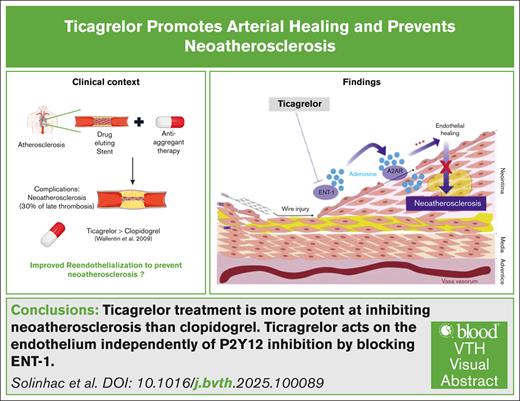
Visual Abstract
TO THE EDITOR:
The development of drug-eluting stents (DES) has revolutionized interventional cardiology over the last 20 years. The delivering of antiproliferative compounds by these devices resulted in considerably reduced incidence of stent restenosis.1 Nevertheless, the introduction of DES is accompanied by increased rates of late thrombosis as well as earlier and more frequent development of neoatherosclerosis compared to bare metal stents.2 It is now well established that neoatherosclerosis is the second leading cause of late stent thrombosis in the newer generation of DES, contributing to late stent failure.3 The PRESTIGE (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort) Consortium clearly incriminated neoatherosclerosis in >30% of the cases of very-late stent thrombosis4,5 likely due to delayed endothelial healing and functions.2,6,7 These data indicate that promoting better reendothelialization and vascular function is a promising therapeutic strategy to circumvent complications that arise following stent placement. However, the main treatment used in patients with DES is clopidogrel, a P2Y12 inhibitor that efficiently inhibits platelet aggregation, without any known beneficial effect on the endothelium. Recently, ticagrelor, a potent inhibitor directly acting on and reversibly binding the P2Y12 receptor, arose as a particularly interesting alternative to clopidogrel. This compound is currently recommended for the prevention of atherothrombotic events in acute coronary syndromes and in patients with coronary artery diseases.8 According to the PLATO (Platelet Inhibition and Patient Outcomes) study, ticagrelor significantly reduced both the total and cardiovascular mortalities compared to clopidogrel, highlighting its potential in cardiovascular disease prevention.9 van der Hoeven et al found that ticagrelor improved endothelial function more efficiently than prasugrel in patients with ST-elevation myocardial infarction after 1 year.10 In addition, in diabetic patients with prior acute coronary syndromes, ticagrelor showed superior effects on peripheral arterial function over clopidogrel and prasugrel.11 These findings suggest that the benefits of ticagrelor upon vascular function are P2Y12-independent. Nevertheless, the mechanisms underlying this beneficial effect have yet to be established.
To investigate ticagrelor's impact on vascular function, we have used several in vivo and ex vivo approaches to mimic endothelial injury in different contexts. First, we used an in vivo model of carotid injury in wild-type mice (Envigo, Harlan Laboratories). Animals were force-fed morning and evening for 5 days, either with water (group control), clopidogrel, or ticagrelor (AZ11939728-032; AstraZeneca).12 Additionally, a wire-induced endovascular injury of the carotid artery 12 performed in high cholesterol diet–fed male LDLR–/– mice (Jackson Laboratory) was used to emulate neoatherosclerosis. Carotid arteries were embedded in optimal cutting temperature compound, frozen for cross-section preparation and stained with Nile red (lipids) and DAPI (4′,6-diamidino-2-phenylindole) (5 μg/mL). For all experiments, 7- to 10-week-old mice were used. Ticagrelor concentration measurement and tail bleeding time were measured as described previously.13,14
For ex vivo experiments, carotid arteries were incubated with control medium or medium containing CGS21680.
For in vivo and ex vivo reendothelialization analysis, carotid arteries were stained with vascular endothelial (VE)-cadherin antibody (clone 11D4.1; BD Pharmingen) and Evans blue (sc-203736; Santa Cruz) as previously described.12 Images were acquired by confocal microscopy using a Zeiss LSM780 microscope equipped with a PLAN APO 20× objective (numerical aperture, 0.8). Quantification of deendothelialized areas was analyzed using ImageJ software. Briefly, the VE-cadherin–negative area was manually drawn and reported on the corresponding Evans blue staining image to have an acute visualization of the deendothelialized areas.
