou, 20 Rue Leblanc, 75015 Paris, France; email: nicolas.gendron@aphp.fr.
References
Author notes
C.R. and N.G. are joint first authors.
The data that support the findings of this study are available on reasonable request from the corresponding author, Nicolas Gendron (nicolas.gendron@aphp.fr).
Presented as a poster at the Congress of the International Society on Thrombosis and Haemostasis, Washington, DC, 21 June 2025.