Visual Abstract
TO THE EDITOR:
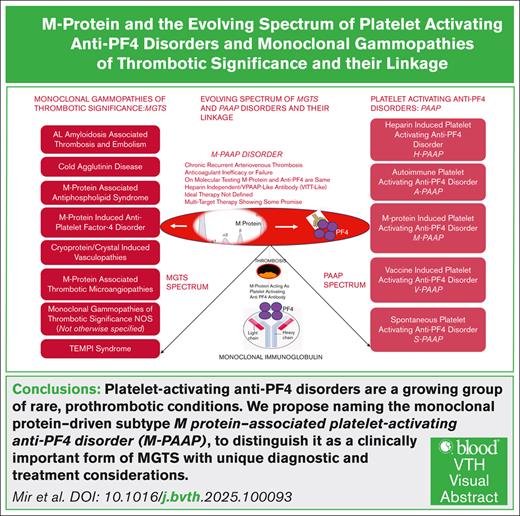
Platelet-activating anti–platelet factor 4 (anti-PF4) antibody disorders are rare yet challenging prothrombotic conditions in which predominantly immunoglobulin G anti-PF4 antibodies are targeted against a complex of positively charged PF4 and negatively charged polymers (polyanions) like heparin. Platelet-activating anti-PF4 disorders encompass the classic heparin-induced thrombocytopenia (HIT) and HIT-like disorders triggered by similar nonheparin polyanionic pharmaceuticals, autoimmune HIT, heparin-independent spontaneous HIT, or vaccine-induced immune thrombotic thrombocytopenia (VITT) following exposure to adenovirus-based COVID-19 vaccine, and a newly described entity, monoclonal gammopathy–associated platelet-activating anti-PF4 disorder.1 This rapid expansion in our understanding of these distinct but related disorders with overlapping pathophysiology make it challenging to expand understanding and awareness of the diseases. Moreover, different nomenclatures have been used by different authors to describe this newly described M protein triggered disorder, which to a nonhematologist reader could be confusing.2-5
Recently a few cases of this so called “VITT-like monoclonal gammopathy of thrombotic significance” have been published, where unlike the classic HIT, this recalcitrant thrombotic disorder was successfully treated and controlled with multitarget therapy including clone directed therapy, IV immunoglobulin, and Bruton tyrosine kinase inhibitor, rather than anticoagulants and antiplatelets, opening new diagnostic and therapeutic possibilities for patients with unexplained refractory thrombotic disorders.5 These disorders are characterized by the presence of a monoclonal protein detected on serum protein electrophoresis driven by a clonal expansion of plasma cells in the marrow. The monoclonal protein plays a central role in the pathogenesis of the thrombotic disorder by causing platelet reactivity through PF4 activation in a heparin-independent manner. The PF4 binding sites have been shown to be similar to VITT, although the antibody signature is distinct from those seen in VITT. Similar to VITT the antibodies may be detected on enzyme-linked immunosorbent assays or serotonin release assays to detect PF4 antibodies developed to diagnose patients with HIT. However, in some patients these tests can be negative and can be identified with PF4-dependent functional assays such as PF4-dependent P-selectin expression assay.6 Antibodies in HIT and VITT are usually transient after the acute episode coinciding with exposure to heparin and vaccine, respectively.7.8 However, in patients where the pathogenic process is driven by the monoclonal protein (M protein) they are usually persistent, and thus repeat testing can be used to distinguish these conditions (Figure 1).
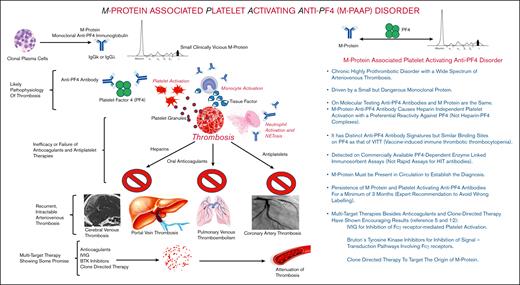
M protein–associated platelet-activating anti-PF4 disorder pathophysiology and clinical characteristics. BTK, Bruton tyrosine kinase; Ig, immunoglobulin; IVIG, IV immunoglobulin.
