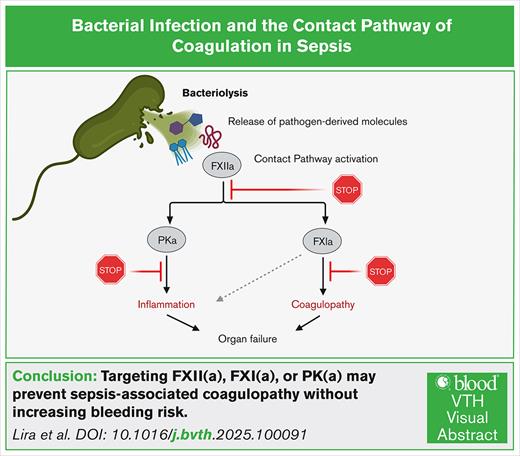
Visual Abstract
The excessive inflammatory and prothrombotic response to a bacterial infection creates a life-threatening medical condition in sepsis. The transition from localized to systemic thrombin generation is a hallmark of disseminated intravascular coagulation (DIC), a severe prothrombotic disorder resulting in microvascular thrombosis and subsequent multiorgan failure. The contact pathway of coagulation has been shown to play roles both in the initiation and amplification of thrombin generation in models of sepsis. The contact pathway consists of the coagulation factors XII (FXII) and XI (FXI), prekallikrein, and high- molecular-weight kininogen. Activation of the contact pathway can be triggered by binding to anionic surfaces, such as the ionized phosphoryl and carboxylate groups present on bacterial surface macromolecules. In particular, FXII has been shown to bind to the components of the bacterial envelope, including adhesins, peptidoglycan, lipopolysaccharides from gram-negative bacteria, and lipoteichoic acids from gram-positive bacteria. This review discusses the molecular pathways linking activation of the contact pathway by the envelope structures of bacterial and downstream thrombin generation and inflammation associated with sepsis-induced coagulopathies. We highlight the potential for FXII and FXI as potential therapeutic options for safely preventing DIC in sepsis.
Introduction
Bacteria are classified as gram positive or gram negative based on their cell wall structure and ability to retain crystal violet stain.1,2 Gram-positive bacteria possess a thick peptidoglycan (PGN) layer, whereas gram-negative bacteria have a thinner PGN layer and an outer membrane rich in lipopolysaccharides (LPSs).2 Historically, gram-negative bacteria (eg, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas spp) from the gastrointestinal and urogenital microbiotas were the leading cause of sepsis-related deaths.3 However, recent epidemiological shifts have seen a rise in mortality due to gram-positive pathogens, such as Staphylococcus aureus and Enterococcus spp.4 This trend highlights the need for a deeper understanding of how different bacterial pathogens interact with host defenses.
Bacteria interact with the blood microenvironment at 3 scales: (1) macromolecular components shed from, or embedded in, the bacterial cell wall; (2) individual planktonic (free-floating) cells; or (3) organized communities within biofilms. At each scale, host immune and coagulation systems detect distinct molecular patterns, triggering responses that range from transient, localized control to pathological immunothrombosis, a maladaptive interplay of innate immunity and thrombosis. In severe cases, this cascade escalates into systemic inflammation and disseminated coagulation, overwhelming host defenses.
Immunothrombosis is a physiological host defense mechanism that locally restrains pathogens by integrating the coagulation cascade, platelet activation, and innate immune responses (eg, neutrophil extracellular traps and complement). However, dysregulation of this process can precipitate systemic immunothrombosis, leading to widespread microthrombosis and organ dysfunction characteristic of sepsis.5,6 These systems work synergistically to detect pathogens, amplify inflammation, and contain infections via thrombus formation.7 The coagulation cascade produces fibrin to trap and limit microbial spread,8 whereas the complement system opsonizes pathogens, recruits leukocytes, and enhances inflammatory signaling.9 Platelets serve as immune sentinels, releasing antimicrobial peptides, engaging with immune cells, and providing a procoagulant surface for thrombin generation.10 During bacterial infections, these pathways form a tightly regulated network that, when dysregulated, can escalate into pathologic thrombosis, coagulopathy, and multiorgan failure.11-13
This review focuses on the molecular interplay between bacterial surface structures, such as LPS, PGN, teichoic acids (TAs), and adhesins, and the activation of the contact pathway of coagulation. The contact pathway is initiated when factor XII (FXII) binds to negatively charged surfaces, such as polyphosphates, DNA, or bacterial cell wall components, leading to autoactivation and subsequent activation of FXI and FIX.14,15 This cascade converges with the common coagulation pathway to generate thrombin, a central mediator of fibrin formation and platelet activation. Additionally, FXII-driven plasma kallikrein (PKa) activation results in bradykinin release, linking coagulation to inflammatory responses.15 We explore how these interactions promote thrombin generation, bradykinin release, and amplification of inflammatory responses during sepsis, with an emphasis on the emerging role of bacterial components in driving infection-associated coagulopathies.
