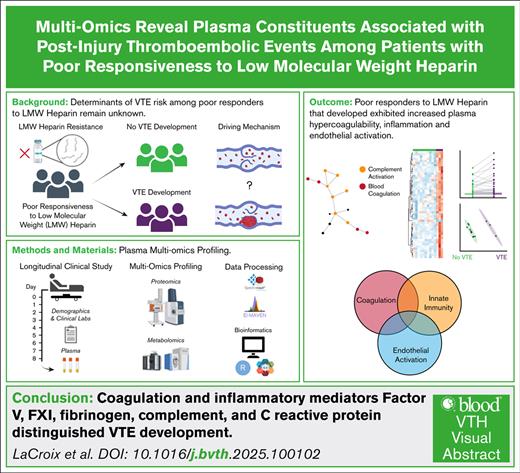
Key Points
Poor responsiveness to low molecular weight heparin is prevalent in the surgical trauma population.
Coagulation, innate immunity, and endothelial activation distinguish VTE development in patients who do not respond to LMWH.
Visual Abstract
Venous thromboembolism (VTE) is among the most common complications affecting patients with severe injuries. Although enoxaparin resistance is common, predicting which patients among this subgroup will develop VTE remains a clinical challenge. The goal of this study was to use multiomics to identify plasma constituents associated with VTE development among trauma patients exhibiting poor responses to enoxaparin. We conducted a secondary analysis of trauma patients at high risk of VTE based on injury characteristics. Plasma was collected daily for the first 8 hospital days. Patients who never responded to enoxaparin were defined as not achieving an anti-factor Xa (FXa) level of ≥0.2 IU/mL during the sampling period. A total of 145 patients were included in the analysis, of whom 24 (16.6%) developed VTE. Proteomics analyses identified increased proteins involved in coagulation, complement activation, and immune response pathways among patients with VTE compared to those without VTE. A subset of patients (n = 26) never responded to enoxaparin; among them, 8 (30.8%) developed VTE. Partial least squares discriminant analysis on multiomics of never responders revealed clear separation based on VTE status. The top 25 differentially abundant proteins were involved in coagulation, fibrin cross-linking, inflammation, and acute phase responses. Differences were particularly robust on hospital day 5, at which we observed significant increases in FV, FXI, fibrinogen, complement, and C-reactive protein. In conclusion, proteomics signatures distinguish VTE development among trauma patients who do not respond to enoxaparin. These findings implicate novel pathways in postinjury VTE pathogenesis and new candidate proteins for future therapeutic intervention.
Introduction
Venous thromboembolism (VTE) remains a leading cause of in-hospital morbidity and postdischarge preventable mortality among patients who survive major traumatic injuries.1 With the widespread adoption of institutional chemoprophylaxis protocols, the incidence of posttrauma VTE has decreased from a staggering 58% to ∼18% to 30% in the most severely injured patients2-4; however, few advances have been made over the past several decades to further improve VTE prevention. Current VTE prophylaxis protocols across surgical trauma services include twice-daily subcutaneous prophylactic doses (30-40 mg) of the low-molecular-weight heparin (LMWH) enoxaparin as standard-of-care chemoprophylaxis in conjunction with mobilization devices. Although utilization and timing of enoxaparin monitoring vary across institutions, adequate responses are commonly determined by peak anti-factor Xa (FXa) levels, collected 4 hours after administration of the third dose of enoxaparin, with thresholds for acceptable responses defined within the range of 0.2 to 0.4 IU/mL. Importantly, several studies have demonstrated poor responsiveness to enoxaparin in the trauma population, with many failing to achieve the recommended anti-FXa level of 0.2 IU/mL or greater, despite enoxaparin dose escalations.3,5-8
Our group recently demonstrated in a prospective longitudinal study that responsiveness to enoxaparin is strongly associated with VTE incidence and the importance of the circulating anticoagulant, antithrombin (AT), in determining these responses.3 In this study, anti-FXa levels were measured over time in trauma patients receiving standard-of-care enoxaparin and categorized as “always responders,” defined as those having an anti-FXa level of ≥0.2 IU/mL following each dose of enoxaparin after the third dose, “transient responders,” those having at least 1 anti-FXa level of ≥0.2 IU/mL, and “never responders,” those who never had an anti-FXa level of ≥0.2 IU/mL during the 8 day sampling period. Never responders had a fivefold increased incidence of VTE compared to always and transient responders, in addition to significantly lower levels of AT, which is essential to the mechanism of action of heparins.3 Importantly, although 30.8% of never responders developed VTE, 69.2% did not, with no clinical factors identified that distinguished risk among never responders. Thus, it is critical to understand mechanisms that promote or protect from VTE among patients who do not respond to enoxaparin, to enable development of novel therapeutics to improve VTE prevention after major injury. The goal of this study was to use an unbiased mass spectrometry (MS)–based metabolomics and proteomics (multiomics) approach to identify plasma constituents that distinguish VTE development among trauma patients who do not respond to enoxaparin.