M protein–associated platelet-activating anti-PF4 disorder pathophysiology and clinical characteristics. BTK, Bruton tyrosine kinase; Ig, immunoglobulin; IVIG, IV immunoglobulin.
Monoclonal gammopathies of clinical significance are a class of diseases triggered by a circulating M protein. The physicochemical properties of this M protein can bring forth different diseases through multiple mechanisms such as its antigen specificity, its tendency toward tissue deposition, or its complement activation property. This clonal heterogeneity generates dozens of unique disorders involving the kidneys, nervous system, skin, ocular system, cardiovascular system, etc.9 In addition monoclonal proteins have the potential to produce bleeding or thrombotic phenotypes through a variety of complex pathophysiologic interactions with coagulation factors, platelets, or the vascular endothelium. Monoclonal gammopathies of thrombotic significance (MGTS) includes many thrombotic disorders driven by the M protein via unique and diverse mechanisms rather than being a single entity representing MGTS as a whole. Although monoclonal gammopathies have been identified as a thrombotic risk factors the mechanisms appear to be diverse including cold agglutination, cryoglobulinemia, hyperviscosity, autoantibodies against naturally occurring anticoagulants (antithrombin, protein C, protein S), and antiphospholipid antibodies.10 Although Gkalea et al10 have discussed the pathophysiology and thrombotic spectrum of M protein–associated disorders, they ultimately restrict the definition of MGTS to patients with monoclonal gammopathy of undetermined significance and unexplained recurrent thrombosis after excluding known M protein–related causes—effectively describing only the tip of the iceberg.
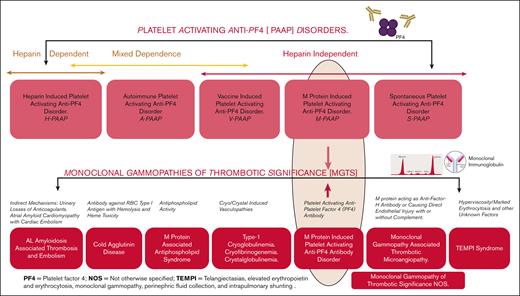
In contrast, we propose the term MGTS—not otherwise specified for this subset—following exclusion of more defined entities such as M protein–associated antiphospholipid syndrome, VITT-like MGTS/M-PAAP (M protein–induced platelet-activating anti-PF4 disorder), AL amyloidosis, and other established thrombotic conditions. (Figure-2). In one study, among 134 patients with monoclonal gammopathy of undetermined significance who developed a thrombotic event, banked plasma was subjected to PF4 antigen–based and functional assays and 4 patients demonstrated HIT-like antibodies, described as PF4-dependent platelet activation that could be inhibited with high concentrations of heparin (100 U/mL), and blockade of the platelet immunoglobulin G receptor, FcγRIIa.11 This study demonstrates that only a small number of patients with MGTS may have PF4-dependent platelet-activating antibodies, and this highlights the need to use distinct terminology to allow accurate diagnosis and clinical decisions as well as among educators and researchers as our understanding expands. Our focus is on emphasizing the broader and underrecognized spectrum of MGTS and highlighting their connection to the challenging entity of M-PAAP.12 A key goal is to dispel the misconception that MGTS refers to a single disorder. Thus, we believe that it would be appropriate to classify anti-PF4 disorder caused by a monoclonal gammopathy as M-PAAP, as an important subtype of MGTS, rather than a standalone MGTS entity (Figures 1 and 2).
Proposed pathophysiology and nomenclature-based classification of platelet-activating anti-PF4 disorders and MGTS and their linkage.
Proposed pathophysiology and nomenclature-based classification of platelet-activating anti-PF4 disorders and MGTS and their linkage.
Contribution: T.H.M. conceptualized, drafted, and wrote the manuscript, approved the final manuscript, and created the figures; R.P. contributed to writing the manuscript and approved and edited the final manuscript; and J.A.F. contributed to writing the manuscript and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tajamul H. Mir, Division of Nephrology and Lupus and Vasculitis Centre, Government Medical College Associated Super Speciality Hospital, Srinagar 190010, Jammu and Kashmir, India; email: thmir@rediffmail.com.
References
Author notes
Literature-related information is available from the corresponding author, Tajamul H. Mir (thmir@rediffmail.com), on request.