Electrostatic properties of bacterial cell surface
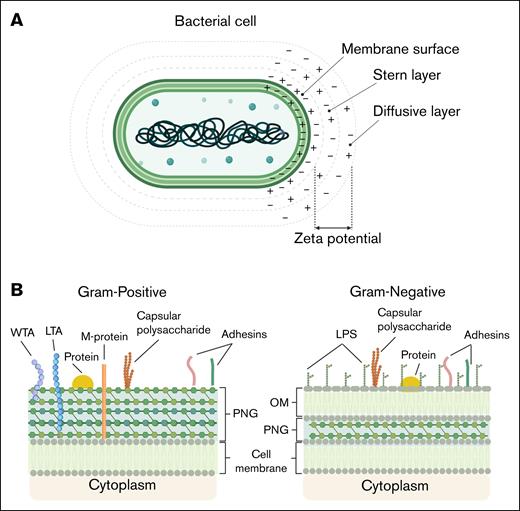
Bacteria are unicellular microorganisms lacking membrane-bound intracellular compartments and, as such, their outer envelope interacts directly with their microenvironment.16 The bacterial surface mediates adhesion, exchange processes, and immune interactions, while supporting growth and division. The bacterial surface contains functional groups including carboxyl, phosphate, and amino moieties that ionize in response to pH, generating a negatively charged layer.16,17 In aqueous environments, hydration and electrostatic charge influence nutrient flux and waste removal. At physiological pH, bacterial surfaces form a negatively charged double layer, with bound and diffuse counter-ions mediating electrostatic interactions.17 The inner region of this double layer includes the Stern layer, in which counter-ions are tightly bound to the bacterial surface, contributing to surface charge stabilization. The ζ-potential approximates the electrostatic potential within the inner region of the diffuse layer (Figure 1A).17 The cell envelope, external to the cytoplasm, is composed of lipids, carbohydrates, and proteins, used to classify bacteria as either gram positive or gram negative (Figure 1B).16,18,19 The envelope architecture of these bacterial groups defines their surface charge. Gram-positive bacteria have a thick PGN layer with lipoteichoic acids (LTAs) and wall teichoic acids (WTAs), whose phosphate and carboxylate groups create an overall net negative charge.18 In contrast, gram-negative bacteria have a thin PGN layer in the periplasmic space, surrounded by an outer membrane. Their anionic surface charge mainly comes from LPSs, especially the phosphate and carboxyl groups of 3-deoxy-d-manno-octulosonic acid residues (Figure 1B).19 Extracellular layers also modulate surface charge in both bacterial types. Many bacteria produce acidic polysaccharides, forming dense capsules or loose slime layers. These proteinaceous or glycoprotein layers, stabilized by divalent cations, associate with the cell wall and affect surface charge.16,17
Schematic depiction of bacterial membrane surface properties. (A) Simplified model of a bacterial cell and its surrounding electrostatic layers. The membrane surface carries fixed charges, followed by the Stern layer with tightly bound counterions and the diffusive layer with a dynamic ion distribution. The ζ-potential, measured at the slipping plane, reflects the effective surface charge, influencing bacterial adhesion and interactions. (B) Schematic representation of the bacterial cell envelope components in gram-positive and gram-negative bacteria. Gram-positive bacteria possess a thick PGN layer, decorated with WTA and LTA, as well as surface-exposed adhesins, membrane proteins, and capsular polysaccharides. In contrast, gram-negative bacteria feature a thinner PGN layer located within the periplasmic space, an OM containing LPS, and the absence of TAs. Both bacterial types contain cytoplasmic membranes and can express adhesins and capsular polysaccharides, which contribute to interactions with host cells and immune evasion. OM, outer membrane.
Schematic depiction of bacterial membrane surface properties. (A) Simplified model of a bacterial cell and its surrounding electrostatic layers. The membrane surface carries fixed charges, followed by the Stern layer with tightly bound counterions and the diffusive layer with a dynamic ion distribution. The ζ-potential, measured at the slipping plane, reflects the effective surface charge, influencing bacterial adhesion and interactions. (B) Schematic representation of the bacterial cell envelope components in gram-positive and gram-negative bacteria. Gram-positive bacteria possess a thick PGN layer, decorated with WTA and LTA, as well as surface-exposed adhesins, membrane proteins, and capsular polysaccharides. In contrast, gram-negative bacteria feature a thinner PGN layer located within the periplasmic space, an OM containing LPS, and the absence of TAs. Both bacterial types contain cytoplasmic membranes and can express adhesins and capsular polysaccharides, which contribute to interactions with host cells and immune evasion. OM, outer membrane.