Methods
Study design
We conducted a secondary analysis of a prospective observational study previously performed at The University of Texas Health Science Center at Houston and Memorial Hermann Hospital–Texas Medical Center in Houston, Texas. Full details on enrollment are reported.3 Briefly, we enrolled 150 highest level activation trauma patients at risk of developing VTE based on injury patterns. Blood samples were collected upon admission to the emergency department and once daily for 8 days. Blood was drawn 4 hours after the morning dose of enoxaparin and plasma was stored at −80°C until analysis. Plasma was assessed for anti-FXa levels, AT activity, and fibrinogen levels by ACL TOP Coagulation Analyze and thrombin generation was measured by calibrated automated thrombogram. Admission thrombelastography (TEG) values, daily platelet counts, and patient outcomes were collected from the medical records. VTE was defined as the presence of deep vein thrombosis, diagnosed by duplex ultrasound, and/or pulmonary embolism, diagnosed by computed tomography of the chest.
Ultrahigh-pressure liquid chromatography (UHPLC)-MS–based metabolomics frozen plasma aliquots (10 μL) were extracted 1:25 in ice-cold extraction solution (methanol, acetonitrile, and water in a 5:3:2 ratio by volume). Samples were vortexed for 30 minutes at 4°C before centrifugation for 10 minutes at 15 000g at 4°C. Analyses were performed using a Vanquish UHPLC system coupled online to a Q Exactive mass spectrometer (Thermo Fisher), as previously described.9,10
Nano-UHPLC-MS/MS–based proteomics
Workflows for MS-based proteomics were used as previously described.11 Briefly, plasma samples were tryptic digested using S-Trap 96-well plates. Peptides were lyophilized, resuspended in 0.1% formic acid, and loaded onto Evotips. Peptides were analyzed using the Evosep One system coupled to a timsTOF Pro mass spectrometer (diaPASEF mode) via the nanoelectrospray ion source. Raw DIA files were searched in Spectronaut using a project specific spectral library, and data were presented as units of relative intensity.
Bioinformatics
An in-house data analytics pipeline was developed in R (version 4.3.1). Missing omics values were imputed with 20% of the lowest value per feature. Then each omics layer was mean centered and divided by the standard deviation. Linear discriminant analysis was performed using the MASS package. Longitudinal trends and box plots were generated using the ggplot2 package. Partial least squares discriminant analyses (PLS-DA), as well as heat map generation were performed in MetaboAnalyst 5.0.12 Resulting P values from univariate analyses (ie, t test and analysis of variance) were adjusted using the false discovery rate. Protein networks were developed using OmicsNet,13 and biological processes were analyzed using Gene Ontology.14
This study was conducted with approval from The University of Texas Health Science Center at Houston Institutional Review Board.
Results
Cohort clinical characteristics by VTE status
Of the 150 patients enrolled in the original study,3 5 had no plasma remaining, leaving a total of 145 patients in this analysis. Additionally, we included plasma samples from 20 healthy volunteers. No differences in age, sex, or race/ethnicity were identified between healthy and injured participants. Of the 145 patients included, 24 (16.6%) developed VTE. The median time of VTE diagnosis was hospital day 3 (1-7 days). No differences were identified in age, sex, race/ethnicity, body mass index, admission vital signs, and injury mechanism/severity between patients who did and did not develop VTE (Table 1). Furthermore, no differences in admission TEG parameters were identified between groups, highlighting key challenges in stratification of patients at high risk of VTE using clinically available coagulation tests (supplemental Figure 1A). Compared to patients who did not develop VTE, those who developed VTE received a significantly larger number of red blood cell (0 [0-3] vs 5 [1-10] units, respectively; P = .001), plasma (1 [0-3] vs 2 [1-13] units, respectively; P = .005), platelet (0 [0-0] vs 0 [0-3] units, respectively; P = .037), and cryoprecipitate (0 [0-0] vs 0 [0-0] units, respectively; P = .008; Table 1) transfusions. In addition, we observed fewer intensive care unit–free days (20 [8-27] vs 24 [21-27] days, respectively; P = .036) and hospital-free days (6 [0-16] vs 17 [9-21] days, respectively; P < .0003) among patients who developed VTE compared to those without VTE.
