Key Points
Female mice are more vulnerable to initial LPS challenge, but show greater resistance upon rechallenge.
Neutrophils protect against LPS shock in both sexes, and are key mediators of sex-based differences in response.
Visual Abstract
Lipopolysaccharide (LPS) exposure in mice induces robust morbidity and mortality, and is widely used as a model for sepsis. However, the role of biological sex in modulating immune responses during LPS-induced sepsis remains incompletely understood. In this study, we investigated how sex influences immune responses following LPS challenge in mice. Using age-matched mice, we found that during primary LPS challenge, females exhibited significantly higher mortality than males. This difference correlated with greater production of proinflammatory cytokines in females. Further analysis revealed that female myeloid cells expressed higher levels of Toll-like receptor 4, and displayed enhanced activation of NF-κB and MAPK signaling. Additionally, compared with males, female macrophages expressed significantly more inducible nitric oxide synthase but less arginase, supporting a sex-based divergence in inflammatory response to LPS. Interestingly, during lethal LPS rechallenge, the sex bias was reversed, with higher mortality observed in males than in females. These findings suggest that males had a survival advantage during the primary LPS challenge, while females exhibited greater resistance during rechallenge, emphasizing the need for careful consideration of sex-based differences in sepsis models. Neutrophils played a critical role in these sex-based differences. Neutrophil depletion significantly increased susceptibility to both primary and secondary LPS challenge. Notably, the sex bias in LPS-induced shock disappeared in neutrophil-depleted mice, highlighting a previously unrecognized role for neutrophils in mitigating LPS-induced mortality and maintaining sex-based differences in sepsis outcome.
Introduction
Sex-based differences in inflammation, immunity, and disease susceptibility are well documented.1 These disparities are due to genetic and hormonal differences, including the unequal distribution of X and Y chromosomes, and the influence of sex hormones on immune function.2-6 The X chromosome encodes several key inflammatory genes, such as Toll-like receptor 4 (TLR7), TLR8 and IRAK1, which contribute to sex-based immune disparities.7 Sex differences are evident in the incidence of infectious and autoimmune diseases. Autoimmune disorders, including Sjögren syndrome, systemic sclerosis, and systemic lupus erythematous, occur severalfold more frequently in females than in males.8-11 Additionally, females exhibit stronger immune responses to multiple vaccines and, thus, are more prone to vaccine-associated adverse effects.12 In contrast, males have higher susceptibility to various infections, including bacterial, viral, fungal, and parasitic diseases.13,14 Recent data on COVID-19–associated morbidity and mortality further support this trend, with significantly lower rates observed in females.15,16 On the other hand, males exhibit a higher incidence of many cancers compared with females.17,18 The stronger inflammatory responses observed in females may underlie their higher prevalence of autoimmune diseases, enhanced vaccine efficacy, and improved infection control.19,20 These differences highlight the critical role of sex as a biological variable in disease susceptibility and immune regulation.
Despite well-documented sex-based differences in disease susceptibility, where some conditions disproportionately affect males while others favor females, research on sex as a biological variable remains limited. A significant proportion of studies fail to account for sex-based variables, limiting our understanding of their impact.21 A 2009 review revealed that <10% of immunology papers reported sex as a study variable, highlighting a persistent gap in the field.21 Given the clear disparities in immune responses and disease outcomes between males and females, rigorous cellular and molecular investigations are necessary to elucidate the mechanisms driving these differences. To address this gap, we examined sex-based differences in immune responses during lipopolysaccharide (LPS)-induced sepsis in mice.
Sepsis is a severe medical condition characterized by an uncontrolled immune response to systemic infection, leading to excessive proinflammatory cytokine production (ie, cytokine storm), multiorgan failure and, ultimately, death.22 Epidemiological and experimental studies have reported higher sepsis incidence and mortality in males,22-26 although some studies have found no significant sex-based differences.27 In humans, postsurgical sepsis has been associated with greater morbidity and mortality in males than females.24,28-32 Similarly, in a 2-hit trauma-induced shock model followed by sepsis, male mice consistently exhibit higher morbidity and mortality.33 A related study, in which mice received beta-glucan therapy 1 hour after cecal ligation and puncture (CLP) surgery, also demonstrated poorer survival in males.34 However, findings from standard sepsis models, including CLP, bacterial sepsis, or LPS-induced shock, have been inconsistent. In CLP-induced sepsis, mortality rates did not significantly differ between male and female BALB/c mice, though females exhibited ∼25% lower survival.35 In Streptococcus pyogenes–induced sepsis, female mice exhibited higher levels of interleukin-6 (IL-6) and tumor necrosis factor (TNF), and suffered more rapid mortality than males.36 Similarly, in a TNF-induced shock model, female mice had significantly greater mortality than males.36 Supporting the notion that females mount stronger inflammatory responses, one study reported that LPS-induced plasma levels of IL-1β, TNF and IL-10 at 6 hours were significantly higher in females than in males.37 However, in neonatal mice, LPS treatment did not reveal major sex differences in the expression of TLR4 or proinflammatory cytokines.38 Given these conflicting findings, further rigorous, well-controlled studies are warranted to clarify the role of biological sex in sepsis susceptibility.
We investigated the role of biological sex in a standard LPS-induced sepsis model using adult male and female mice, and identified several cellular and molecular differences that may account for the observed sex-based disparities. During primary LPS challenge, female mice exhibited greater mortality than males, aligning with previous findings that females generally mount stronger immune responses, display lower susceptibility to infections, and exhibit greater vaccine responsiveness than males.1 However, in LPS rechallenge experiments, we observed a striking reversal of susceptibility, where female mice demonstrated increased resistance to LPS-induced shock compared with males. Notably, our LPS rechallenge results are consistent with epidemiological studies in patients with surgical sepsis and of 2-hit trauma-sepsis models, both of which report higher susceptibility in males.39 These findings suggest that sex-based differences in sepsis outcomes are influenced by prior immune exposure, emphasizing the need to consider exposure history when evaluating sex bias in sepsis susceptibility. At baseline and following LPS challenge, we identified key cellular and molecular differences between male and female mice that provide new insights into sex-specific immune responses and survival outcomes in LPS-induced sepsis. Additionally, our findings highlight a critical protective role for neutrophils during both primary and secondary LPS challenge–induced sepsis. Importantly, our data demonstrate that neutrophils contribute to the observed sex bias in LPS-induced mortality, further underscoring their key role in shaping sepsis outcomes.
Methods
For detailed methods, please refer to the supplemental Information.
Sex as a biological variable
This study specifically investigates the role of sex as a biological variable. To ensure comparability, all experiments utilized sex- and age-matched C57BL/6 mice (8-16 weeks old).