Contact activation and its interplay with bacterial surfaces
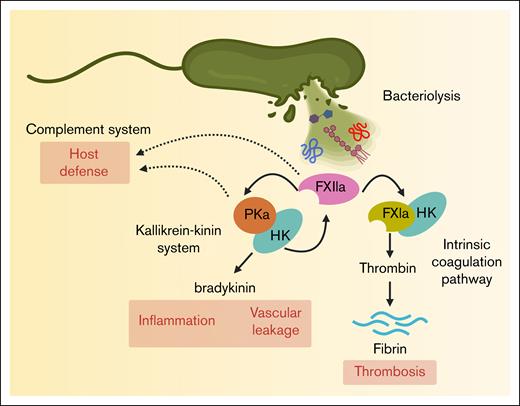
The contact pathway of coagulation plays a vital role linking coagulation and inflammation. Three serine zymogens, coagulation FXII and FXI, and plasma prekallikrein (PK), along with the nonenzymatic cofactor high-molecular-weight kininogen (HK) comprise the contact pathway. Unlike the extrinsic pathway of coagulation, which is triggered by tissue factor,20 activation of the contact pathway is initiated by the binding of FXII to negatively charged surfaces. For instance, upon binding to anionic surfaces such as biopolymers,21,22 bio(nano)materials,23,24 or microbial structures,25 FXII undergoes conformational changes that expose the proteolytic site to form the active species of FXII.26 Activated FXII (FXIIa) then converts PK into PKa, which amplifies FXII activation through a feedback loop. This cascade next activates FXI, leading to FIX activation and ultimately converging with the extrinsic pathway at FX.27,28 The activation of FX drives thrombin generation, fibrin formation, and clot stabilization (Figure 2).20 The generation of fibrin has been suggested to play a key role in the innate immune response by encapsulating and trapping bacteria or bacterial components,29 whereas excessive fibrin formation is a driver of inflammation and coagulopathy.8
Activation of the human contact pathway by bacterial cell envelope components. Bacterial lysis releases membrane components such as LPS, PGN, and LTA, which interact with FXII and promote its activation to FXIIa. FXIIa initiates the intrinsic coagulation pathway by generating FXIa, contributing to thrombosis. FXIIa also activates plasma kallikrein (PKa), triggering the kallikrein-kinin system and leading to the release of the proinflammatory peptide bradykinin. In addition, FXIIa, FXIa, and PKa have a suggestive of a role in the host defense mechanism.
Activation of the human contact pathway by bacterial cell envelope components. Bacterial lysis releases membrane components such as LPS, PGN, and LTA, which interact with FXII and promote its activation to FXIIa. FXIIa initiates the intrinsic coagulation pathway by generating FXIa, contributing to thrombosis. FXIIa also activates plasma kallikrein (PKa), triggering the kallikrein-kinin system and leading to the release of the proinflammatory peptide bradykinin. In addition, FXIIa, FXIa, and PKa have a suggestive of a role in the host defense mechanism.
FXII, FXI, and PK play interconnected roles in coagulation and inflammation, suggesting a broader function in host defense. For instance, the activation of PK to PKa generates bradykinin, a proinflammatory peptide that increases vascular permeability, dilates blood vessels, and facilitates leukocyte extravasation to infected or injured tissues. Moreover, PKa itself can cleave complement component C3 and thus produce C3a and C3b to enhance immune cell recruitment and opsonization of pathogens.30 Similarly, activated FXI (FXIa) directly interacts with the complement system by enzymatically inactivating complement factor H. Factor H controls the alternative pathway by inhibiting the formation of the C3 convertase and allowing the cleavage of C3b, thus preventing excess complement activation and protection of the host cells from injury.31,32 In addition, FXIa has been shown to directly modulate endothelial barrier function by cleaving endothelial junction proteins (eg, vascular endothelial–cadherin)33 promoting vascular leakage, a key pathological feature of sepsis-induced organ dysfunction. Thus, by taking off this natural braking system of complement activity, activation of the contact pathway and FXI may play a role in amplifying the host defense system in certain infections (Figure 2).