Demographics, admission vital signs, and outcome data for all patients by VTE group
| . | No VTE (n = 121) . | VTE (n = 24) . | P value . |
|---|---|---|---|
| Demographics | |||
| Age (y) | 35 (28, 50) | 40 (25, 54) | .728 |
| Male, n (%) | 94 (77.7) | 15 (62.5) | .116 |
| Race, White, n (%) | 91 (75.2) | 18 (75.0) | .558 |
| Ethnicity, Hispanic/Latino, n (%) | 40 (33.1) | 9 (37.5) | .674 |
| Body mass index | 28 (24, 31) | 29 (26, 32) | .219 |
| Admission vital signs and laboratory tests | |||
| Heart rate (bpm) | 105 (85, 124) | 108 (94, 133) | .308 |
| Systolic blood pressure (mm Hg) | 123 (115, 150) | 110 (69, 136) | .243 |
| Base excess/deficit (mmol/L) | −2 (−4, −1) | −4.5 (−6.5, −2.0) | .188 |
| Injury | |||
| Blunt, n (%) | 110 (90.9) | 21 (87.5) | .732 |
| Glasgow Coma Scale | 14 (3, 15) | 3 (3, 15) | .058 |
| Injury Severity Score | 27 (17, 34) | 29 (20, 41) | .218 |
| AIS head >2, n (%) | 52 (43.0) | 11 (45.8) | .796 |
| Transfusion volumes (units) | |||
| 24-hour whole blood cells | 0 (0, 0) | 0 (0, 1) | .094 |
| 24-hour RBCs | 0 (0, 3) | 5 (1, 10) | .001∗ |
| 24-hour plasma | 1 (0, 3) | 2 (1, 13) | .005∗ |
| 24-hour platelets | 0 (0, 0) | 0 (0, 3) | .037∗ |
| 24-hour cryoprecipitate | 0 (0, 0) | 0 (0, 0) | .008∗ |
| Outcomes | |||
| Ventilator-free days | 28 (26, 30) | 27 (14, 30) | .186 |
| ICU-free days | 24 (21, 27) | 20 (8, 27) | .036∗ |
| Hospital-free days | 17 (9, 21) | 6 (0, 16) | .000∗ |
| In-hospital mortality, n (%) | 4 (3.3) | 2 (8.3) | .259 |
| . | No VTE (n = 121) . | VTE (n = 24) . | P value . |
|---|---|---|---|
| Demographics | |||
| Age (y) | 35 (28, 50) | 40 (25, 54) | .728 |
| Male, n (%) | 94 (77.7) | 15 (62.5) | .116 |
| Race, White, n (%) | 91 (75.2) | 18 (75.0) | .558 |
| Ethnicity, Hispanic/Latino, n (%) | 40 (33.1) | 9 (37.5) | .674 |
| Body mass index | 28 (24, 31) | 29 (26, 32) | .219 |
| Admission vital signs and laboratory tests | |||
| Heart rate (bpm) | 105 (85, 124) | 108 (94, 133) | .308 |
| Systolic blood pressure (mm Hg) | 123 (115, 150) | 110 (69, 136) | .243 |
| Base excess/deficit (mmol/L) | −2 (−4, −1) | −4.5 (−6.5, −2.0) | .188 |
| Injury | |||
| Blunt, n (%) | 110 (90.9) | 21 (87.5) | .732 |
| Glasgow Coma Scale | 14 (3, 15) | 3 (3, 15) | .058 |
| Injury Severity Score | 27 (17, 34) | 29 (20, 41) | .218 |
| AIS head >2, n (%) | 52 (43.0) | 11 (45.8) | .796 |
| Transfusion volumes (units) | |||
| 24-hour whole blood cells | 0 (0, 0) | 0 (0, 1) | .094 |
| 24-hour RBCs | 0 (0, 3) | 5 (1, 10) | .001∗ |
| 24-hour plasma | 1 (0, 3) | 2 (1, 13) | .005∗ |
| 24-hour platelets | 0 (0, 0) | 0 (0, 3) | .037∗ |
| 24-hour cryoprecipitate | 0 (0, 0) | 0 (0, 0) | .008∗ |
| Outcomes | |||
| Ventilator-free days | 28 (26, 30) | 27 (14, 30) | .186 |
| ICU-free days | 24 (21, 27) | 20 (8, 27) | .036∗ |
| Hospital-free days | 17 (9, 21) | 6 (0, 16) | .000∗ |
| In-hospital mortality, n (%) | 4 (3.3) | 2 (8.3) | .259 |
Data are presented as median (Q1, Q3) for continuous variables.
AIS, abbreviated injury scale; ICU, intensive care unit; RBC, red blood cell; Q, quartile.
Denotes P < .05.
Thromboinflammatory alterations distinguish VTE status after traumatic injury
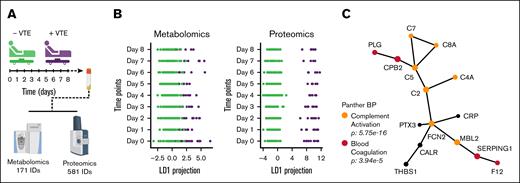
We first examined the entire cohort of 145 patients, regardless of enoxaparin responsiveness, to determine global omics signatures that differed between trauma patients who did and did not develop VTE, yielding high-confidence identifications of 171 metabolites and 581 proteins (Figure 1A). Metabolome and proteome wide linear discriminant analysis (LDA) were performed across the monitored time points and indicated distinct profiles dependent on patient VTE status (Figure 1B). Metabolites that significantly contributed to the separation observed in the LDA model did not form a known interaction network, indicating that subtle differences were detected. Conversely, the proteins identified from LDA formed an extensive network that highlighted biological processes including complement activation (C2, C5, C4A, C7, C8A, and MBL2) and blood coagulation (FXII, PLG, SERPING1, and CPB2; Figure 1C). Network analysis also identified related roles for C-reactive protein (CRP), pentaxin 3, calreticulin, and thrombospondin 1, which all have known or putative functions in regulating acute inflammation. Together, these data suggest that in addition to hypercoagulability, postinjury VTE is driven by enhanced innate immunity and thromboinflammation.
Multiomics analyses reveal distinct molecular signatures depended on VTE status. (A) The cohort comprised 145 patients, including 121 who did not develop VTE (green) and 24 who developed VTE (purple). Blood samples were collected from each patient at 8 time points, spanning from arrival to 8 days after admission. The plasma fraction of these samples was subjected to MS-based multiomics analyses. (B) LDA was performed on the metabolomic and proteomic profiles of the patients across the monitored time points. (C) Protein network detected by the LDA model, annotated with the top significantly enriched biological processes, highlighting the functional implications of the proteomic alterations.