In vivo LPS challenge
Mice were intraperitoneally (IP) challenged with 3 to 7.5 mg/kg LPS. In some experiments, LPS-challenged mice were allowed to recover for 7 days before being rechallenged with a higher dose of LPS (35 mg/kg, IP). Mice were monitored for survival or euthanized 6 hours after challenge for serum cytokine analysis. For additional analyses, PBL, spleen, and peritoneal lavage samples were collected at 6 hours or 1 day after LPS challenge. Prepared samples were analyzed using flow cytometry, western blotting, and enzyme-linked immunosorbent assay (ELISA).
Neutrophil depletion
To deplete neutrophils, mice were treated with 400 μg of anti–Ly6G antibody on days −1 and 0 relative to LPS treatment. Control mice received an equivalent dose of rat immunoglobulin G2a isotype control antibody. Neutrophil depletion was confirmed by flow cytometry.
Bone marrow–derived macrophages (BMDMs)
Bone marrow cells were harvested from femur femurs and tibias as previously described,40 with slight modifications.
Study approval
All experimental procedures were approved by the Office of Animal Resources of the University of Iowa under Institutional Animal Care and Use Committee protocol 3032004 (principal investigator, P.G.).
Results
Female mice exhibit increased susceptibility to primary LPS challenge
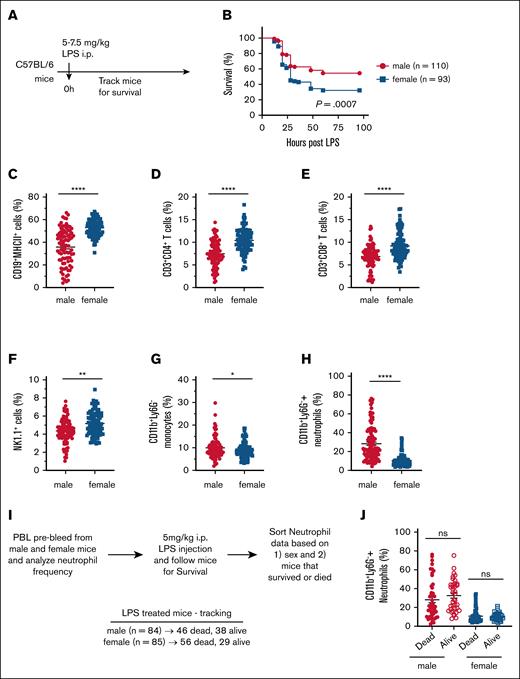
Sex-based differences in immune responses have been widely reported across various infections and disease conditions.1,14,39,41,42 However, the role of sex in sepsis remains unclear, with conflicting reports indicating resistance in either males or females.1,14,39 To address this gap, we examined sex differences in sepsis-induced mortality using a well-established LPS-induced shock model in adult C57BL/6 mice. To objectively assess the impact of sex on LPS-induced mortality, we conducted a retrospective analysis of survival data from C57BL/6 mice subjected to LPS challenge. Mice were injected IP with 5 to 7.5 mg/kg LPS, and monitored for survival (Figure 1A). Our analysis revealed a significantly higher susceptibility to LPS-induced mortality in female mice compared with males. Specifically, the mortality rate was 67.74% in females, whereas males exhibited a lower mortality rate of 45.45% (Figure 1B). Given the unbiased approach and the large sample size (∼100 per sex), our findings strongly indicate that female mice are more vulnerable to primary LPS–induced shock than male mice.
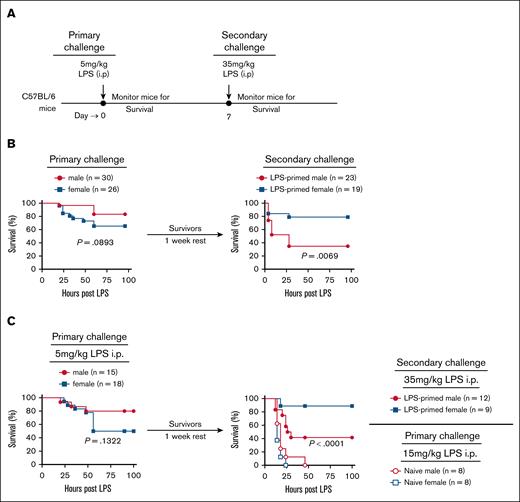
Increased susceptibility of female mice to LPS-induced endotoxic shock. (A) Experimental design for survival analysis following LPS-induced endotoxic shock. (B) Retrospective survival analysis of 8- to 16-week-old C57BL/6 male (n = 110) and female (n = 93) mice after IP injection of 5 to 7.5 mg/kg LPS. (C-H) Baseline frequencies of immune cell populations in the PBL of male (n = 104) and female (n = 102) mice: (C) CD19+MHCII+ B cells, (D) CD3+CD4+ T cells, (E) CD3+CD8+ T cells, (F) NK1.1+ NK cells, (G) CD11b+Ly6G– monocytes, and (H) CD11b+Ly6G+ neutrophils. (I) Experimental outline for assessing CD11b+Ly6G+ neutrophil frequency in relation to sex and survival following LPS shock. (J) Baseline frequency of CD11b+Ly6G+ neutrophils in PBLs, stratified by survival outcome after LPS-induced endotoxic shock. Each dot represents an individual mouse. Data are presented as mean ± standard error of the mean (SEM). Survival curves were analyzed using the log rank (Mantel-Cox) test. Statistical significance of immune cell frequency differences was determined using 2-tailed t tests (Mann-Whitney U tests). P values < .05 were considered to indicate statistical significance. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. ns, not significant.
Increased susceptibility of female mice to LPS-induced endotoxic shock. (A) Experimental design for survival analysis following LPS-induced endotoxic shock. (B) Retrospective survival analysis of 8- to 16-week-old C57BL/6 male (n = 110) and female (n = 93) mice after IP injection of 5 to 7.5 mg/kg LPS. (C-H) Baseline frequencies of immune cell populations in the PBL of male (n = 104) and female (n = 102) mice: (C) CD19+MHCII+ B cells, (D) CD3+CD4+ T cells, (E) CD3+CD8+ T cells, (F) NK1.1+ NK cells, (G) CD11b+Ly6G– monocytes, and (H) CD11b+Ly6G+ neutrophils. (I) Experimental outline for assessing CD11b+Ly6G+ neutrophil frequency in relation to sex and survival following LPS shock. (J) Baseline frequency of CD11b+Ly6G+ neutrophils in PBLs, stratified by survival outcome after LPS-induced endotoxic shock. Each dot represents an individual mouse. Data are presented as mean ± standard error of the mean (SEM). Survival curves were analyzed using the log rank (Mantel-Cox) test. Statistical significance of immune cell frequency differences was determined using 2-tailed t tests (Mann-Whitney U tests). P values < .05 were considered to indicate statistical significance. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. ns, not significant.