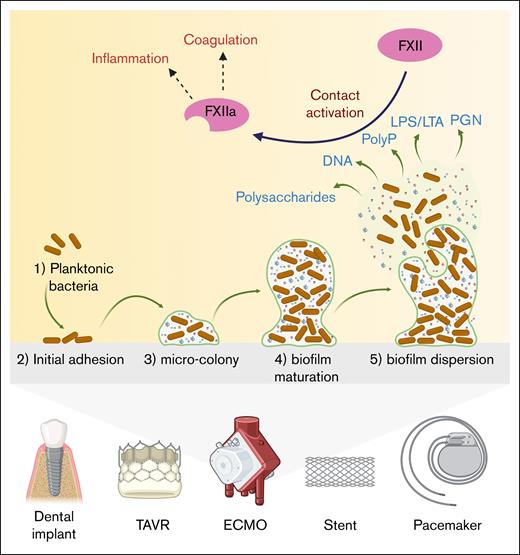
The members of the contact pathway interact with host cells, such as endothelial cells, by binding proteoglycans, specifically glycosaminoglycans.34 Similarly, bacteria, whether as isolated cell wall components, intact planktonic cells, or organized biofilm communities, possess highly anionic components with structural and functional similarities to host proteoglycans. This molecular mimicry potentiates the binding and activation of the members of the contact pathway by bacterial cell wall components.35 For instance, the gram-negative bacteria E coli,36-38,Salmonella spp,36,37,Moraxella catarrhalis,36,K pneumoniae,36,Haemophilus influenzae,36,Yersinia enterocolitica,36,Porphyromonas gingivalis,39,Bacteroides spp,40,Fusobacterium necrophorum,41 and Leptospira spp42,43 have been shown to bind either and, in select settings, activate FXII, FXI, PK, or HK. Similarly, for gram-positive bacteria, S aureus,36,Streptococcus spp,36,44-48 and group G streptococci45 exhibit surface components that promote binding and activation of coagulation factors including FXII. Moreover, bacteriolysis or partial bacterial disintegration can release large amounts of negatively charged cell wall fragments (eg, LPS and PGN).49 which are capable of triggering FXII autoactivation. As a consequence, massive surges in bacterial fragment release (as occurs in septicemia) can drive extensive clotting and inflammation. Intact bacteria elicit immunological responses through release of toxins or surface expression of adhesins and capsules, whereas the unique complexity of bacterial biofilm formation on biomedical devices and implants, such as extracorporeal membrane oxygenation circuits, dental implants, and implantable cardiovascular devices serves to amplify both the immune and inflammatory response to infection (Figure 3).50-53 Biofilm surfaces selectively concentrate negatively charged polysaccharides, TAs, LPS, and extracellular DNA and release of polyphosphates,54,55 which may act in concert to assemble and activate the contact pathway members to initiate and amplify local thrombin generation. As a result, biofilm formation on medical devices has been associated with increased risk of deep vein thrombosis and other cardiovascular complications.56,57 As such, understanding the mechanistic interplay between bacteria and biofilm formation, the immune response and pathways leading to thrombin generation is essential for development of therapies to safely preventing thrombotic disorders associated with bacterial infections of medical devices.58,59
Biofilm formation on intravascular implants and devices and its role in coagulation and inflammation. The illustration shows how biofilms form on intravascular implants and devices, and how they can trigger blood clotting and inflammation. (1) Planktonic bacteria: planktonic bacteria move to the surface of the implant. (2) Initial adhesion: bacteria attach to the implant surface, starting the colonization process. (3) Microcolony formation: the attached bacteria start to grow and form small clusters, producing a protective slime. (4) Biofilm maturation: the biofilm develops into a structured, organized colony with a matrix composed of polysaccharides, extracellular DNA, LPS, or LTA, and PGN. (5) Biofilm dispersion: bacteria and matrix fragments are released from the biofilm, dispersing into the bloodstream and potentially inducing new infections. Released bacterial molecules, including LPS, LTA, and PGN, are capable of activating FXII, starting the blood clotting process. At the same time, bacterial fragments can trigger inflammation, creating a high-risk environment for thrombosis and immune reactions around the implant. ECMO, extracorporeal membrane oxygenation; PolyP, polyphosphates; TAVR, transcatheter aortic valve replacement.
Biofilm formation on intravascular implants and devices and its role in coagulation and inflammation. The illustration shows how biofilms form on intravascular implants and devices, and how they can trigger blood clotting and inflammation. (1) Planktonic bacteria: planktonic bacteria move to the surface of the implant. (2) Initial adhesion: bacteria attach to the implant surface, starting the colonization process. (3) Microcolony formation: the attached bacteria start to grow and form small clusters, producing a protective slime. (4) Biofilm maturation: the biofilm develops into a structured, organized colony with a matrix composed of polysaccharides, extracellular DNA, LPS, or LTA, and PGN. (5) Biofilm dispersion: bacteria and matrix fragments are released from the biofilm, dispersing into the bloodstream and potentially inducing new infections. Released bacterial molecules, including LPS, LTA, and PGN, are capable of activating FXII, starting the blood clotting process. At the same time, bacterial fragments can trigger inflammation, creating a high-risk environment for thrombosis and immune reactions around the implant. ECMO, extracorporeal membrane oxygenation; PolyP, polyphosphates; TAVR, transcatheter aortic valve replacement.
Bacterial envelope structures and activation of the contact pathway of coagulation
The evolution of the bacterial envelope serves to protect these organisms from the environment, and in particular in the setting of the blood microenvironment the immune response to infection. As either a direct or bystander effect, the coagulation cascade is activated by the principal components of the bacterial envelope including LPS, PGN, TAs, and adhesins. Here, we review the molecular mechanisms and potential relevance of each of these bacterial envelope components to the host response, as well as maladies including sepsis-induced coagulopathies.