Multiomics analyses reveal distinct molecular signatures depended on VTE status. (A) The cohort comprised 145 patients, including 121 who did not develop VTE (green) and 24 who developed VTE (purple). Blood samples were collected from each patient at 8 time points, spanning from arrival to 8 days after admission. The plasma fraction of these samples was subjected to MS-based multiomics analyses. (B) LDA was performed on the metabolomic and proteomic profiles of the patients across the monitored time points. (C) Protein network detected by the LDA model, annotated with the top significantly enriched biological processes, highlighting the functional implications of the proteomic alterations.
VTE development among patients who do not respond to enoxaparin is associated with elevated coagulation factors, inflammatory mediators, and markers of endothelial activation
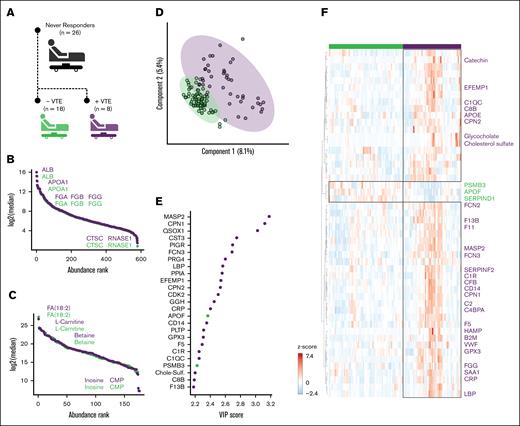
Our prior work demonstrated that VTE rates are highest among patients who exhibit poor responsiveness to enoxaparin3; however, it remains unknown what determines VTE development among this subgroup. Given our observation that VTE after trauma is associated with plasma thromboinflammatory markers, we next focused our analysis on patients who did not respond to enoxaparin, defined as never achieving an anti-FXa level ≥0.2 IU/mL over the first 8 days of hospitalization. Of the 145 patients included in the study, 26 were classified as never responders, among whom 8 patients developed VTE (30.8%; Figure 2A). The median time of VTE diagnosis was hospital day 5 (4-10). Similar to the entire cohort, no differences in age, sex, race/ethnicity, body mass index, admission vital signs, or injury mechanism/severity were identified between never responders who did and did not develop VTE (Table 2). Within this subgroup, there were also no differences in 24-hour transfusion; however, we did observe significantly fewer hospital-free days among never responders who developed VTE compared to those who did not develop VTE (10 [3-19] vs 20 [16-23] days, respectively; P = .042; Table 2).
Multiomics profiling of enoxaparin never responders. (A) Twenty-six patients were identified as never responders to enoxaparin therapy. Among these, 18 patients did not develop VTE, whereas 8 patients developed VTE (purple). (B) Ranked log2-transformed median protein abundances for the never responders, grouped by VTE development status. (C) Similarly, ranked log2-transformed median metabolite abundances displayed for the never responders, grouped by VTE development status. (D) PLS-DA was used to classify the integrated metabolomic and proteomic signatures of patients based on VTE development status. (E) The top 25 molecular features identified by the PLS-DA model contributed to the differentiation between VTE development status groups. (F) Heat map depicting the top 50 molecular features that showed significant differences between the VTE development status groups, as determined by the t test. Feature names were colored according to key thromboinflammatory pathways of interest to this analysis. Orange, complement activation; red, blood coagulation; dark blue, immune response; light blue, acute phase response. The analysis incorporated data from all time points.
Multiomics profiling of enoxaparin never responders. (A) Twenty-six patients were identified as never responders to enoxaparin therapy. Among these, 18 patients did not develop VTE, whereas 8 patients developed VTE (purple). (B) Ranked log2-transformed median protein abundances for the never responders, grouped by VTE development status. (C) Similarly, ranked log2-transformed median metabolite abundances displayed for the never responders, grouped by VTE development status. (D) PLS-DA was used to classify the integrated metabolomic and proteomic signatures of patients based on VTE development status. (E) The top 25 molecular features identified by the PLS-DA model contributed to the differentiation between VTE development status groups. (F) Heat map depicting the top 50 molecular features that showed significant differences between the VTE development status groups, as determined by the t test. Feature names were colored according to key thromboinflammatory pathways of interest to this analysis. Orange, complement activation; red, blood coagulation; dark blue, immune response; light blue, acute phase response. The analysis incorporated data from all time points.