To investigate potential baseline immune differences contributing to this disparity, we retrospectively analyzed PBL composition in naïve male and female C57BL/6 mice (Figure 1C-H). It should be noted that both male and female mice have similar number of total white blood cell count (data not shown). However, females had significantly higher frequencies of lymphocytes and natural killer (NK) cells that include CD4+ T cells, CD8+ T cells, CD19+ B cells and NK1.1+ cells (Figure 1C-F). In contrast, males exhibited significantly greater abundance of myeloid cells, specifically monocytes and neutrophils (Figure 1G-H). Given that neutrophils have been implicated in mitigating LPS-induced toxicity and mortality,43 we further examined whether baseline neutrophil frequency correlated with survival following LPS challenge (Figure 1I-J). We hypothesized that higher baseline neutrophil levels would be associated with increased survival. However, our analysis revealed no significant correlation between neutrophil frequency and survival outcomes, as neutrophil levels were comparable between surviving and deceased mice (Figure 1J).
LPS challenge induces elevated cytokine production in female mice
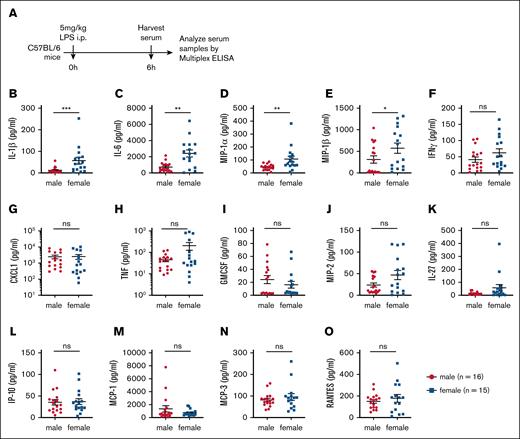
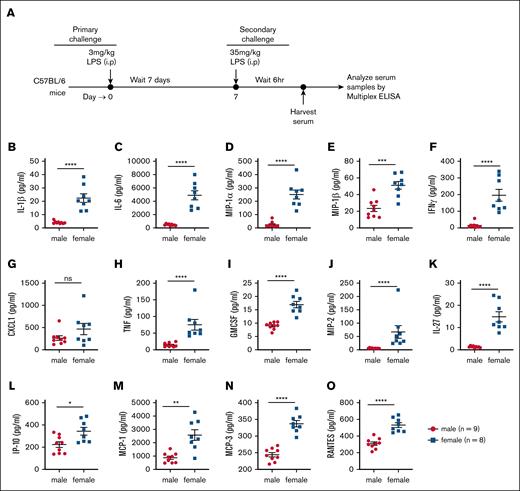
Sepsis is characterized by an exaggerated immune response to systemic infection, often leading to multiorgan failure and death.44 Similarly, LPS-induced mortality is driven by excessive immune activation and systemic cytokine production.45 Given our finding that female mice exhibit greater susceptibility to LPS-induced mortality (Figure 1), we hypothesized that their cytokine response to LPS challenge would be significantly elevated. To test this hypothesis, age-matched C57BL/6 male and female mice were injected IP with 5 mg/kg LPS, and serum cytokine levels were measured 6 hours after injection using multiplex ELISA kits (Figure 2A). Female mice exhibited significantly higher levels of proinflammatory cytokines, including IL-1β, IL-1α, IL-6, MIP-1α and MIP-1β (Figure 2B-E; supplemental Figure 1). Additionally, interleukins such as IL-7, IL-7RA, IL-19, IL-25 and IL-28 were significantly elevated in female mice compared with males (supplemental Figure 1). Analysis of a broader panel of cytokines [interferon-gamma, C-X-C motif chemokine ligand 1 (CXCL1), TNF, granulocyte-macrophage colony-stimulating factor (GMCSF), macrophage inflammatory protein 2 (MIP2), IL-27, interferon gamma-inducible protein 10 (IP-10), monocyte chemoattractant protein (MCP)-1, MCP-3, RANTES, granulocyte colony-stimulating factor (GSCF), IL-18, LIF, leptin, IL-33 and IL-33R] revealed a similar trend, with female mice producing higher levels than males (Figure 2; supplemental Figure 1). Notably, no cytokine measured in this study was found to be more abundant in male mice than in females (Figure 2; supplemental Figure 1). Taken together, these findings demonstrate that female mice exhibit heightened sensitivity to LPS challenge in vivo, which is accompanied by an amplified production of multiple proinflammatory cytokines.
Female mice exhibit elevated serum levels of LPS-induced cytokines and chemokines compared with male mice. (A) Experimental timeline outlining the assessment of inflammatory responses following LPS (5 mg/kg) challenge. (B-O) Serum concentrations of proinflammatory cytokines and chemokines in male (n = 16) and female (n = 15) mice. Each dot represents an individual mouse. Statistical significance was determined using 2-tailed t tests (Mann-Whitney U tests) for 2 groups. P values < .05 were considered to indicate statistical significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, not significant.
Female mice exhibit elevated serum levels of LPS-induced cytokines and chemokines compared with male mice. (A) Experimental timeline outlining the assessment of inflammatory responses following LPS (5 mg/kg) challenge. (B-O) Serum concentrations of proinflammatory cytokines and chemokines in male (n = 16) and female (n = 15) mice. Each dot represents an individual mouse. Statistical significance was determined using 2-tailed t tests (Mann-Whitney U tests) for 2 groups. P values < .05 were considered to indicate statistical significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, not significant.
BMDMs from age-matched male and female mice were analyzed for MAPK and NF-κB pathway activation following LPS stimulation (supplemental Figure 2A). No significant sex-based differences were observed in signaling pathway activation or cytokine production (supplemental Figure 2B-D). LPS tolerance was also comparable between sexes (supplemental Figure 3A-B), indicating that sex does not affect LPS priming or tolerance in vitro.
While male mice have high levels of testosterone and low estrogen, female mice exhibit high estrogen and low testosterone.46,47 To assess the role of sex hormones, male and female BMDMs were treated with dihydrotestosterone or 17β-estradiol (estrogen), and then stimulated with LPS (supplemental Figure 3C). Both hormones similarly enhanced LPS-induced MAPK and NF-κB activation in BMDMs from both sexes (supplemental Figure 3D). These findings suggest that while sex hormones modulate LPS responses, male and female BMDMs exhibit similar signaling and tolerance profiles.