LPS
LPS is a key component of the gram-negative outer membrane, consisting of lipid A, the core oligosaccharide, and the O-antigen.60,61 Lipid A anchors LPSs, whereas the core maintains membrane integrity. The highly anionic lipid A and inner core contribute to LPSs’ negative charge.62 The O-antigen affects solubility, immune recognition, and surface hydrophilicity, with variations influencing pathogenicity.63 LPS is classified as smooth (full O-antigen), rough (absent O-antigen), or semirough (1 repeating unit), with these structural forms shaping bacterial adaptation and immune interactions.60,61 These structural distinctions play a crucial role in bacterial adaptation, influencing interactions with the host immune system and resistance to external stressors.
The link between LPS and FXII activation was first identified in 1974 by Morrison and Cochrane, who found that E coli LPS directly activates FXII in purified systems.64 They suggested that this activation is influenced by the negative charges of phosphate groups or fatty acids in lipid A. Later, in the early 1980s, Kalter at al further explored this connection, emphasizing the role of lipid A and variations in LPS polysaccharide structure in regulating FXII activation.65 Studies by Yang et al have demonstrated that LPS is capable of associating with HK as an additional mechanism for activation of the contact pathway.66
Using a nonhuman primate (NHP) model, we recently demonstrated that FXII was activated after an E coli infection.38 In vitro, we demonstrated that the physicochemical properties LPS dictate the kinetics of FXII binding and activation. Specifically, our data suggest that the polysaccharide chains of LPS may shield negatively charged regions essential for FXII activation. We observed that only the semirough variant was sufficiently capable of potentiating FXII activation. Consistent with a role for heterogeneity within bacterial strains dictating their thrombogenicity, we found that the ability of LPS to bind FXI and PK and modulate FXIa and PKa activity was chemotype dependent.38
The amphipathic properties of LPS creates a transition from monomers in aqueous solutions to the aggregation of LPS into micelles beyond a critical micelle concentration.62,67 These LPS micelles are characterized by a hydrophobic lipid A core surrounded by a hydrophilic shell of polysaccharides, held together by intermolecular interactions.68 The presence of divalent cations such as Ca2+ and Mg2+ plays a crucial role in stabilizing LPS aggregates and facilitating bilayer formation. These cations form ionic bridges between the negatively charged phosphate groups of lipid A, reducing electrostatic repulsion and promoting a more compact arrangement of LPS molecules.69,70 This stabilization supports the structural transition from micelles to bilayers under physiological conditions. The addition of chelating agents or detergents disrupts these interactions, leading to disaggregation and the release of monomeric LPS.71 Building on the concept that biological activity is dependent on the biophysical properties of LPS,62,72,73 we recently demonstrated that the ability to activate FXII is likewise dependent on the supramolecular architecture of LPS. We found that only the micellar form and not the monomeric form of semirough LPS is capable of promoting FXII proteolytic activation. The addition of Ca2+ increased the hydrodynamic diameter and ζ-potential of LPS, directly reducing the ability of the vesicle-like LPS to activate FXII.74 These findings support a model whereby activation of the contact pathway is modulated by structural transitions of LPS in a concentration-dependent manner.
PGN
PGN is a key structural component of bacterial cell walls, providing shape and mechanical strength to gram-positive and gram-negative bacteria. PGN consists of repeating disaccharides (N-acetylglucosamine and N-acetylmuramic acid) crosslinked by short peptides, with crosslinking and side chain composition varying by species.75 The anionic nature of PGN, due to carboxyl and amino groups, facilitates interactions with host biomolecules. The amphiphilic properties of PGN enable interactions with both hydrophilic and hydrophobic environments, essential for bacterial survival and host interactions.75,76
PGN has been implicated in coagulopathic events in rodent models; however, the underlying mechanisms remain poorly understood. In rats, systemic inflammation, shock, and multiorgan failure have been observed following exposure to S aureus cell wall preparations containing TAs and PGN.77 Interestingly, neither component alone could fully replicate the observed pathophysiology. In vitro studies have shown that human and NHP monocytes, unlike murine cells, respond robustly to purified Bacillus anthracis PGN by producing proinflammatory cytokines, perhaps underscoring a limitation of use of rodent models for studying the effects of PGN on organ systems.78-82 Beyond monocytes, PGN has been shown to be capable of stimulating platelets to generate procoagulant activity, including aggregation and exposure of anionic phospholipids, perhaps as an extension of the immune response or as part of a prothrombotic phenotype of bacteria that express PGN.83
The procoagulant phenotype of PGN is dependent on the physicochemical and structural variations including charge density and degree of crosslinking. These biophysical characteristics regulate the ability of PGN to adsorb and activate of members of the contact pathway and kallikrein-kinin system. In particular, S aureus PGN has been shown to activate FXII to induces PK activation, providing a mechanistic link between bacterial cell wall components and activation the contact pathway and inflammatory pathways during an infection.65 The study by Popescu et al demonstrated that the interaction of PGN from B anthracis with FXII and PK enhanced the enzymatic activity of FXIIa and PKa while preventing FXIIa inhibition by antithrombin. In addition, PGN was found to induce the expression of tissue factor on monocytes both in vitro as well in vivo in a NHP model, activating the extrinsic coagulation cascade. Thus, the procoagulant properties of PGN include activation of both the contact and extrinsic pathways of coagulation to promote thrombin generation, platelet activation, consumptive coagulopathy, thrombosis, increased vascular permeability, and multiorgan failure in NHPs.84