Demographics, admission vital signs, and outcome data for patients who did not respond to enoxaparin by VTE group
| . | Never responder No VTE (n = 18) . | Never responder VTE (n = 8) . | P value . |
|---|---|---|---|
| Demographics | |||
| Age (y) | 36 (28, 53) | 31 (23, 49) | .469 |
| Male, n (%) | 13 (72.2) | 6 (75.0) | .883 |
| Race, White, n (%) | 14 (77.8) | 5 (62.5) | .435 |
| Ethnicity, Hispanic/Latino, n (%) | 5 (27.8) | 2 (25.0) | .883 |
| Body mass index | 27 (24, 31) | 26 (26, 32) | .573 |
| Admission vital signs and laboratory tests | |||
| Heart rate (bpm) | 92 (72, 123) | 108 (81, 125) | .531 |
| Systolic blood pressure (mm Hg) | 123 (115, 150) | 110 (69, 136) | .243 |
| Base excess/deficit (mmol/L) | −2 (−4, −1) | −4.5 (−6.5, −2.0) | .188 |
| Injury | |||
| Blunt, n (%) | 13 (72.2) | 6 (75.0) | .883 |
| Glasgow Coma Scale | 15 (3, 15) | 3 (3, 15) | .371 |
| Injury Severity Score | 25 (16, 34) | 26 (20, 42) | .541 |
| Transfusion volumes (units) | |||
| 24-hour whole blood | 0 (0, 1) | 0 (0, 1) | 1.000 |
| 24-hour RBC | 0 (0, 2) | 1 (0, 6) | .384 |
| 24-hour plasma | 0.5 (0, 2) | 1 (0, 2) | .807 |
| 24-hour platelets | 0 (0, 1) | 0 (0, 0) | .757 |
| 24-hour cryoprecipitate | 0 (0, 0) | 0 (0, 0) | .745 |
| Outcomes | |||
| Ventilator-free days | 29 (27, 30) | 27 (19, 30) | .266 |
| ICU-free days | 27 (25, 30) | 24 (15, 29) | .218 |
| Hospital-free days | 20 (16, 23) | 10 (3, 19) | .042∗ |
| In-hospital mortality, n (%) | 1 (5.6) | 0 (0.0) | .497 |
| . | Never responder No VTE (n = 18) . | Never responder VTE (n = 8) . | P value . |
|---|---|---|---|
| Demographics | |||
| Age (y) | 36 (28, 53) | 31 (23, 49) | .469 |
| Male, n (%) | 13 (72.2) | 6 (75.0) | .883 |
| Race, White, n (%) | 14 (77.8) | 5 (62.5) | .435 |
| Ethnicity, Hispanic/Latino, n (%) | 5 (27.8) | 2 (25.0) | .883 |
| Body mass index | 27 (24, 31) | 26 (26, 32) | .573 |
| Admission vital signs and laboratory tests | |||
| Heart rate (bpm) | 92 (72, 123) | 108 (81, 125) | .531 |
| Systolic blood pressure (mm Hg) | 123 (115, 150) | 110 (69, 136) | .243 |
| Base excess/deficit (mmol/L) | −2 (−4, −1) | −4.5 (−6.5, −2.0) | .188 |
| Injury | |||
| Blunt, n (%) | 13 (72.2) | 6 (75.0) | .883 |
| Glasgow Coma Scale | 15 (3, 15) | 3 (3, 15) | .371 |
| Injury Severity Score | 25 (16, 34) | 26 (20, 42) | .541 |
| Transfusion volumes (units) | |||
| 24-hour whole blood | 0 (0, 1) | 0 (0, 1) | 1.000 |
| 24-hour RBC | 0 (0, 2) | 1 (0, 6) | .384 |
| 24-hour plasma | 0.5 (0, 2) | 1 (0, 2) | .807 |
| 24-hour platelets | 0 (0, 1) | 0 (0, 0) | .757 |
| 24-hour cryoprecipitate | 0 (0, 0) | 0 (0, 0) | .745 |
| Outcomes | |||
| Ventilator-free days | 29 (27, 30) | 27 (19, 30) | .266 |
| ICU-free days | 27 (25, 30) | 24 (15, 29) | .218 |
| Hospital-free days | 20 (16, 23) | 10 (3, 19) | .042∗ |
| In-hospital mortality, n (%) | 1 (5.6) | 0 (0.0) | .497 |
Data are presented as median (Q1, Q3) for continuous variables.
Denotes P < .05.
Further, no differences in admission TEG values were identified (supplemental Figure 1B). When comparing time-dependent changes in fibrinogen and platelet counts, no differences were observed between never responders who did and did not develop VTE (supplemental Figure 2A-B), reinforcing the challenges in identifying patients at highest risk of VTE. However, we did observe significant decreases in AT activity among those who developed VTE on admission and hospital day 7 (supplemental Figure 2C), in agreement with our previous work.3 When assessing anti-FXa levels, no differences were found over time within the never-responder subgroup (supplemental Figure 2D). Moreover, no differences in thrombin generation were identified between groups (supplemental Figure 2E-F).
To identify multiomic endotypes associated with VTE in never responders, we used PLS-DA modeling and variable importance in projection (VIP) scores. Overall total plasma protein (Figure 2B) and metabolite (Figure 2C) abundances were not significantly different between never-responder patients who did and did not develop VTE. PLS-DA models were developed using integrated multiomics profiles, which revealed clear separation into 2 distinct cohorts corresponding to patients who did and did not develop VTE. (Figure 2D). Proteins rather than metabolites were the leading contributors to the separation, similar to the entire cohort, as determined through assessment of the VIP scores (Figure 2E). The top 25 features with highest VIP scores highlighted key proteins involved with acute inflammation (eg, CPN1, LBP, CRP, and CD14), complement activation (eg, MASP2, FCN3, C1R, C1QC, and C8B), and coagulation (eg, FV, FXIIIB). Identified metabolites included glycocholate, a bile salt involved in lipid digestion and cholesterol metabolism, cholesterol sulfate, involved in cellular signaling, cholesterol metabolism, and membrane integrity, and catechin, which has a protective role against oxidative stress and inflammation. Hierarchical clustering indicated that the top 50 differentially abundant proteins and metabolites were associated with the same pathways that were identified through PLS-DA (Figure 2F), validating that the significant abundance differences were largely increased in patients with VTE.