Differences in baseline and LPS-induced peritoneal lavage cells suggest an anti-inflammatory environment in males compared with females
Given the lack of sex-based differences in LPS responses in in vitro–derived BMDMs, we investigated whether primary immune cells in vivo exhibit sex-specific differences following LPS challenge. Because LPS is administered IP, immune cells in the peritoneum play a crucial role in initiating immune responses. Thus, we examined differences in peritoneal lavage cells between male and female mice at baseline and after LPS challenge. Peritoneal lavage cells were collected from male and female mice at baseline (control, no LPS treatment) and at 1 day after LPS challenge, followed by flow cytometry analysis (Figure 3A). The total number of peritoneal lavage cells was comparable between males and females, both at baseline and after LPS challenge (Figure 3B). LPS challenge is known to induce lymphopenia, characterized by a marked reduction in both B and T cells.48 Although we did not observe a significant reduction in the total number of peritoneal lavage cells, a trend toward a decrease was noted in the LPS-treated group compared with controls (Figure 3B). Peritoneal lavage cells were primarily analyzed for myeloid cell populations using CD11b and Ly6G markers, revealing 3 major subsets: population A, CD11bmodLy6G– cells (denoted as CD11bmod cells); population B, CD11bhiLy6G– cells (monocyte/macrophage population of the peritoneum); and population C, CD11b+Ly6G+ cells (neutrophils; Figure 3C). At baseline, only CD11bmod and CD11bhiLy6G– cells were present in peritoneal lavage samples (Figure 3D). Notably, the frequencies of these populations differed between males and females, with females exhibiting higher frequency of CD11bmod cells and a lower frequency of CD11bhiLy6G– cells than males (Figure 3D-E). At 1 day after LPS challenge, CD11b+Ly6G+ neutrophils were present in both male and female mice, but the frequency of neutrophil influx was significantly higher in males (Figure 3D-E).
LPS challenge induces greater neutrophil infiltration and reduced iNOS expression in male mice compared with female mice. (A) Schematic of the experimental design. Male and female mice were injected IP with 5 mg/kg LPS, and peritoneal lavage cells were collected 1 day later. Control male and female mice were left untreated to assess baseline immune cell populations. Flow cytometry was used to analyze immune cell composition, as well as iNOS and arginase expression, in lavage cells from control and 1-day LPS-challenged male (n = 6) and female (n = 6) mice. (B) Total peritoneal lavage cell counts from control and 1-day LPS-treated male and female mice. (C) Gating strategy for flow cytometry analysis of peritoneal lavage cells, identifying distinct myeloid cell subsets: (A) CD11bmod cells; (B) CD11bhiLy6G– cells (peritoneal macrophages); (C) CD11b+Ly6G+ cells (neutrophils). (D) Representative flow cytometry plots of CD11bmod, CD11bhiLy6G– and CD11b+Ly6G+ cells in peritoneal lavage fluid from control and 1-day LPS-treated male and female mice. (E) Quantification of the frequency of CD11bmod, CD11bhiLy6G– and CD11b+Ly6G+ cells in peritoneal lavage fluid from control and 1-day LPS-treated male and female mice (n = 6 per group). (F) Representative flow cytometry plots showing iNOS and arginase expression in CD11bhiLy6G– macrophages from peritoneal lavage fluid of control and 1-day LPS-treated male and female mice. (G) Quantification of the frequency of iNOS-positive, arginase-positive and arginase-positive/iNOS-positive populations within the CD11bhiLy6G– macrophages in peritoneal lavage fluid from control and 1-day LPS-treated male and female mice. Statistical significance was determined using 2-tailed t tests (Mann-Whitney U tests) for 2-group comparisons. P values < .05 were considered to indicate statistical significance. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. ns, not significant.
LPS challenge induces greater neutrophil infiltration and reduced iNOS expression in male mice compared with female mice. (A) Schematic of the experimental design. Male and female mice were injected IP with 5 mg/kg LPS, and peritoneal lavage cells were collected 1 day later. Control male and female mice were left untreated to assess baseline immune cell populations. Flow cytometry was used to analyze immune cell composition, as well as iNOS and arginase expression, in lavage cells from control and 1-day LPS-challenged male (n = 6) and female (n = 6) mice. (B) Total peritoneal lavage cell counts from control and 1-day LPS-treated male and female mice. (C) Gating strategy for flow cytometry analysis of peritoneal lavage cells, identifying distinct myeloid cell subsets: (A) CD11bmod cells; (B) CD11bhiLy6G– cells (peritoneal macrophages); (C) CD11b+Ly6G+ cells (neutrophils). (D) Representative flow cytometry plots of CD11bmod, CD11bhiLy6G– and CD11b+Ly6G+ cells in peritoneal lavage fluid from control and 1-day LPS-treated male and female mice. (E) Quantification of the frequency of CD11bmod, CD11bhiLy6G– and CD11b+Ly6G+ cells in peritoneal lavage fluid from control and 1-day LPS-treated male and female mice (n = 6 per group). (F) Representative flow cytometry plots showing iNOS and arginase expression in CD11bhiLy6G– macrophages from peritoneal lavage fluid of control and 1-day LPS-treated male and female mice. (G) Quantification of the frequency of iNOS-positive, arginase-positive and arginase-positive/iNOS-positive populations within the CD11bhiLy6G– macrophages in peritoneal lavage fluid from control and 1-day LPS-treated male and female mice. Statistical significance was determined using 2-tailed t tests (Mann-Whitney U tests) for 2-group comparisons. P values < .05 were considered to indicate statistical significance. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. ns, not significant.
We next assessed inducible nitric oxide synthase (iNOS) and arginase expression within the CD11bhiLy6G– population, as these markers were negligible in CD11bmod or CD11b+Ly6G+ cells at both baseline and after LPS challenge (data not shown). At baseline, only arginase was expressed by CD11bhiLy6G– cells, and its expression was significantly higher in males than in females (Figure 3F-G). At 1 day after LPS, CD11bhiLy6G– cells began expressing iNOS, leading to the emergence of iNOS-positive, arginase-positive, dual iNOS-positive/arginase-positive populations (Figure 3F-G). Importantly, the frequency of iNOS-positive cells was significantly higher, while the frequency of arginase-positive cells was lower, in female mice (Figure 3G). These findings suggest that female mice harbor a higher proportion of M1-type CD11bhiLy6G– cells, which are known to be more inflammatory during LPS challenge.49 In contrast, male mice had a greater frequency of M2-type myeloid cells (determined by arginase expression), both baseline and post-LPS treatment. The heightened iNOS expression and reduced M2-like myeloid cell presence in females may underlie their hyperresponsiveness to LPS challenge in vivo.
