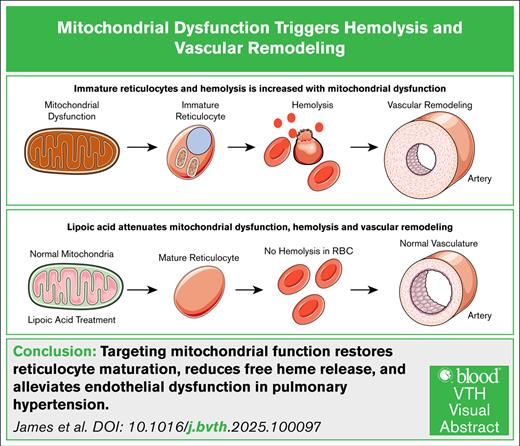
Visual Abstract
TO THE EDITOR:
Pulmonary arterial hypertension and/or pulmonary hypertension (PH) is a lethal yet incurable cardiovascular disease.1 Mitochondrial dysfunction (MD) has been central to the pathogenesis of several lung diseases,2 including PH. Humans with mutations in NFU1, a mitochondrial iron-sulfur cluster (Fe-S) chaperone, develop multiple MD syndrome and exhibit a strikingly high prevalence of PH (70%),3 far exceeding that of the general population and suggesting a causal link between mitochondrial impairment and PH. Another group of individuals with a high association of PH are patients with blood diseases such as sickle cell disease.4 It was also recently demonstrated that elevated levels of circulating free heme are significantly associated with preclinical models of PH and clinical PH.5 However, the association between MD and hemolysis has not been explored.
Reticulocytes are precursors to red blood cells (RBCs). The reticulocytes undergo extensive organelle removal in the transition to RBCs, and mitochondrial energy aids in this transition.6 Intriguingly, mitochondrial retention is upregulated in sickle cell disease, making RBCs highly susceptible to hemolysis.7-9 Given the essential role of efficient mitochondrial function in reticulocyte maturation, we propose that MD may impair this process, resulting in increased reticulocyte fragility and hemolysis. We demonstrated that hemolysis rapidly disrupts the endothelial barrier10 and exacerbates PH through vascular leakage, proliferation, and inflammation.5,11 Furthermore, we showed increased hemolysis and elevated free heme levels in patients with PH.11 Moreover, a recent study12 reveals that the immature reticulocyte fraction is a promising novel biomarker for assessing hemodynamic severity in pulmonary arterial hypertension, with several clinically relevant implications. Thus, our study provides a mechanistic link explaining the immature reticulocyte fraction, hemolysis, and PH.
Our previous reports demonstrated a link between MD and PH by studying rats with a homologous mutation in NFU1.13 These NFU1 (G206C)-mutant rats with marked MD spontaneously develop PH, well characterized in previous studies.14,15 MD results from a pyruvate dehydrogenase (PDH) deficiency, a crucial mitochondrial enzyme that requires lipoic acid (LA) as a cofactor. Because the NFU1 protein is necessary for a functional lipoic acid synthase (LAS), mutations in NFU1 impair LAS’s ability to incorporate LA into PDH, exacerbating MD. This allows us to target MD by LA supplementation. Thus, the NFU1 model offers a unique genetic model that recapitulates the human condition and underscores the potential causal relationship between mitochondrial defects and PH development. In this study, we investigate whether MD, hemolysis, and PH are interconnected.