Time-dependent changes in proteins involved in coagulation, inflammation, and acute phase responses between never responders who did and did not develop VTE
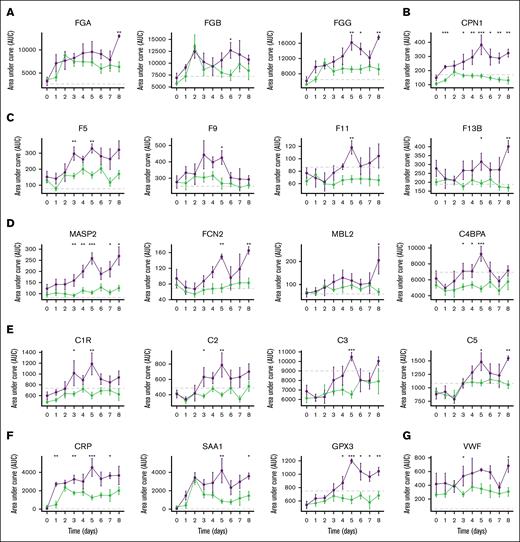
Direct t test comparisons between VTE status were then performed for patient time point samples to determine specific multiomic differences. Two-way analysis of variance (ie, time and VTE status) showed significant differences in the longitudinal trends of fibrinogen chains (Figure 3A), CPN1 (Figure 3B), coagulation proteins (Figure 3C), complement activation (Figure 3D), complement pathway (Figure 3E), the acute phase response (Figure 3F), as well as the endothelial marker von Willebrand factor (VWF; Figure 3G). No differences in liver function markers, aspartate aminotransferase and alanine aminotransferase, were identified between groups. Further, no differences in platelet count or platelet activation markers, including platelet factor 4, were observed between cohorts.
Longitudinal protein abundances among never responders who do and do not develop VTE. Median plasma protein levels with standard error across monitored time points, categorized by VTE development status: no VTE and VTE (purple). The analysis specifically focused on patients who responded poorly to enoxaparin treatment. (A) Fibrinogen chain levels. (B) Carboxypeptidase N catalytic chain. (C) Coagulation factors. (D) Complement activators. (E) Complement proteins. (F) Acute phase proteins. (G) VWF.
Longitudinal protein abundances among never responders who do and do not develop VTE. Median plasma protein levels with standard error across monitored time points, categorized by VTE development status: no VTE and VTE (purple). The analysis specifically focused on patients who responded poorly to enoxaparin treatment. (A) Fibrinogen chain levels. (B) Carboxypeptidase N catalytic chain. (C) Coagulation factors. (D) Complement activators. (E) Complement proteins. (F) Acute phase proteins. (G) VWF.
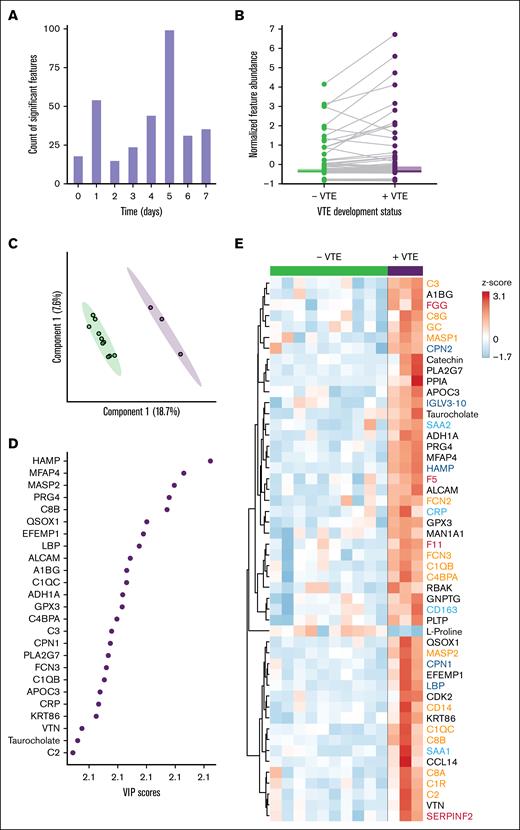
The time dependency of protein and metabolite circulating abundance differences observed between VTE status was further assessed. Specifically, by summing the number of multiomics features with significant differences per time point (Figure 4A). Day 5 showed the greatest number of significant differences (n = 99 features) between VTE status (Figure 4A), which was the median day of VTE occurrence. This result guided us to focus on key multiomics differences between VTE status that occurred on day 5. Direct comparison of the 99 significantly different features showed that the major normalized abundance alterations reflected increases in patients with VTE (Figure 4B). Importantly, the variation explained between VTE status increased in the PLS-DA model (Figure 4C), and the features with highest VIP scores were similarly thromboinflammatory markers (Figure 4D). Focusing on the top 50 significantly different features between never responders who did, or did not, develop VTE revealed notable increases in omics biomarkers in patients with VTE (Figure 4E). Of note, significantly increased proteins in the VTE group included those involved in coagulation (fibrinogen gamma chain, FV, FXI, α2-antiplasmin), implicating key roles for hypercoagulability and reduced fibrinolysis. We also observed significant increases in proteins that regulate inflammation, including complement activators (MASP2, C1R, C2, C3, C4BPA, C8A, C8B, and C8G), CD14, and CRP. Collectively, these results implicate a combined role for enhanced coagulation and innate immunity in VTE development among patients with poor responsiveness to enoxaparin.