Finally, endothelial cell migration was evaluated by scratch assays using HUAEC cells. The wounded cells were incubated with medium containing vehicle or CSG21680 at 1 μM. Cell migration was then followed using a video microscope for 24 hours (Cell Observer; Zeiss) and maintained at 37°C. Images were acquired using the Zen software (Zeiss) every 30 minutes. Migration speed was calculated using ImageJ.
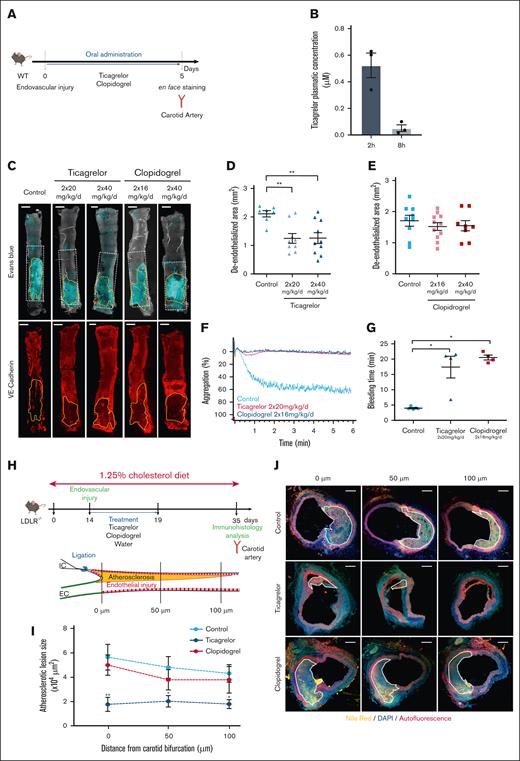
To interrogate the effect of ticagrelor vs clopidogrel on endothelial recovery, we used a mouse model of endovascular injury of the carotid artery12 (Figure 1A). The plasmatic concentration of ticagrelor 2 hours after oral administration was 0.52 ± 0.09 μM, whereas it dropped to only 0.05 ± 0.02 μM after 8 hours, demonstrating a rapid decay of the compound in circulating blood (Figure 1B). On the basis of these observations, all the following experiments were performed in mice treated with ticagrelor twice daily. Treatment of mice with 2 different doses of ticagrelor (20 and 40 mg/kg) resulted in a significant decrease in the unhealed area, as attested by Evans blue and VE-cadherin staining analyzed by confocal microscopy, as compared to placebo-treated mice (Figure 1C-D). Unlike ticagrelor, clopidogrel did not achieve the same beneficial effect on endothelial recovery, even at the highest dose (Figure 1C,E), while both treatments led to a total inhibition of adenosine 5′-diphosphate (ADP)–induced platelet aggregation and increased bleeding time, as expected (Figure 1F-G). To investigate the possible long-term benefits of ticagrelor on the endothelium, we assessed the impact of the 2 treatments on the development of neoatherosclerosis in a new mouse model. Neoatherosclerosis was induced by submitting LDLR–/– mice fed an atherogenic diet to an endothelial injury of the carotid artery,12 mimicking angioplasty in patients.12 Twenty-one days after the surgery, the injured carotid arteries showed the development of an atherosclerotic plaque at the site of the lesion. Mice were treated daily for a short time corresponding to the arterial healing phase (from day 1 to day 5 after surgery) with either ticagrelor, clopidogrel, or vehicle (Figure 1H). Twenty-one days after the surgery, ticagrelor, but not clopidogrel, treatment significantly decreased the size of the atheromatous lesion at all distances measured from the carotid bifurcation (Figure 1I-J). Altogether, these results indicate that ticagrelor displays a specific beneficial effect on neoatherosclerosis after arterial injury and confirm the inverse correlation between endothelial healing and neoatherosclerosis.