Systemic analysis of immune cells following LPS challenge reveals increased activation of LPS-induced signaling in female mice
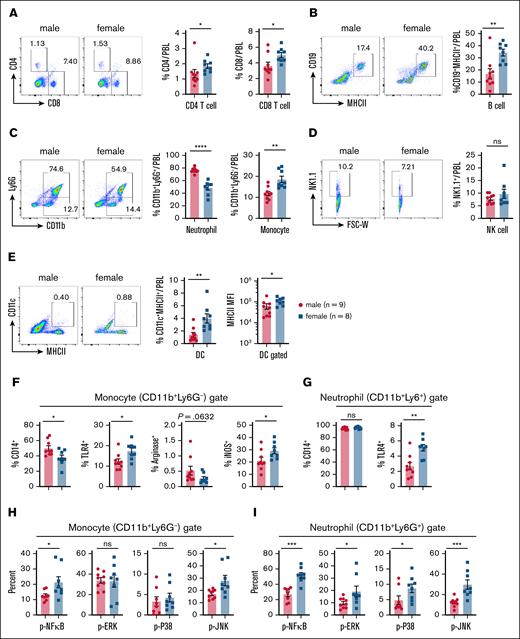
To assess the systemic effects of LPS treatment in vivo, we administered 5 mg/kg LPS IP to male and female mice. Peripheral blood leukocytes (PBLs) and spleens were harvested 6 hours after injection, and were analyzed for immune cell composition using flow cytometry (supplemental Figure 4A). Analysis of PBLs revealed significantly higher frequencies of CD4+ and CD8+ T cells, CD19+MHCII+ B cells, CD11b+Ly6G– monocytes, and CD11c+MHCII+ dendritic cells (DCs) in females compared with males at 6 hours after LPS injection (Figure 4A-E). The frequency of NK1.1+ cells remained similar between sexes (Figure 4D). In contrast, males exhibited a significantly higher frequency of neutrophils, which constituted nearly 80% of total PBL immune cells, compared with ∼50% in females (Figure 4C). Notably, female-derived DCs expressed higher levels of major histocompatibility complex class II (MHCII), indicating greater activation in response to LPS (Figure 4E).
LPS challenge induces higher iNOS expression and greater MAPK and NF-κB activation in peripheral myeloid cells from female mice compared with male mice. Experimental setup: age-matched male and female mice were injected IP with 5 mg/kg LPS. PBLs were collected 6 hours after injection, and analyzed via flow cytometry for immune cell frequencies, activation markers and signaling pathway activation. (A) Representative flow cytometry plots (left) and cumulative frequencies (right) of CD4+ and CD8+ T cells in PBLs from 1-day LPS-treated male and female mice. (B) Representative flow plots (left) and cumulative frequency (right) of CD19+MHCII+ B cells in PBLs from 1-day LPS-treated male and female mice. (C) Representative flow plots (left) and cumulative frequency (right) of CD11b+Ly6G+ neutrophils and CD11b+Ly6G– monocytes in PBLs from 1-day LPS-treated male and female mice. (D) Representative flow plots (left) and cumulative frequency (right) of NK1.1+ NK cells in PBLs from 1-day LPS-treated male and female mice. (E) Representative flow plots (left) and cumulative frequency (middle) of CD11c+MHCII+ DCs in PBLs from 1-day LPS-treated male and female mice. Mean fluorescence intensity of MHCII expression on DCs (right). (F) Frequencies of CD14+, TLR4+, arginase-positive, and iNOS-positive populations among CD11b+Ly6G– monocytes from 1-day LPS-treated male and female mice. (G) Frequencies of CD14+ and TLR4+ populations among CD11b+Ly6G+ neutrophils from 1-day LPS-treated male and female mice. (H) Frequencies of the phosphorylated (p)–NF-κB+, p-ERK+, p-P38+ and p-JNK+ populations among CD11b+Ly6G– monocytes from 1-day LPS-treated male and female mice. (I) Frequencies of the p–NF-κB+, p-ERK+, p-P38+ and p-JNK+ populations among CD11b+Ly6G+ neutrophils from 1-day LPS-treated male and female mice. Each dot represents data from an individual mouse. Statistical significance was determined using 2-tailed t tests (Mann-Whitney U tests) for 2-group comparisons. P values < .05 were considered to indicate statistical significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
LPS challenge induces higher iNOS expression and greater MAPK and NF-κB activation in peripheral myeloid cells from female mice compared with male mice. Experimental setup: age-matched male and female mice were injected IP with 5 mg/kg LPS. PBLs were collected 6 hours after injection, and analyzed via flow cytometry for immune cell frequencies, activation markers and signaling pathway activation. (A) Representative flow cytometry plots (left) and cumulative frequencies (right) of CD4+ and CD8+ T cells in PBLs from 1-day LPS-treated male and female mice. (B) Representative flow plots (left) and cumulative frequency (right) of CD19+MHCII+ B cells in PBLs from 1-day LPS-treated male and female mice. (C) Representative flow plots (left) and cumulative frequency (right) of CD11b+Ly6G+ neutrophils and CD11b+Ly6G– monocytes in PBLs from 1-day LPS-treated male and female mice. (D) Representative flow plots (left) and cumulative frequency (right) of NK1.1+ NK cells in PBLs from 1-day LPS-treated male and female mice. (E) Representative flow plots (left) and cumulative frequency (middle) of CD11c+MHCII+ DCs in PBLs from 1-day LPS-treated male and female mice. Mean fluorescence intensity of MHCII expression on DCs (right). (F) Frequencies of CD14+, TLR4+, arginase-positive, and iNOS-positive populations among CD11b+Ly6G– monocytes from 1-day LPS-treated male and female mice. (G) Frequencies of CD14+ and TLR4+ populations among CD11b+Ly6G+ neutrophils from 1-day LPS-treated male and female mice. (H) Frequencies of the phosphorylated (p)–NF-κB+, p-ERK+, p-P38+ and p-JNK+ populations among CD11b+Ly6G– monocytes from 1-day LPS-treated male and female mice. (I) Frequencies of the p–NF-κB+, p-ERK+, p-P38+ and p-JNK+ populations among CD11b+Ly6G+ neutrophils from 1-day LPS-treated male and female mice. Each dot represents data from an individual mouse. Statistical significance was determined using 2-tailed t tests (Mann-Whitney U tests) for 2-group comparisons. P values < .05 were considered to indicate statistical significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
LPS sensing relies on a coordinated response involving multiple molecules, including LPS-binding protein, which extracts LPS monomers from LPS micelles, and CD14, which facilitates LPS transfer to the TLR4/MD2 complex.50 LPS binding to TLR4 on immune cells ultimately triggers NF-κB and MAPK signaling pathways.50 Given the central role of myeloid cells in the LPS response, we analyzed CD14 and TLR4 expression in CD11b+Ly6G– monocytes and CD11b+Ly6G+ neutrophils (Figure 4F-G; supplemental Figure 4B). Female mice exhibited a significantly lower frequency of CD14+ monocytes, but a higher frequency of TLR4+ monocytes than males (Figure 4F). In contrast, nearly all neutrophils expressed CD14 at high levels, with no sex-based differences observed (Figure 4G). However, the frequency of TLR4+ neutrophils was significantly higher in females than in males (Figure 4G), reinforcing the notion that female myeloid cells exhibit heightened LPS responsiveness. Consistent with our findings in peritoneal lavage cells (Figure 3), we observed a lower frequency of arginase-positive monocytes, and a significantly higher frequency of iNOS-positive CD11b+Ly6G– monocytes in females at 6 hours after LPS injection (Figure 4F). Given the proinflammatory role of iNOS in shaping M1 monocyte/macrophage responses, these data further support the notion of an exaggerated LPS-induced immune response in females.