Day 5 multiomics signatures in never responders highlight key altered pathways in the VTE cohort. (A) Significant differences in protein and metabolite levels between VTE development status groups were assessed specifically for patients who responded poorly to enoxaparin, at each monitored time point. The day 5 time point exhibited the highest number of significantly different features. (B) Pairwise comparison of features detected in both VTE status groups, specifically for day 5 plasma samples. (C) PLS-DA of day 5 multiomic signatures, categorized by VTE status. (D) The top 25 molecular features identified by the PLS-DA model that contributed to the differentiation between VTE status. (E) Heat map presentation of the top 50 molecular features that showed significant differences between the VTE status groups at day 5, as determined by the t test. Feature names were colored according to key thromboinflammatory pathways of interest to this analysis. Orange, complement activation; red: blood coagulation; dark blue, immune response; light blue, acute phase response.
Day 5 multiomics signatures in never responders highlight key altered pathways in the VTE cohort. (A) Significant differences in protein and metabolite levels between VTE development status groups were assessed specifically for patients who responded poorly to enoxaparin, at each monitored time point. The day 5 time point exhibited the highest number of significantly different features. (B) Pairwise comparison of features detected in both VTE status groups, specifically for day 5 plasma samples. (C) PLS-DA of day 5 multiomic signatures, categorized by VTE status. (D) The top 25 molecular features identified by the PLS-DA model that contributed to the differentiation between VTE status. (E) Heat map presentation of the top 50 molecular features that showed significant differences between the VTE status groups at day 5, as determined by the t test. Feature names were colored according to key thromboinflammatory pathways of interest to this analysis. Orange, complement activation; red: blood coagulation; dark blue, immune response; light blue, acute phase response.
Discussion
The identification of injured patients at highest risk for developing VTE, remains a major clinical challenge and is particularly common among patients exhibiting resistance or poor responsiveness (never responders) to the standard-of-care thromboprophylactic, LMWH.3,4 Our work addresses this diagnostic conundrum via endotype identification of proteins driving postinjury VTE. Our analysis resulted in clear distinction between these 2 groups, identifying key plasma constituents and molecular pathways uniquely enriched among patients with VTE, including those involved in coagulation, suppression of fibrinolysis, innate immunity, endothelial activation, and sustained acute phase responses.
Prior work has firmly established elevated coagulation factor levels, reduced AT activity, and enhanced thrombin generation among trauma patients who develop VTE compared to those who do not.3,15-17 Here, we identified greater abundance of FV, FIX, FXI, FXIIIB, and fibrinogen α, β, and γ chains among never responders who developed VTE compared to those who did not. Interestingly, significant differences were observed between hospital days 3 to 8, beyond the time at which most coagulation monitoring is conducted clinically, but during which time most VTE occur. FXIIIB was among the top differentially abundant proteins between patients who did and did not develop VTE. FXIII is a heterodimer, including 2 A subunits and 2 B subunits.18 It is unclear from this analysis why enrichment of FXIIIB was detected and not FXIIIA; however, this could be related to increased overall abundance of circulating FXIIIB compared to FXIIIA, altered clearance/consumption of FXIIIA, or changes in FXIIIA-B complexing. We also observed significant increases in VWF among patients who developed VTE. As VWF is stored within endothelial Weibel-Palade bodies and released upon activation,19 our findings could indicate that increased (or unresolved) endothelial activation or dysregulation is a key mechanism distinguishing VTE development among patients who do not respond to enoxaparin, in agreement with prior work.20 We also observed significant differences in several serine protease inhibitors, including SERPINF2 and SERPIND1. SERPINF2, or α2-antiplasmin, regulates fibrinolysis through its inhibition of plasmin and can become incorporated into developing clots through FXIII cross links, bringing it into close proximity to plasmin generated by fibrin-bound tissue plasminogen activator and plasminogen.21 The fact that we observed concomitant increases in FXIII and SERPINF2 could indicate that elevations in these proteins cooperatively stabilize fibrin clot structure and protect them from fibrinolysis. We also observed a significant reduction in abundance of SERPIND1, also known as heparin cofactor II, which both inhibits thrombin and serves as a cofactor for heparin anticoagulants22; however, given the weak association between heparin cofactor II deficiency and thrombosis,23 the precise physiologic role is unclear.
Our analyses determined that most proteins separating the VTE and non-VTE cohorts were involved in inflammation, including complement activation, innate immune pathways, and immunoglobulins. Among the top 50 differentially abundant proteins that distinguish VTE development among never responders on hospital day 5, 10 are regulators of the complement system. Previous work has demonstrated a strong association between complement activation and risk of thromboembolic events, including increased C3, C5, and MASP2.24-26 Complement mediators C3 and C4 have been shown to augment leukocyte recruitment, activation, and neutrophil extracellular trap formation, promoting immunothrombosis.24 Indeed, prior work has shown that C3 and C4 knockout mice exhibit reduced thrombosis and targeting complement mediators does not affect hemostasis, reducing bleeding risks.24 Moreover, recent work has demonstrated that immunoglobulins can promote thrombosis through their interaction with endothelial cells, promoting platelet and leukocyte adhesion, and augmenting complement activation.24 Here, we observed significant increases in C1r, which activates C1, C2, C3, C4 binding protein α, and C8 among never responders who developed VTE. Notably, we also observed significant increases in the acute phase protein, CRP, which is known to activate complement, specifically via the classical pathway mediators C1, C2, C3, and C4.27 This could indicate complement activation is an inflammatory augmentation mechanism driven by acute phase response proteins, which are mobilized in response to systemic cytokine release. We did observe significant increases in mannan-binding lectin serine protease 2 (MASP2), which regulates the lectin pathway by cleaving C2 and C4 to ultimately produce the membrane attack complex. Interestingly, we did not observe changes in C9, indicating a lack of terminal attack complex generation. This could be due to the sterile etiology of postinjury inflammation rather than pathogen-associated inflammation. Past research has demonstrated MASP2 can promote lectin pathway activation in the absence of C4 to promote inflammation in sterile conditions such as ischemia reperfusion injury,28 suggesting a combination of classical and lectin complement pathway activation in the trauma setting could robustly influence ongoing inflammation.