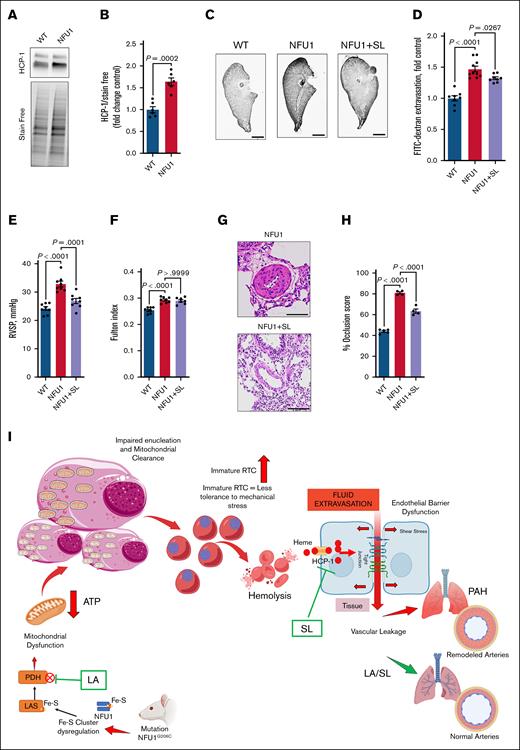
Reticulocytes are precursors to RBCs; they lose their mitochondria and nuclei to become mature RBCs.6 Mitochondrial function is critical to this maturation process, so we assessed mitochondrial activity in reticulocytes isolated from animals.14 We observed significantly reduced adenosine triphosphate production by mitochondria in reticulocytes from the NFU1 group (Figure 1A). To investigate whether MD disrupts the reticulocyte maturation process, we measured the proportion of immature reticulocytes in both wild-type and NFU1 rats. Our data indicate that NFU1 rats had significantly increased immature reticulocytes (Figure 1B-C). We also observed a twofold increase in reticulocyte numbers in the NFU1 rats (Figure 1D). Interestingly, a recent study found that the number of immature reticulocytes was significantly elevated in patients with PH, identifying them as a novel biomarker for PH severity.12 Because reticulocyte production is tightly regulated by erythropoietin (EPO),16 and we found that EPO levels were significantly elevated (Figure 1E).17 Furthermore, we observed a dramatic increase in carbonic anhydrase-9 expression in the lungs (Figure 1F-G), the hypoxia marker. This can be explained by impaired angiogenesis recently identified in the lungs of NFU1 rats.15 Therefore, lung tissue hypoxia could explain the increased EPO levels.
The role of mitochondrial dysfunction in reticulocytes and hemolysis. (A) ATP production: mitochondrial ATP production, as measured by the Seahorse XF assay, is significantly decreased in NFU1 reticulocytes. ATP production is measured as the difference in the OCR after the addition of oligomycin, a complex V inhibitor (n = 5-7). (B-C) Immature reticulocytes: the number of immature reticulocytes, identified by the persistence of mitochondria under microscopy, is markedly increased in the blood of NFU1 rats. Scale bar, 50 μm (n = 8). (D) Circulating reticulocytes: reticulocyte counts are significantly elevated in NFU1 rats, indicating increased production, decreased clearance, and maturation of reticulocytes (n = 10). (E) EPO levels: EPO levels, which control reticulocyte and RBC production, determined by enzyme-linked immunosorbent assay are significantly elevated in the plasma of NFU1 rats (n = 10). (F-G) Carbonic anhydrase 9 (CA9) expression: immunohistochemical staining shows increased CA9 expression in the lungs of NFU1 rats, indicating a potential hypoxic environment. Scale bar, 200 μm (n = 4). (H) Circulating free heme: free heme levels in the plasma are significantly elevated in NFU1 rats, indicating hemolysis (n = 10). (I-J) Barrier disruption: FITC-dextran extravasation assays reveal significant barrier disruption in the lungs of NFU1 rats. Scale bar, 5 mm (n = 7-8). (K) RVSP: RVSP is significantly increased in NFU1 rats, indicating significant PH (n = 8). (L) Fulton index: the Fulton index, indicative of right ventricular remodeling (calculated as follows: right ventricle/left ventricle + septum), is significantly elevated in NFU1 rats (n = 8). (M-N) Vascular occlusion score: the vascular occlusion score, a measure of vascular remodeling, is markedly increased in NFU1 lungs. Scale bar, 100 μm (n = 5). (O) LA treatment: LA supplementation in NFU1 rats significantly improves mitochondrial ATP production (n = 5). LA is a cofactor of PDH, a mitochondrial enzyme. LAS synthesizes LA. The NFU1 protein inserts Fe-S clusters in LAS, enabling LA synthesis. The NFU1 mutation impairs LA synthesis because of LAS dysfunction. LA supplementation directly circumvents the LAS dysfunction. (P) Reticulocyte count posttreatment: reticulocyte numbers are normalized in NFU1 rats after LA