While in vitro-differentiated BMDMs from male and female mice exhibited no significant differences in NF-κB and MAPK activation (supplemental Figures 2 and 3), we next investigated whether these pathways are differentially activated in vivo. Using flow cytometry, we analyzed the activation of NF-κB and MAPK signaling pathways in monocytes (CD11b+Ly6G–) and neutrophils (CD11b+Ly6G+) 6 hours after LPS challenge (supplemental Figure 4). A significantly greater proportion of monocytes in female PBLs expressed p–NF-κB and p-JNK compared with males, while no significant differences were observed for ERK or P38 phosphorylation (Figure 4H). In neutrophils, females exhibited higher frequencies of p–NF-κB+, p-ERK+, p-P38+ and p-JNK+ cells than males (Figure 4I). Additionally, analysis of splenic immune populations 6 hours after LPS revealed that males harbored significantly more neutrophils than females (supplemental Figure 5A). Similar to PBLs, splenic monocytes and neutrophils from females exhibited higher TLR4 expression than those from males (supplemental Figure 5B). Assessment of neutrophil activation via CD63 expression51 demonstrated a significantly greater proportion of CD63+ neutrophils in females than in males (supplemental Figure 5C). Flow cytometry analysis of IL-1β production in splenic monocyte and neutrophil populations further revealed a higher frequency of IL-1β-positive cells in females than in males (supplemental Figure 5B-C). Taken together, these findings demonstrate that LPS induces significantly greater activation of NF-κB and MAPK signaling pathways in female mice than in male mice in vivo (Figure 4).
Sexual dimorphism in LPS tolerance confers increased resistance to secondary LPS challenge in female mice
Previous studies have demonstrated that mice primed with LPS develop resistant to a subsequent lethal LPS challenge due to an acquired state of hyporesponsiveness.52 Given that female mice exhibit heightened susceptibility to primary LPS challenge in vivo (Figure 1), we investigated whether sex-based differences persist during LPS tolerance. To address this, male and female C57BL/6 mice were administered a primary LPS challenge (5 mg/kg, IP) and monitored for survival. Survivors were subsequently rechallenged with a lethal dose of LPS (35 mg/kg, IP), and survival was assessed (Figure 5A). Consistent with prior findings, female mice displayed significantly greater mortality than males following the primary LPS challenge in 2 independent experiments (Figure 5B-C, left panel). However, following the secondary LPS challenge, this pattern was reversed, with significantly more female mice surviving compared with males (Figure 5B-C, right panel). Importantly, naïve (non–LPS-primed) mice exhibited extreme susceptibility to lower doses (15 mg/kg, IP), with 100% mortality in both sexes (Figure 5C, right panel).
LPS rechallenge–induced mortality is significantly higher in male mice than in female mice. (A) Schematic of the experimental design. Age-matched male and female mice were initially challenged IP with 5 mg/kg LPS on day 0, survival was monitored. On day 7, surviving mice were rechallenged with a lethal dose of LPS (35 mg/kg, IP) and monitored for survival. (B) Left: survival curve of male (n = 30) and female (n = 26) mice following the initial 5 mg/kg LPS challenge. Right: survival curve of male (n = 23) and female (n = 19) survivors rechallenged with 35 mg/kg LPS. (C) Independent repeat of the experiment in panel B, including naïve control mice for the LPS rechallenge studies. Left: survival curve of male (n = 15) and female (n = 18) mice following 5 mg/kg LPS challenge. Right: survival curve of male (n = 12) and female (n = 9) survivors rechallenged with 35 mg/kg LPS. As additional control, naïve males (n = 8) and females (n = 8) were challenged with 15 mg/kg LPS to assess baseline susceptibility. Survival curves were analyzed using the log rank (Mantel-Cox) test. P values < .05 were considered to indicate statistical significance.
LPS rechallenge–induced mortality is significantly higher in male mice than in female mice. (A) Schematic of the experimental design. Age-matched male and female mice were initially challenged IP with 5 mg/kg LPS on day 0, survival was monitored. On day 7, surviving mice were rechallenged with a lethal dose of LPS (35 mg/kg, IP) and monitored for survival. (B) Left: survival curve of male (n = 30) and female (n = 26) mice following the initial 5 mg/kg LPS challenge. Right: survival curve of male (n = 23) and female (n = 19) survivors rechallenged with 35 mg/kg LPS. (C) Independent repeat of the experiment in panel B, including naïve control mice for the LPS rechallenge studies. Left: survival curve of male (n = 15) and female (n = 18) mice following 5 mg/kg LPS challenge. Right: survival curve of male (n = 12) and female (n = 9) survivors rechallenged with 35 mg/kg LPS. As additional control, naïve males (n = 8) and females (n = 8) were challenged with 15 mg/kg LPS to assess baseline susceptibility. Survival curves were analyzed using the log rank (Mantel-Cox) test. P values < .05 were considered to indicate statistical significance.
We next hypothesized that the increased resistance of female mice to secondary LPS challenge would be associated with lower levels of circulating proinflammatory cytokines. To test this, male and female C57BL/6 mice were primed with LPS, rested for 7 days, and then rechallenged. Mice were euthanized 6 hours after secondary LPS challenge, and serum cytokine levels were quantified via multiplex ELISA (Figure 6A). Contrary to our hypothesis, female mice exhibited significantly higher levels of all measured proinflammatory cytokines, including IL-1β, IL-6, MIP-1α, MIP-1β, interferon-gamma, CXCL1, TNF, GMCSF, MIP-2, IL-27, IP-10, MCP-1, MCP-3, RANTES, GCSF, IL-1α, IL-7, IL-7RA, IL-18, IL-19, IL-25, IL-28, LIF, leptin, IL-33 and IL-33R (Figure 6B-O; supplemental Figure 6). These findings demonstrate that female mice develop increased tolerance to lethal LPS upon secondary challenge, despite exhibiting elevated proinflammatory cytokine levels.