TAs
LTA and WTA are anionic glycopolymers in the cell walls of gram-positive bacteria. The roles of TAs range from providing cell wall integrity, evasion of the immune system, as well as mediating host interactions.18 LTA is tethered to the cytoplasmic membrane via a glycolipid anchor and consists of repeating glycerol phosphate units, whereas WTA is covalently linked to PGN and typically composed of ribitol or glycerol phosphate repeats. Both can be modified with d-alanine and sugars, influencing the charge and binding properties of LTA and WTA.18,85 LTA contributes to membrane stability and immune evasion, whereas WTA plays a key role in PGN integrity and resistance to host defenses. TA, a key component of the S aureus cell wall, has been shown to induce activation of the plasma kallikrein-kinin system. In a study evaluating the activation of plasma PK by bacterial cell wall fractions in purified systems, TA was found to induce PKa activity in a FXII-dependent manner.65 These findings highlight the ability of TA to activate the contact pathway, potentially contributing to coagulation and inflammation during S aureus infections.
LTA self-assembles into different supramolecular structures depending on concentration. Similar to LPS, this amphiphilic polymer can exist as monomers, micelles, or other aggregates, with structural organization influencing interactions with biological systems.86,87 Distinct physical forms appear to be critical for biological activity, but their specific roles in the contact pathway and blood coagulation still need further investigation.
Adhesins
Bacterial adhesion and invasion are key to pathogenicity, enabling resistance to clearance and stable colonization. Adhesins, critical to virulence, include MSCRAMMs (microbial surface components recognizing adhesive matrix molecules), which anchor to the cell wall, and SERAMs (secretable expanded repertoire adhesive molecules), which are secreted but reassociate with the bacterial surface for adhesion.88
S aureus expresses adhesins, including clumping factor A (ClfA), clumping factor B (ClfB), and fibronectin-binding proteins, which bind fibrinogen and fibrin to promote platelet aggregation and clot formation.89 In addition, the secretion of coagulases, including coagulase (Coa) and von Willebrand factor–binding protein (vWbp), directly activates prothrombin, leading to fibrin deposition.90 These interactions contribute to S aureus pathogenesis by enhancing bacterial dissemination, immune evasion, and the establishment of infection-associated thrombotic events.89,91 In Streptococcus pyogenes, M protein binds fibrinogen, inhibiting complement activation and promoting fibrin deposition, which facilitates immune evasion and thrombus formation.92 Furthermore, M protein triggers tissue factor expression in monocytes, increasing thrombin and fibrin formation, thereby linking S pyogenes infection to hypercoagulability and thrombosis.48,93 The adhesins curli and fimbriae expressed by E coli and Salmonella have been shown to facilitate assembly of FXII, PK, and HK, leading to FXII autoactivation and subsequent PKa generation, HK cleavage, and the release of bradykinin.37 These findings highlight a mechanistic link between bacterial adhesion and the pathological activation of the contact pathway, contributing to the inflammatory and thrombotic complications observed in severe infections.
Therapeutic and diagnostic perspectives
Bacteria and bacterially derived molecules and their role in contact activation during bacterial infections have significant consequences for the host. Excessive contact activation can lead to bradykinin overproduction, promoting vasodilation, increased vascular permeability, and bacterial dissemination. In severe infections, such as sepsis, uncontrolled contact pathway activation contributes to disseminated intravascular coagulation, characterized by microthrombosis, and organ failure.11,12,94 Bacterial components that trigger FXII activation, including LPS and PGN, can amplify inflammatory responses, leading to cytokine storms and endothelial damage.
Although early administration of antibiotics is critical in sepsis management,95 the selection of antimicrobial agents significantly affects outcomes. In high-burden settings, bacteriostatic antibiotics or combination therapies may mitigate toxin release. Adjunctive therapies to limit bacteriolysis products include antiendotoxin measures like polymyxin B hemoperfusion96 or high-volume hemofiltration,97 although these remain under investigation. It is also important to recognize that certain broad-spectrum antibiotics can themselves influence the coagulation cascade and confound the effects observed during sepsis.98 For instance, β-lactam antibiotics, such as piperacillin-tazobactam and cefoperazone, have been associated with hypoprothrombinemia and bleeding due to interference with vitamin K metabolism.98 In addition, antibiotics such as linezolid may induce thrombocytopenia, whereas others such as vancomycin have been linked to platelet dysfunction and prothrombotic complications.99,100 These drug-induced effects may mask or exacerbate coagulopathy driven by bacterial infections, complicating the interpretation of laboratory findings and management of patients with sepsis. Acknowledging these potential confounding factors is critical when studying the interplay between bacterial components, coagulation, and inflammation.