treatment (n = 10-12). (Q-R) Immature reticulocytes posttreatment: LA treatment significantly reduces the number of immature reticulocytes in NFU1 rats, indicating improved reticulocyte maturation (n = 10-12). (S) Free heme posttreatment: free heme levels are significantly reduced in NFU1 rats after LA treatment, indicating decreased hemolysis (n = 9-10). (T-U) Barrier protection posttreatment: LA treatment significantly protects NFU1 rats from barrier disruption, as shown by reduced FITC-dextran extravasation in the lungs. Scale bar, 10 mm (n = 8). (V) RVSP posttreatment: LA treatment significantly protects NFU1 rats from increased RVSP, indicating a protection from the induction of PH (n = 8). (W) Fulton index posttreatment: LA treatment prevents the rise in the Fulton index in NFU1 rats (n = 8). (X-Y) Vascular remodeling posttreatment: LA treatment in NFU1 rats significantly reverses pulmonary vascular remodeling. Scale bar, 100 μm (n = 5-6). For panels A-R, a P value of <.05 was considered significant as determined by an unpaired t test. For panel S, significance was determined using 1-way analysis of variance with selected comparisons between NFU1 vs NFU1-LA and NFU1 vs wild-type (WT) groups, followed by a Holm-Sidak post hoc test. (T-Y) used an unpaired t test to determine significance, with a P value of < .05 considered significant. ATP, adenosine triphosphate; FITC, fluorescein isothiocyanate; OCR, oxygen consumption rate.
The role of mitochondrial dysfunction in reticulocytes and hemolysis. (A) ATP production: mitochondrial ATP production, as measured by the Seahorse XF assay, is significantly decreased in NFU1 reticulocytes. ATP production is measured as the difference in the OCR after the addition of oligomycin, a complex V inhibitor (n = 5-7). (B-C) Immature reticulocytes: the number of immature reticulocytes, identified by the persistence of mitochondria under microscopy, is markedly increased in the blood of NFU1 rats. Scale bar, 50 μm (n = 8). (D) Circulating reticulocytes: reticulocyte counts are significantly elevated in NFU1 rats, indicating increased production, decreased clearance, and maturation of reticulocytes (n = 10). (E) EPO levels: EPO levels, which control reticulocyte and RBC production, determined by enzyme-linked immunosorbent assay are significantly elevated in the plasma of NFU1 rats (n = 10). (F-G) Carbonic anhydrase 9 (CA9) expression: immunohistochemical staining shows increased CA9 expression in the lungs of NFU1 rats, indicating a potential hypoxic environment. Scale bar, 200 μm (n = 4). (H) Circulating free heme: free heme levels in the plasma are significantly elevated in NFU1 rats, indicating hemolysis (n = 10). (I-J) Barrier disruption: FITC-dextran extravasation assays reveal significant barrier disruption in the lungs of NFU1 rats. Scale bar, 5 mm (n = 7-8). (K) RVSP: RVSP is significantly increased in NFU1 rats, indicating significant PH (n = 8). (L) Fulton index: the Fulton index, indicative of right ventricular remodeling (calculated as follows: right ventricle/left ventricle + septum), is significantly elevated in NFU1 rats (n = 8). (M-N) Vascular occlusion score: the vascular occlusion score, a measure of vascular remodeling, is markedly increased in NFU1 lungs. Scale bar, 100 μm (n = 5). (O) LA treatment: LA supplementation in NFU1 rats significantly improves mitochondrial ATP production (n = 5). LA is a cofactor of PDH, a mitochondrial enzyme. LAS synthesizes LA. The NFU1 protein inserts Fe-S clusters in LAS, enabling LA synthesis. The NFU1 mutation impairs LA synthesis because of LAS dysfunction. LA supplementation directly circumvents the LAS dysfunction. (P) Reticulocyte count posttreatment: reticulocyte