Female mice produce significantly greater levels of cytokines and chemokines following LPS rechallenge. (A) Schematic of the experimental design. Age-matched male (n = 9) and female (n = 8) mice were initially challenged with 3 mg/kg LPS via IP injection on day 0. After 7 days, the surviving mice were rechallenged with a lethal dose of 35 mg/kg LPS (IP). Serum samples were collected 6 hours after the second LPS challenge, and multiple cytokines and chemokines were analyzed using multiplex ELISA. (B-O) Cytokine and chemokine concentrations in serum samples 6 hours after rechallenge, measured via multiplex ELISA. Each dot represents data from a single mouse. Statistical significance was determined using 2-tailed Student t tests and Mann-Whitney tests. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
Female mice produce significantly greater levels of cytokines and chemokines following LPS rechallenge. (A) Schematic of the experimental design. Age-matched male (n = 9) and female (n = 8) mice were initially challenged with 3 mg/kg LPS via IP injection on day 0. After 7 days, the surviving mice were rechallenged with a lethal dose of 35 mg/kg LPS (IP). Serum samples were collected 6 hours after the second LPS challenge, and multiple cytokines and chemokines were analyzed using multiplex ELISA. (B-O) Cytokine and chemokine concentrations in serum samples 6 hours after rechallenge, measured via multiplex ELISA. Each dot represents data from a single mouse. Statistical significance was determined using 2-tailed Student t tests and Mann-Whitney tests. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
Neutrophils mediate sex-based differences in LPS-induced shock in mice
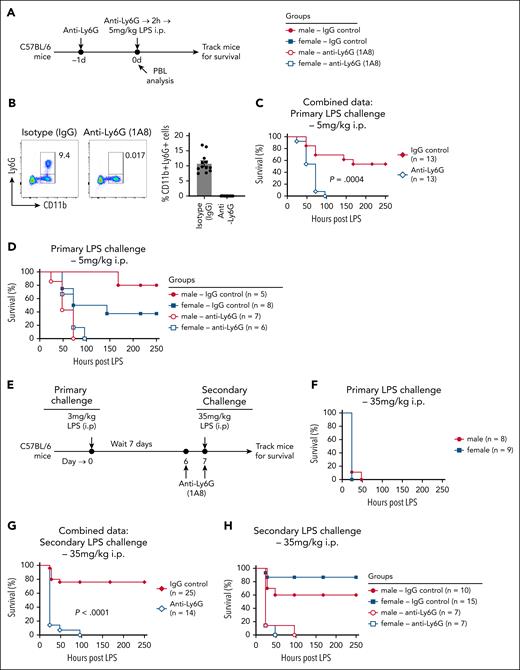
Given the significant differences in neutrophil frequency and mobilization between male and female mice during LPS treatment (Figures 1-3), and the central role of neutrophils in innate immune responses, we questioned whether these cells contribute to the observed sex bias in LPS responses. To assess the role of neutrophils during the primary LPS response, we depleted neutrophils using anti–Ly6G antibody (Clone: 1A8) and examined LPS-induced responses (Figure 7A). Flow cytometry analysis of PBLs on day 0 confirmed efficient deletion of neutrophils in the anti–Ly6G-treated mice (Figure 7B). Compared with IgG isotype control-treated mice, neutrophil-depleted mice exhibited significantly greater susceptibility to primary LPS challenge (Figure 7C). While 50% of IgG-treated control mice succumbed to LPS challenge (5 mg/kg, IP), 100% of neutrophil-depleted mice died more rapidly following LPS administration (Figure 7C). These findings highlight a counterintuitive protective role for neutrophils during LPS-induced shock. As observed previously (Figures 1 and 5), IgG-treated male mice exhibited greater resistance to primary LPS challenge than female mice (Figure 7D). However, this sex difference was abolished in neutrophil-depleted mice, with both male and female mice succumbing to LPS with similar rapid kinetics (Figure 7D). Collectively, these data demonstrate that neutrophils not only provide protection against LPS-induced mortality, but also mediate sex-based differences in primary LPS responses.
Neutrophils maintain sex differences and provide protection during LPS challenge–induced mortality. (A) Schematic of the experimental design for neutrophil depletion during primary LPS challenge. Age-matched male and female mice received anti–Ly6G monoclonal antibody (mAb; 400 μg per mouse) to deplete neutrophils or isotype control antibody on day −1 and day 0. On day 0, mice were challenged with 5 mg/kg LPS (IP) and monitored for survival. (B) Left: representative flow cytometry plot showing depletion of neutrophils in PBLs of mice treated with anti–Ly6G Ab. Right: cumulative data of CD11b+Ly6G+ neutrophil frequencies in all isotype and anti–Ly6G-treated mice. (C) Survival curve of control and neutrophil-depleted mice following LPS challenge. (D) Data from panel C analyzed separately for male and female survival curves in IgG control and anti–Ly6G-treated groups. (E) Schematic of the experimental design for survival analysis of mice rechallenged with LPS. Age-matched male and female mice were primed with 3 mg/kg LPS (IP) on day 0. On day 7, survivors were rechallenged with a lethal dose of 35 mg/kg LPS (IP) and monitored for survival. Some LPS-primed mice were treated with anti–Ly6G Ab (400 μg/mouse) or isotype control Ab on day 6 and day 7 to deplete neutrophils before rechallenge. (F) Naïve male and female mice were treated with 35 mg/kg LPS (IP) and monitored for survival to assess baseline susceptibility. (G) Survival curve of control or neutrophil-depleted LPS-tolerized mice, rechallenged with 35 mg/kg LPS. (H) Data in panel G analyzed separately for male and female survival curves in IgG control and anti–Ly6G-treated groups. Survival curves were analyzed using the log rank (Mantel-Cox) test. P values < .05 were considered to indicate statistical significance.
Neutrophils maintain sex differences and provide protection during LPS challenge–induced mortality. (A) Schematic of the experimental design for neutrophil depletion during primary LPS challenge. Age-matched male and female mice received anti–Ly6G monoclonal antibody (mAb; 400 μg per mouse) to deplete neutrophils or isotype control antibody on day −1 and day 0. On day 0, mice were challenged with 5 mg/kg LPS (IP) and monitored for survival. (B) Left: representative flow cytometry plot showing depletion of neutrophils in PBLs of mice treated with anti–Ly6G Ab. Right: cumulative data of CD11b+Ly6G+ neutrophil frequencies in all isotype and anti–Ly6G-treated mice. (C) Survival curve of control and neutrophil-depleted mice following LPS challenge. (D) Data from panel C analyzed separately for male and female survival curves in IgG control and anti–Ly6G-treated groups. (E) Schematic of the experimental design for survival analysis of mice rechallenged with LPS. Age-matched male and female mice were primed with 3 mg/kg LPS (IP) on day 0. On day 7, survivors were rechallenged with a lethal dose of 35 mg/kg LPS (IP) and monitored for survival. Some LPS-primed mice were treated with anti–Ly6G Ab (400 μg/mouse) or isotype control Ab on day 6 and day 7 to deplete neutrophils before rechallenge. (F) Naïve male and female mice were treated with 35 mg/kg LPS (IP) and monitored for survival to assess baseline susceptibility. (G) Survival curve of control or neutrophil-depleted LPS-tolerized mice, rechallenged with 35 mg/kg LPS. (H) Data in panel G analyzed separately for male and female survival curves in IgG control and anti–Ly6G-treated groups. Survival curves were analyzed using the log rank (Mantel-Cox) test. P values < .05 were considered to indicate statistical significance.