Ticagrelor but not clopidogrel increases endothelial healing and prevents neoatherosclerosis. (A) Protocol used to compare the effects of ticagrelor and clopidogrel effect on endothelial healing after vascular injury. The endovascular injury was performed in carotid arteries of wild-type (WT) mice, followed by administration of water (control), oral ticagrelor (20 mg/kg), or clopidogrel (16 mg/kg or 40 mg/kg) twice a day for 5 days. Carotid artery endothelial healing was then analyzed by en face staining. (B) Ticagrelor plasmatic concentration in mice 2 hours and 8 hours after administration. (C-E) Representative confocal images (C) and quantification of deendothelialized area of en face carotid arteries (E) stained with Evans blue or anti–VE-cadherin from the indicated treatments. Scale bar: 0.5 mm. The zone outlined in white represents the initial deendothelialized area, and the zone outlined in yellow represents the area that has not been recovered with endothelial cells. (F) Platelet aggregation of WT mice treated with ticagrelor and clopidogrel following ADP stimulation (10 μM). (G) Quantification of tail bleeding time of mice treated with vehicle (control), ticagrelor (n = 4), or clopidogrel (n = 4). (H) Protocol used to investigate ticagrelor impact on atherosclerosis development after vascular injury in vivo. Seven-week-old LDLR–/– mice were fed a 1.25% cholesterol diet for 35 days. Endovascular injury was performed in carotid arteries on day 14 and then treated by oral administration of ticagrelor (20 mg/kg) or clopidogrel (16 mg/kg) twice a day for 5 days (upper panel). Atherosclerotic plaque development was then analyzed by immunohistochemistry on day 35 as schematized in the lower panel. (I) Atherosclerotic lesions were measured on histological sections located 0, 50, and 100 μm from the carotid bifurcation as schematized in panel H. (J) Representative confocal images of atherosclerosis stained with Nile red (yellow) and DAPI (blue) (scale bar: 50 μm). Data are expressed as means ± standard error of the mean (SEM) and compared using an analysis of variance (ANOVA) test followed by Bonferroni’s post hoc test. Values of P <.05 were considered statistically significant.
Ticagrelor but not clopidogrel increases endothelial healing and prevents neoatherosclerosis. (A) Protocol used to compare the effects of ticagrelor and clopidogrel effect on endothelial healing after vascular injury. The endovascular injury was performed in carotid arteries of wild-type (WT) mice, followed by administration of water (control), oral ticagrelor (20 mg/kg), or clopidogrel (16 mg/kg or 40 mg/kg) twice a day for 5 days. Carotid artery endothelial healing was then analyzed by en face staining. (B) Ticagrelor plasmatic concentration in mice 2 hours and 8 hours after administration. (C-E) Representative confocal images (C) and quantification of deendothelialized area of en face carotid arteries (E) stained with Evans blue or anti–VE-cadherin from the indicated treatments. Scale bar: 0.5 mm. The zone outlined in white represents the initial deendothelialized area, and the zone outlined in yellow represents the area that has not been recovered with endothelial cells. (F) Platelet aggregation of WT mice treated with ticagrelor and clopidogrel following ADP stimulation (10 μM). (G) Quantification of tail bleeding time of mice treated with vehicle (control), ticagrelor (n = 4), or clopidogrel (n = 4). (H) Protocol used to investigate ticagrelor impact on atherosclerosis development after vascular injury in vivo. Seven-week-old LDLR–/– mice were fed a 1.25% cholesterol diet for 35 days. Endovascular injury was performed in carotid arteries on day 14 and then treated by oral administration of ticagrelor (20 mg/kg) or clopidogrel (16 mg/kg) twice a day for 5 days (upper panel). Atherosclerotic plaque development was then analyzed by immunohistochemistry on day 35 as schematized in the lower panel. (I) Atherosclerotic lesions were measured on histological sections located 0, 50, and 100 μm from the carotid bifurcation as schematized in panel H. (J) Representative confocal images of atherosclerosis stained with Nile red (yellow) and DAPI (blue) (scale bar: 50 μm). Data are expressed as means ± standard error of the mean (SEM) and compared using an analysis of variance (ANOVA) test followed by Bonferroni’s post hoc test. Values of P <.05 were considered statistically significant.