numbers are normalized in NFU1 rats after LA treatment (n = 10-12). (Q-R) Immature reticulocytes posttreatment: LA treatment significantly reduces the number of immature reticulocytes in NFU1 rats, indicating improved reticulocyte maturation (n = 10-12). (S) Free heme posttreatment: free heme levels are significantly reduced in NFU1 rats after LA treatment, indicating decreased hemolysis (n = 9-10). (T-U) Barrier protection posttreatment: LA treatment significantly protects NFU1 rats from barrier disruption, as shown by reduced FITC-dextran extravasation in the lungs. Scale bar, 10 mm (n = 8). (V) RVSP posttreatment: LA treatment significantly protects NFU1 rats from increased RVSP, indicating a protection from the induction of PH (n = 8). (W) Fulton index posttreatment: LA treatment prevents the rise in the Fulton index in NFU1 rats (n = 8). (X-Y) Vascular remodeling posttreatment: LA treatment in NFU1 rats significantly reverses pulmonary vascular remodeling. Scale bar, 100 μm (n = 5-6). For panels A-R, a P value of <.05 was considered significant as determined by an unpaired t test. For panel S, significance was determined using 1-way analysis of variance with selected comparisons between NFU1 vs NFU1-LA and NFU1 vs wild-type (WT) groups, followed by a Holm-Sidak post hoc test. (T-Y) used an unpaired t test to determine significance, with a P value of < .05 considered significant. ATP, adenosine triphosphate; FITC, fluorescein isothiocyanate; OCR, oxygen consumption rate.
Retention of organelles in reticulocytes is a known contributor to fragility and subsequent hemolysis.18 Furthermore, recent research has highlighted significant structural and functional alterations in RBCs of patients with PH, although the implications of these changes remain underexplored.19 Consistently, with a corresponding increase in immature reticulocytes, we also observe markedly elevated levels of circulating free heme in the NFU1 group (Figure 1H). Free heme is a known instigator of barrier dysfunction and vascular leakage, pathological events that can potentiate PH.5 Notably, NFU1 rats exhibited severe barrier dysfunction, as evidenced by fluorescein isothiocyanate-dextran extravasation into the lungs (Figure 1I-J). This was accompanied by significantly increased right ventricular systolic pressure (RVSP) (Figure 1K) and right ventricular remodeling (Fulton index) (Figure 1L). Histological analysis of the lungs further showed a higher occlusion score in NFU1 rats, indicative of pulmonary vascular remodeling (Figure 1M-N). Thus, we show a link between MD, reticulocyte maturation, hemolysis, and vascular dysfunction in the context of PH, which highlights how these interconnected processes.
The NFU1 G206C mutation impairs Fe-S cluster insertion into client proteins such as LAS, leading to reduced lipoylation of mitochondrial proteins and subsequent dysfunction.13,14 Given the critical role of mitochondrial function in reticulocyte maturation, we tested whether improvement of mitochondrial function could restore reticulocyte maturation and prevent hemolysis. To address the LA deficiency caused by the mutation, we supplemented NFU1 mutant rats with LA; this supplementation significantly mitigated MD (Figure 1O), reduced reticulocyte numbers (Figure 1P), and facilitated their maturation process (Figure 1Q-R). Furthermore, LA treatment showed a significant decrease in circulating free heme levels, reflecting reduced hemolysis (Figure 1S). This reduction in hemolysis effectively prevented vascular leakage (Figure 1T-U) and significantly countered the increase in RVSP (Figure 1V) and Fulton index (Figure 1W). LA treatment reverses severe vascular remodeling (Figure 1X-Y).