LPS priming induces tolerance, conferring resistance to a subsequent lethal LPS challenge (secondary LPS challenge). More importantly, our data show that the sex bias is reversed during secondary LPS challenge, with female mice exhibiting greater resistance than males (Figure 5). To determine whether neutrophils play a similar role during secondary LPS challenge, both in providing protection from lethal LPS doses and in driving sex-based differences, we administered an LPS priming dose (3 mg/kg, IP), followed by a lethal LPS challenge (35 mg/kg, IP) 7 days later (Figure 7E). Notably, 35 mg/kg is a highly lethal dose, with 100% mortality in naïve mice (both males and females) within the first 24 hours (Figure 7F). In contrast, LPS-primed mice exhibited robust resistance, with ∼80% of the mice surviving the lethal dose (Figure 7G). However, neutrophil depletion prior to secondary LPS challenge rendered LPS-primed mice highly susceptible, indicating a critical protective role for neutrophils in LPS-tolerized mice (Figure 7G). Consistent with previous findings (Figure 5), female LPS-primed mice exhibited significantly greater resistance to lethal LPS challenge than male mice (Figure 7H). However, this sex difference was abolished in neutrophil-depleted mice (Figure 7H).
Taken together, our results demonstrate that neutrophils play an essential role in protecting against LPS-induced mortality in both naïve and LPS-tolerized mice. More importantly, neutrophils are key mediators of sex-based differences in LPS responses and survival outcomes.
Discussion
LPS, a major component of gram-negative bacteria, is widely used to model sepsis due to its potent induction of systemic inflammation.50 LPS-induced sepsis (LPS shock) is characterized by a “cytokine storm” that can lead to multiorgan failure and death.45,53-55 Human studies show higher sepsis incidence in males, yet findings in animal models are mixed.22-26,28-37 In our retrospective analysis of LPS shock experiments, female mice exhibited greater mortality during primary LPS challenge, a result confirmed by repeated experiments. Interestingly, during secondary LPS challenge, females had increased survival compared with males, indicating that sex differences in sepsis susceptibility are context dependent. This may reconcile prior conflicting findings in the literature.
Sex hormones modulate immune responses,41,56,57 with testosterone generally suppressing inflammation.58 Given male resistance to primary LPS challenge, we hypothesized testosterone-mediated immunosuppression as a factor. However, in vitro addition of testosterone or estrogen to macrophages enhanced their responsiveness to LPS, suggesting both hormones amplify, rather than suppress, LPS-driven activation. Thus, sex hormones alone are unlikely to explain in vivo differences, though they may act alongside other physiological factors. To dissect hormonal vs genetic contributions, models like SRY transgenics or gonadectomy (ovariectomy/orchiectomy) may be useful.59
In vitro, male and female BMDMs responded similarly to LPS. This could be due to (1) inherently similar activation in macrophages, with sex differences arising in other immune cells like neutrophils, (2) systemic influences in vivo absent in isolated cell culture, and (3) diminished sex-specific traits in BMDMs due to the differentiation process. In contrast, in vivo analyses showed sex-based differences in myeloid populations, particularly neutrophils.
Neutrophils play a protective role in LPS-induced sepsis.43,60 Reber et al. demonstrated that neutrophil deficiency, whether due to antibody-mediated depletion or genetic ablation, renders mice highly susceptible to LPS shock, as evidenced by increased weight loss and mortality.43 Conversely, adoptive transfer of LPS-primed neutrophils into naïve C57BL/6 mice provided superior protection from lethal LPS shock.60 Similar protective roles for neutrophils have also been observed in mouse models of neuroinflammation.39,61,62 We found male mice had higher baseline circulating neutrophils and greater neutrophil infiltration after LPS challenge in the spleen and peritoneal cavity. This enhanced neutrophil response may contribute to male resistance in the primary LPS challenge. However, baseline neutrophil frequency alone did not predict survival.
Cytokine storms, often associated with sepsis mortality,55 were more pronounced in females during primary LPS challenge, consistent with their higher mortality. However, during secondary challenge, female mice showed both increased survival and elevated cytokine levels compared with males. This suggests that cytokine levels may not always correlate with outcomes, especially in secondary sepsis contexts.
We identified several sex-based differences in LPS responses: (1) neutrophil influx and recruitment, (2) arginase and iNOS expression, (3) TLR4 expression, (4) NF-κB and MAPK activation, and (5) cytokine production. While each may influence susceptibility to LPS shock, further study is needed to define their individual roles. In summary, females are more susceptible to primary LPS challenge, but exhibit increased resistance in secondary challenges. Neutrophils are protective and appear to underlie some of the observed sexual dimorphism. Our findings highlight the need to consider sex as a biological variable in sepsis research, and may inform the development of sex-specific treatments.
Acknowledgments
The authors thank Kristina Greiner for her assistance with the scientific editing of the manuscript. The authors also acknowledge the University of Iowa Flow Cytometry Facility for their support in processing the multiplex enzyme-linked immunosorbent assay samples.
This work was supported, in part, by Veterans Affairs (VA) Merit Review Award I01BX006091 from the United States Department of Veterans Affairs, Biomedical Laboratory Research and Development Service; National Institutes of Health (NIH) NIAID grant R01AI155425; University of Iowa Startup Funds; and a University of Iowa Investment in Strategic Priorities award (P.G.). C.P. is supported by a VA IK2BX004532 award, and H.P. is supported by the NIH T32AI007511 Training in Mechanisms of Parasitism grant.
Authorship
Contribution: P.G. conceived of the project; P.S., L.M., S.S., C.P., J.B., and P.G. designed the experiments; P.S., L.M., S.S., and P.G. wrote the manuscript; P.S., L.M., S.S., N.G., A.P., H.P., P.B., and J.D. performed the experiments; C.P. and J.B. provided expert advice and essential reagents; and all authors assisted with reviewing and editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prajwal Gurung, Inflammation Program, Department of Internal Medicine, University of Iowa, 431 Newton Rd, 429 EMRB, Iowa City, IA 52242; email: prajwal-gurung@uiowa.edu.
References
Author notes
P.S., L.M., and S.S. contributed equally to this work.
Uncropped western blot images are available in supplemental Figures 7-9.
The full-text version of this article contains a data supplement.