Our group has demonstrated that complement-mediated bacteriolysis has a detrimental effect by inducing release of LPS and fulminant inflammation. Inhibition of C5 cleavage and subsequent formation of the lytic terminal complex C5b-9 diminished LPS release, blocked sepsis-induced inflammation, decreased the associated coagulopathy, protected organ function, and significantly improved the survival of septic baboons, suggesting a potentially important strategy to treat bacteremic sepsis.101
Additional interventions include antibodies against endotoxin,102 CD14,103 Toll-like receptor 4 (TLR4),104 or tumor necrosis factor α (TNF-α),105 and immunomodulators like IV immunoglobulin106 to neutralize bacterial toxins without impairing clearance. Low-dose corticosteroids may help control excessive inflammation,107 yet the choice to use corticosteroids comes at the risk of immunosuppression.
Therapeutically, modulating the activation of the contact pathway by targeting bacterial components provides a dual benefit: attenuating excessive coagulation and reducing inflammation. In recent years, significant progress has been made in developing targeted inhibitors of the contact pathway proteins, such as FXII and FXI. Our group demonstrated that pharmacological blockade of FXI activation108 and FXI activation by FXIa109 prevented organ failure and death of baboons challenged with heat-inactivated S aureus.
An emerging area of research focuses on the development of small molecules and peptides capable of neutralizing LPS and LTA by disrupting their interactions with host proteins.110,111 These molecules may act by directly binding to LPS and LTA, shielding their negatively charged moieties, or altering their structural conformation, thereby potentially reducing their ability to trigger contact pathway activation and subsequent coagulation. This approach is particularly relevant given our findings that LPS monomers do not trigger FXII autoactivation,38,74 highlighting the importance of targeting LPS aggregates in modulating contact pathway activation. Table 1 summarizes therapeutic strategies targeting immunothrombosis in bacterial infections, including mechanisms of action, representative agents, and clinical progress.
Therapeutic strategies targeting the interplay between bacterial components, the contact pathway of coagulation, and immunothrombosis
| Therapeutic strategy . | Target . | Mechanism . | Clinical status . |
|---|---|---|---|
| FXI inhibitors | FXI | Inhibit FXI activation or activity to prevent thrombosis | Phase 2/3 trials24,112-115 |
| FXII inhibitors | FXII | Block FXII activation | Phase 2 trials112,116 |
| PK inhibitors | Prekallikrein | Inhibit kallikrein-kinin system | Approved for HAE, not yet tested in sepsis117-119 |
| Complement inhibitors | Complement components | Reduce inflammatory response, limit LPS release | Preclinical to early clinical trials101; some agents approved for paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome120 |
| Antiendotoxin therapies | LPS | Remove endotoxins | Investigational/clinical use in select cases96,97,102,110 |
| Broad-spectrum antibiotics | Bacteria | Target pathogens to reduce infection burden | Standard of care95,98 |
| Immunomodulatory therapies | Cytokines, immune receptors | Modulate immune response | Clinical trials/variable efficacy102-106 |
| Therapeutic strategy . | Target . | Mechanism . | Clinical status . |
|---|---|---|---|
| FXI inhibitors | FXI | Inhibit FXI activation or activity to prevent thrombosis | Phase 2/3 trials24,112-115 |
| FXII inhibitors | FXII | Block FXII activation | Phase 2 trials112,116 |
| PK inhibitors | Prekallikrein | Inhibit kallikrein-kinin system | Approved for HAE, not yet tested in sepsis117-119 |
| Complement inhibitors | Complement components | Reduce inflammatory response, limit LPS release | Preclinical to early clinical trials101; some agents approved for paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome120 |
| Antiendotoxin therapies | LPS | Remove endotoxins | Investigational/clinical use in select cases96,97,102,110 |
| Broad-spectrum antibiotics | Bacteria | Target pathogens to reduce infection burden | Standard of care95,98 |
| Immunomodulatory therapies | Cytokines, immune receptors | Modulate immune response | Clinical trials/variable efficacy102-106 |
The table summarizes the primary targets, mechanisms of action, examples of therapeutic agents, and their current status in clinical development or use.
HAE, hereditary angioedema.