Heme carrier protein-1 (HCP-1) mediates free heme entry. Our analysis revealed increased HCP-1 expression in NFU1 rats (Figure 2A-B), suggesting that enhanced heme uptake might contribute to pathological events.10 To investigate this, we tested the effects of sulfasalazine, an HCP-1 inhibitor, on NFU1-mediated spontaneous PH. Sulfasalazine treatment significantly reduced the harmful effects of elevated free heme on lung vascular leakage (Figure 2C-D). It also markedly reversed elevated RVSP (Figure 2E). However, we did not find a reversal in the Fulton index (Figure 2F) but did find a significant attenuation of the severe vascular remodeling (Figure 2G-H). These findings highlight the critical role of heme in PH pathobiology.
Attenuation of heme in hemolysis-induced PH. (A-B) HCP1 expression: HCP1 expression is significantly increased in NFU1 rat lungs, indicating an increased availability of free heme to vascular cells (n = 6). (C-D) Barrier dysfunction: FITC-dextran extravasation is markedly increased in the lungs of NFU1 rats, indicating barrier dysfunction. Using an HCP-1 inhibitor, sulfasalazine (SL) in NFU1 rats significantly decreased FITC-dextran extravasation, indicating that preventing the cellular entry of free heme attenuates barrier dysfunction. Scale bar, 10 mm (n = 8-10). (E) RVSP: RVSP is significantly elevated in NFU1 rats and is attenuated after SL treatment, showing that controlling free heme entry can attenuate PH (n = 8). (F) Fulton index: the elevated Fulton index observed in NFU1 rats is not significantly reduced by SL treatment (n = 7-8). (G-H) Vascular remodeling: severe vascular remodeling in NFU1 rats is significantly reversed by SL treatment. Scale bar, 100 μm (n = 4-5). (I) Schematic overview: this illustrates how MD, leading to reduced ATP levels, impairs the clearance of nuclei and organelles in RTC, increasing their susceptibility to stress and subsequent hemolysis. The resulting free heme entry into cells contributes to barrier dysfunction, vascular leakage, and pulmonary arterial hypertension (PAH). LA supplementation improves mitochondrial function, enhances reticulocyte maturation, and prevents hemolysis. SL treatment blocks heme entry, mitigating barrier dysfunction and PAH progression. A significance level of P < .05 was considered for all comparisons. An unpaired t test was used for panel A, whereas a 1-way analysis of variance of selected columns (WT vs NFU1 and NFU1 vs SL) followed by Bonferroni post hoc test was applied for panels C-H. ATP, adenosine triphosphate; FITC, fluorescein isothiocyanate; RTC, reticulocytes.
Attenuation of heme in hemolysis-induced PH. (A-B) HCP1 expression: HCP1 expression is significantly increased in NFU1 rat lungs, indicating an increased availability of free heme to vascular cells (n = 6). (C-D) Barrier dysfunction: FITC-dextran extravasation is markedly increased in the lungs of NFU1 rats, indicating barrier dysfunction. Using an HCP-1 inhibitor, sulfasalazine (SL) in NFU1 rats significantly decreased FITC-dextran extravasation, indicating that preventing the cellular entry of free heme attenuates barrier dysfunction. Scale bar, 10 mm (n = 8-10). (E) RVSP: RVSP is significantly elevated in NFU1 rats and is attenuated after SL treatment, showing that controlling free heme entry can attenuate PH (n = 8). (F) Fulton index: the elevated Fulton index observed in NFU1 rats is not significantly reduced by SL treatment (n = 7-8). (G-H) Vascular remodeling: severe vascular remodeling in NFU1 rats is significantly reversed by SL treatment. Scale bar, 100 μm (n = 4-5). (I) Schematic overview: this illustrates how MD, leading to reduced ATP levels, impairs the clearance of nuclei and organelles in RTC, increasing their susceptibility to stress and subsequent hemolysis. The resulting free heme entry into cells contributes to barrier dysfunction, vascular leakage, and pulmonary arterial hypertension (PAH). LA supplementation improves mitochondrial function, enhances reticulocyte maturation, and prevents hemolysis. SL treatment blocks heme entry, mitigating barrier dysfunction and PAH progression. A significance level of P < .05 was considered for all comparisons. An unpaired t test was used for panel A, whereas a 1-way analysis of variance of selected columns (WT vs NFU1 and NFU1 vs SL) followed by Bonferroni post hoc test was applied for panels C-H. ATP, adenosine triphosphate; FITC, fluorescein isothiocyanate; RTC, reticulocytes.