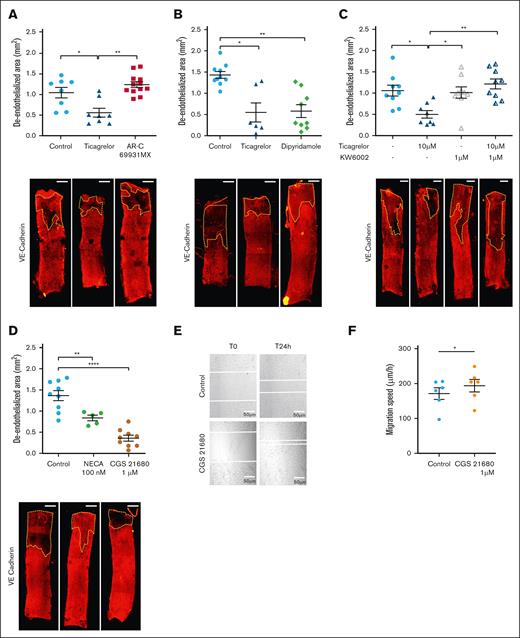
To test whether the inhibition of platelet aggregation was involved in the increased arterial healing in ticagrelor-treated mice, we used an ex vivo model of arterial injury independent from the effect of platelets and other circulating cells.12 Injured carotid arteries from mice were placed in endothelium-specific medium with or without ticagrelor for 3 days. Ticagrelor treatment significantly increased ex vivo reendothelialization as attested by VE-cadherin staining, indicating that ticagrelor-induced arterial healing is due to direct effects on the vascular wall and is independent of circulating blood cells (Figure 2A). To address the involvement of vascular P2Y12 in arterial healing, ex vivo injured arteries were incubated with AR-C69931MX (also called cangrelor), a potent in vitro inhibitor of P2Y12.15 AR-C69931MX did not modify arterial healing compared to control, indicating that P2Y12 is not involved (Figure 2A). Altogether, these experimental data indicate that ticagrelor accelerates arterial healing through platelet- and P2Y12-independent mechanisms. Thus, we explored the alternative underlying molecular mechanisms. ENT1 is an adenosine transporter whose activity is inhibited by ticagrelor without being affected by other P2Y12 inhibitors such as AR-C69931MX.16 This transporter is ubiquitously expressed, in particular on the surface of arterial endothelial cells. To evaluate ENT1 involvement in endothelial healing, we used dipyridamole, a molecule able to directly inhibit ENT1 transporter at the dose of 10 μM.17 Endothelial healing was similar in ex vivo injured arteries incubated with dipyridamole or ticagrelor, leading to the assumption that ENT1 inhibition is responsible for ticagrelor-induced arterial healing (Figure 2B).
Ticagrelor-induced endothelial healing is dependent on adenosine and A2A receptor activation. (A) Quantification of deendothelialized area (upper panel) and representative confocal images of en face carotid arteries (lower panel) cultured ex vivo in control media, or in media containing ticagrelor (10 μM), or P2Y12 inhibitor AR-C69931MX (10 μM) and stained with anti–VE-cadherin. (B) Quantification of deendothelialized area (upper panel) and representative confocal images of en face carotid arteries (lower panel) cultured ex vivo in control media or in media containing ticagrelor (10 μM) or dipyridamole (10 μM) and stained with anti–VE-cadherin. (C) Quantification of deendothelialized area (upper panel) and representative confocal images of en face carotid arteries (lower panel) cultured ex vivo in control media or in media containing ticagrelor (10 μM) with or without KW6002 (1 μM) and stained with anti–VE-cadherin. (D) Quantification of deendothelialized area (upper panel) and representative confocal images of en face carotid arteries cultured ex vivo in control media or in media containing a nonselective adenosine receptor agonist, NECA, or an A2A receptor–specific agonist, CGS21680, and stained with anti–VE-cadherin. The zone outlined in yellow represents the area that has not been recovered with endothelial cells. Scale bar: 0.5 mm. (E) Bright-field microscopy images of scratch wound healing assay in arterial endothelial cells treated with vehicle or CGS21680 (1 mM) at the indicated times. The scratch edge is demarcated by the white dotted line (n = 6). Scale bar = 200 μm. (F) Quantification of migration speed of scratch wound healing assay in the indicated conditions. Data shown are means ± SEM. ∗P < .05. The statistical significance of differences between 2 groups was determined using the unpaired Student t test, and differences among multiple groups were determined using 1-way ANOVA followed by the Bonferroni post hoc test (intragroup comparisons). Values of P <.05 were considered statistically significant. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
Ticagrelor-induced endothelial healing is dependent on adenosine and A2A receptor activation. (A) Quantification of deendothelialized area (upper panel) and representative confocal images of en face carotid arteries (lower panel) cultured ex vivo in control media, or in media containing ticagrelor (10 μM), or P2Y12 inhibitor AR-C69931MX (10 μM) and stained with anti–VE-cadherin. (B) Quantification of deendothelialized area (upper panel) and representative confocal images of en face carotid arteries (lower panel) cultured ex vivo in control media or in media containing ticagrelor (10 μM) or dipyridamole (10 μM) and stained with anti–VE-cadherin. (C) Quantification of deendothelialized area (upper panel) and representative confocal images of en face carotid arteries (lower