Notably, most differentially abundant proteins and metabolites identified herein are synthesized in the liver. No differences in common liver function markers aspartate aminotransferase and alanine aminotransferase were identified between groups, indicating our observations are not likely due to liver injury and dysfunction, but rather greater activation and release of acute phase reactants from the liver. Although the liver is the primary source of coagulation protein production, plasma levels of FV and FXIII can transiently increase during platelet activation. However, we observed no differences in platelet count or platelet activation markers, including platelet factor 4, between cohorts. Alternatively, endothelial cells are activated upon exposure to inflammatory milieus and are important contributors to both thrombus formation and systemic inflammation.29-31 This study confirmed posttrauma VTE is associated with sustained increases in inflammatory markers over time, which correlated with markers of endothelial activation.32 Here, we observed significant increases in VWF levels among never responders who developed VTE, implicating a potential role for activated endothelial cells in promoting both systemic inflammation and local thrombus formation.
Examining never responders as a unique population is highly clinically significant given that 30% of trauma patients who develop VTE belong to this category. Our unbiased multiomics approach has demonstrated that ongoing hypercoagulability, presenting as poor enoxaparin responsiveness, is not the only driver of postinjury VTE but occurs concurrently with thromboinflammation via increased innate immune and endothelial activation. Importantly, these findings indicate that single modal prophylaxis strategies targeting coagulation alone are insufficient in many severely injured patients, suggesting that multimodal therapeutics targeting inflammation and endothelial dysfunction should be explored for improved VTE prevention. Such approaches could leverage existing and approved therapeutics, such as statins, which target inflammation and endothelial activation. The Statin and Aspirin Use in Trauma trial showed that statin administration in conjunction with aspirin reduced postinjury VTE risk compared to standard LMWH alone.33 Sanders et al34 demonstrated trauma patients taking prehospital or in-hospital statins had a significantly reduced incidence of VTE development compared to nonstatin users. Alternatively, development of synthetic heparin molecules that have multitarget properties could have future potential. Liao et al showed that a synthetic heparan sulfate octadecasaccharide with both anticoagulant (through FXa and thrombin inhibition), as well as anti-inflammatory properties (through histones and high mobility group box 1 neutralization), reduced coagulation, inflammation, and improved survival in murine sepsis models.35 Thus, combined therapeutics or novel tailored anticoagulants could provide new avenues for improving postinjury VTE prevention.
This study has several important limitations. First, the number of never responders was small (n = 26), including 8 patients who developed VTE and 18 patients who did not. The small sample size could have limited our ability to identify significant contributors to VTE. Second, measurements of enzymatic coagulation were limited to viscoelastic tests and thrombin generation assays. A more comprehensive analysis including enzyme-serpin complex or markers of fibrin degradation could have increased detectable differences between groups. Finally, patients in this analysis were not excluded based on antiplatelet use. Additional analyses will be conducted to determine their impact of patient endotypes. In addition, participants included in our healthy cohort did not have a recent history of anticoagulant or antiplatelet use, which could have influenced soluble biomarkers that differed between healthy and trauma groups.
Conclusions
In conclusion, unbiased proteomics analyses identified differentially abundant proteins that distinguish VTE development among patients who do not respond to enoxaparin. These findings implicate novel pathways in postinjury VTE pathogenesis, highlight the need for multimodal thromboprophylaxis strategies, and identify new candidate proteins for future therapeutic intervention.
Acknowledgments
This study was supported, in part, by research funding from Grifols (J.C.C.). M.J.C., A.D., and K.H. are supported by an award from the National Institutes of Health, National Institute of General Medical Sciences (RM1GM131968).
Authorship
Contribution: I.S.L. designed the research, conducted experiments, analyzed data, and wrote the manuscript; M.D. and F.C. performed experiments and edited the manuscript; K.S., B.A.C., and M.J.C. interpreted the data and edited the manuscript; C.E.W. assisted with study design and edited the manuscript; A.D. and K.H. assisted with study design, oversaw experiments, interpreted data, and edited the manuscript; J.C.C. conceived the project, designed the research, directed the project, analyzed and interpreted data, and wrote the manuscript; and all authors contributed to and approved the manuscript.
Conflict-of-interest disclosure: J.C.C. and B.A.C. report research funding and speaker honoraria from Grifols. The remaining authors declare no competing financial interests.
Correspondence: Jessica C. Cardenas, University of Colorado, 12700 East 19th Ave, Aurora, CO 80045; email: Jessica.C.Cardenas@cuanschutz.edu.
References
Author notes
Original data are available from the corresponding author, Jessica C. Cardenas (Jessica.C.Cardenas@cuanschutz.edu), upon reasonable request.
The full-text version of this article contains a data supplement.