In summary, our study uncovers a critical link between MD, reticulocyte maturation, hemolysis, and PH. Our findings demonstrate that MD disrupts reticulocyte maturation and elevates hemolysis. Free heme induces significant endothelial barrier dysfunction, contributing to vascular leakage and potentiates PH. LA supplementation effectively restored mitochondrial function in NFU1 mutant rats, improving reticulocyte maturation, reducing hemolysis, lowering free heme levels, reversing vascular leakage, and PH. This suggests that MD can contribute to the pathogenesis of both hemolytic diseases and PH.7,20 Furthermore, inhibiting heme transport mitigated the harmful effects of free heme on vascular leakage and PH. Although our study provides a novel framework linking MD, impaired reticulocyte maturation, hemolysis, and PH, several limitations should be noted. The NFU1 rat model reflects a rare genetic form of MD, and although it is mechanistically informative of MD, it may not fully represent the diverse etiologies of human PH. In addition, our focus on LA synthesis and PDH activity does not capture the broader impact of NFU1 dysfunction on other Fe-S cluster-containing proteins, which may also contribute to the observed phenotype. Independent of LA treatment, we investigated the effect of dichloroacetate, a PDH kinase inhibitor, on mitochondrial function. Interestingly, dichloroacetate enhanced mitochondrial activity in NFU1 reticulocytes (supplemental Figure 1). These findings suggest that further studies are warranted to determine whether improving mitochondrial function through alternative pathways can promote reticulocyte maturation and potentially reverse PH. Inhibition of heme transport with sulfasalazine reduced vascular leakage and RVSP but did not reverse right ventricular remodeling, suggesting that additional heme-independent mechanisms are involved. Although we observed strong associations between MD, reticulocyte immaturity, and PH, we did not directly investigate the molecular mechanisms by which MD impairs reticulocyte stability or how these changes lead to vascular injury. Future studies should explore the roles of other mitochondrial pathways, characterize the cellular mechanisms driving reticulocyte fragility, and assess the translational relevance of our findings in broader PH models. Overall, although our results highlight key mechanistic links, further validation is needed to define their therapeutic potential in PH fully.
Detailed methods used in this study are listed in the supplemental Material.
Acknowledgments: The figure was created in BioRender [James, J. (2025) https://BioRender.com/c3gf4yl] and using elements of Servier Medical Art.
This work was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) R01 grants HL133085 and HL160666 (O.R.), HL132918 and HL151447 (R.R.), NIH/NHLBI K99 grant 1K99HL171869 (J.J.), and American Heart Association grants 23CDA1050843 (M.N.), 834220 (J.J.), and 831538 (M.V.V.).
Contribution: R.R. and O.R. conceived and designed the study; J.J., M.N., M.V.V., and N.M. contributed to analysis and interpretation; and J.J., O.R., and R.R. drafted the manuscript for important intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruslan Rafikov, Indiana University, 980 W Walnut St, R3 Room C414, Indianapolis, Indiana 46202; email: rrafikov@iu.edu; Olga Rafikova, Indiana University, 980 W Walnut St, R3 Room C408, Indianapolis, IN 46202; email: olrafi@iu.edu; and Joel James, Indiana University, 980 W Walnut St, R3 Room C440, Indianapolis, IN 46202; email: jamesjoe@iu.edu.
References
Author notes
J.J. and M.N. contributed equally to this work.
Data are available from the corresponding authors, Ruslan Rafikov (rrafikov@iu.edu), Olga Rafikova (olrafi@iu.edu), and Joel James (jamesjoe@iu.edu), on request.
The full-text version of this article contains a data supplement.