panel) cultured ex vivo in control media or in media containing ticagrelor (10 μM) with or without KW6002 (1 μM) and stained with anti–VE-cadherin. (D) Quantification of deendothelialized area (upper panel) and representative confocal images of en face carotid arteries cultured ex vivo in control media or in media containing a nonselective adenosine receptor agonist, NECA, or an A2A receptor–specific agonist, CGS21680, and stained with anti–VE-cadherin. The zone outlined in yellow represents the area that has not been recovered with endothelial cells. Scale bar: 0.5 mm. (E) Bright-field microscopy images of scratch wound healing assay in arterial endothelial cells treated with vehicle or CGS21680 (1 mM) at the indicated times. The scratch edge is demarcated by the white dotted line (n = 6). Scale bar = 200 μm. (F) Quantification of migration speed of scratch wound healing assay in the indicated conditions. Data shown are means ± SEM. ∗P < .05. The statistical significance of differences between 2 groups was determined using the unpaired Student t test, and differences among multiple groups were determined using 1-way ANOVA followed by the Bonferroni post hoc test (intragroup comparisons). Values of P <.05 were considered statistically significant. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
The effects observed downstream of ENT1 inhibition may be due to an extracellular accumulation of adenosine, acting in a paracrine fashion on one of its 4 known receptors present at the cell surface.18 Adenosine receptors (A1, A2A, A2B, and A3 receptors) belong to the G protein–coupled receptor family. A2A is highly expressed at the surface of endothelial cells.19 To determine whether A2A drives ticagrelor-induced arterial healing, we incubated ex vivo injured carotid arteries with ticagrelor in combination with an inhibitor of the A2A receptor (KW 6002), which reversed the protective effect of ticagrelor, demonstrating a paracrine effect of adenosine through A2A receptor in arterial healing (Figure 2C). To substantiate the possible role of adenosine/A2A receptor on arterial healing, we incubated ex vivo injured carotid arteries with a nonselective adenosine receptor agonist, NECA, or a specific agonist of the A2A receptor (CGS21680). Both compounds were able to increase arterial healing, demonstrating the involvement of adenosine and A2A receptor in these processes (Figure 2D). This positive effect of A2A receptor activation on endothelial healing was confirmed in vitro in a scratch assay performed in arterial endothelial cells (Figure 2E-F).
In conclusion, our results demonstrate that improved endothelial healing by ticagrelor is responsible for the reduced development of neoatherosclerosis in vivo. These findings are consistent with those of various clinical trials demonstrating the superiority of ticagrelor over clopidogrel.9,20 Although the literature continues to show conflicting results on the benefit of ticagrelor vs clopidogrel on cardiovascular events in humans, our results provide mechanistic data opening up new perspectives for the development of future molecules aimed at improving endothelial healing and preventing arterial damage in cardiovascular pathology.
Acknowledgments: The authors are grateful for the technical services of the animal facility (Genotoul Anexplo US006/INSERM/Université Paul Sabatier, Toulouse, France) and the TRI-Genotoul I2MC platform (light microscopy; France-BioImaging, member of the national infrastructure, supported by the French National Research Agency).
This research was supported by Nouvelle Société Francophone d'Athérosclérose and INSERM and conducted with the support of AstraZeneca.
Contribution: R.S., A.W., A.L., C.S., N.F.S., N.M., K.C., and H.C. participated in the methodology and validation of the experiments; M.-P.G. and B.P. supervised platelet aggregation experiment and analysis; S.G., N.A., D.C., T.L., and D.R. were involved in investigation, formal analysis, expertise, and critical reading of the manuscript; D.R. and M.L. wrote the manuscript; and M.L. led project and secured funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Muriel Laffargue, INSERM UMR1297, University of Toulouse 3, Institute of Metabolic and Cardiovascular Diseases, 31432 Toulouse Cedex 04, France; email: muriel.laffargue@inserm.fr; and Damien Ramel, INSERM UMR1297, University of Toulouse 3, Institute of Metabolic and Cardiovascular Diseases, 31432 Toulouse Cedex 04, France; email: damien.ramel@inserm.fr.
References
Author notes
R.S. and A.W. contributed equally to this work.
Original data are available on request from the corresponding author, Muriel Laffargue (muriel.laffargue@inserm.fr).