Although this review emphasizes the contribution of bacterial envelope components, particularly LPS, to contact pathway activation, it is important to acknowledge the broader spectrum of LPS-mediated pathophysiology in sepsis. Beyond coagulation and bradykinin generation, LPS is a potent activator of innate immune responses, including the induction of inducible nitric oxide synthase, leading to excessive nitric oxide production.121 This nitric oxide surge contributes to profound vasodilation, hypotension, and septic shock.122 LPS also disrupts endothelial and epithelial barrier integrity by modulating tight junction proteins, promoting vascular leak (“third-spacing”), and contributing to tissue edema and organ dysfunction.13,123,124 Furthermore, persistent platelet activation and consumption in sepsis leads to platelet exhaustion, exacerbating coagulopathy and impairing hemostatic balance.10 These complex and overlapping mechanisms highlight the multifactorial nature of sepsis pathophysiology, in which contact pathway activation represents 1 of several critical contributors.
Successful integration of these therapeutic and diagnostic innovations into clinical practice will require interdisciplinary collaboration, robust preclinical validation, and well-designed clinical trials. The ultimate goal is to translate these advances into effective strategies to prevent and manage coagulopathies in bacterial infections, improving patient care and clinical outcomes.
Clinical implications of contact activation in sepsis and possibilities for translation
Given the significant morbidity associated with sepsis, substantial effort has been directed toward developing clinically meaningful therapies. However, to date, little progress has been made in identifying druggable targets that improve outcomes in patients with sepsis. One of the most notable examples is activated protein C (drotrecogin alfa), which was initially approved for severe sepsis based on early clinical trial data suggesting a mortality benefit.125 However, subsequent studies failed to replicate these findings, leading to its withdrawal from the market.126 Other anticoagulants, including tissue factor pathway inhibitors and antithrombin supplementation, have similarly not shown consistent efficacy in reducing mortality or mitigating sepsis-associated disseminated intravascular coagulation.127 Currently, anticoagulation and antiplatelet therapy are not directly indicated for the treatment of sepsis, because no randomized trials have conclusively demonstrated a survival benefit. Instead, the primary focus remains infection control, hemodynamic stabilization, and organ support.128 Outside of this, anticoagulation is primarily used in prophylactic doses to prevent thrombosis, provided there is no alternative clinical indication for anticoagulation.
Multiple FXI inhibitors are currently in development, including antisense oligonucleotides (eg, fesomersen), small molecules (eg, asundexian and milvexian), and monoclonal antibodies (eg, abelacimab, osocimab, gruticibart [AB023, 14E11], etc), each offering distinct mechanisms to selectively inhibit FXI and reduce thrombotic risk while minimizing bleeding complications compared with more traditional forms of anticoagulation.24,112 Notably, gruticibart and its murine precursor, 14E11, have demonstrated both antithrombotic and anti-inflammatory effects in animal models of sepsis, as well as survival benefits in murine and primate models of severe sepsis, suggesting potential for future therapeutic applications in infection-driven coagulopathies.11,113-115 However, these promising findings have yet to be translated to human studies of sepsis. In contrast, FXII inhibitors have been explored for their therapeutic potential in viral infections. Garadacimab (CSL312), an FXIIa-neutralizing monoclonal antibody, was evaluated in a phase 2 trial for patients with severe COVID-19, but the study did not demonstrate significant clinical benefits, highlighting the challenges of translating FXII inhibition into effective treatments for infection-driven coagulopathies.116 Additionally, PK inhibitors have been successfully developed and approved for the treatment of hereditary angioedema. Agents such as lanadelumab, berotralstat, and ecallantide effectively reduce bradykinin-mediated swelling episodes by targeting the kallikrein-kinin system117-119 However, despite their established role in hereditary angioedema, PK inhibitors have yet to be evaluated in sepsis, leaving open questions about their potential impact on infection-associated coagulopathies and inflammation.
Conclusions
Host-pathogen interactions are shaped by the dynamic relationship between the contact pathway of coagulation and bacterial envelope components. Although the activation of the contact pathway during an infection may contribute to the innate immune response, the excessive activation of FXII and FXI during bacterial infections can drive thrombosis and inflammatory complications. Modulating these interactions through targeted therapeutics, including inhibitors of select enzymatic steps in the contact pathway including the activation of FXI by FXIIa, represents promising approaches to safely limiting disease severity. Continued research into the biochemical mechanisms of bacterial toxin–coagulation factor interactions will pave the way for innovative therapies to improve outcomes in patients with bacterial infections.
Acknowledgments
The figures were created using BioRender.com.
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (R01AI157037) and National Heart, Lung, and Blood Institute (R01HL101972 and R01HL144133).
Authorship
Contribution: A.L.L. wrote the manuscript; C.P., J.J.S., F.L., and O.J.T.M. edited the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Owen J. T. McCarty, Department of Biomedical Engineering, Oregon Health & Science University, Center for Health & Healing, Building 1, 3303 South Bond Ave, Portland, OR 97239; email: mccartyo@ohsu.edu.