Abstract
This article discusses ways in which pediatric patients with acute lymphoblastic leukemia (ALL) can be stratified to receive intensive and less intensive therapies in order to decrease morbidity and mortality. Specifically, the focus may shift away from current intensive therapies for ultra low-risk patients and away from transplantation for certain patients at relapse. In contrast, infants with ALL comprise an ultra high-risk population in need of specialized approaches.
In Section I Dr. Lange describes the need to identify ultra low-risk children. Groups around the world have improved the outcome of children with ALL by identifying the basic “total therapy” model of the 1970s and stratifying treatment according to risk of relapse. Current first-line treatment cures about 85% of children with standard-risk ALL and 70% of children with high-risk disease. However, all children receive anthracyclines, alkylating agents, or moderate- to high-dose antimetabolite infusions. While randomized clinical trials prove that these intensifications reduce relapses, they also show that half of all children with ALL can be cured with the modest therapy of the 1970s and early 1980s. The patients curable with lesser therapy may be considered an ultra low-risk group. Attempts to use age, gender, white count, morphology, and karyotype to identify the ultra low-risk group of patients with a 90-95% cure rate with minimal therapy have failed. An expanded repertoire of tools such as pharmacogenetic profiling, PCR measurement of minimal residual disease and microarray technology may make this goal achievable in this decade.
In section II Dr. Chessells addresses the management of children with relapsed ALL. The chance of successful re-treatment with conventional chemotherapy for relapse depends on the duration of first remission and the site of relapse. Bone marrow transplantation from a histocompatible sibling or other suitable donor, which is widely accepted as the treatment of choice for children with a first remission of < 24 months, is associated with a high risk of relapse. Bone marrow transplantation for later bone marrow relapse improves leukemia-free survival but has significant short-term and long-term toxicities. The challenges are to develop more effective treatment for early relapse and to identify those children with relapsed ALL who are curable with chemotherapy or, failing this, those children who would be candidates for bone marrow transplantation in third remission.
In Section III Dr. Felix addresses the problem of infant ALL. ALL of infancy is clinically aggressive, and infants continue to have the worst prognosis of all pediatric patients with ALL. High white blood cell count, younger age, bulky extramedullary disease, and CNS disease at diagnosis are unfavorable characteristics. These features occur with MLL gene translocations. The probability of an MLL gene translocation and the probability of poor outcome both are greatest in younger infants. Specialized intensive chemotherapy approaches and bone marrow transplantation in first remission for this disease may lead to improved survival.
Refined recognition of pediatric patients with ALL who need more and less intensive therapies is necessary to increase survival and decrease toxicities.
I. The Ultra Low Risk Child with Acute Lymphoblastic Leukemia
Beverly J. Lange, M.D.*
Division of Oncology, The Children's Hospital of Philadelphia, Wood Building 4th Floor, 34th Street and Civic Center Boulevard, Philadelphia PA 19104
Background and Rationale
The projected cure rate for children with acute lymphoblastic leukemia (ALL) in the United States and Western Europe is now about 80%. Strategies responsible for this accomplishment include 1) years of treatment with two or more sequential combinations of multiagent chemotherapy, 2) CNS prophylaxis, 3) stratification of patients according to prognostic factors that predict risk of relapse, 4) risk-based intensification of therapy, and 5) large, controlled and usually randomized clinical trials. Patients are broadly classified and treated as standard risk or high risk based on age and white blood cell count (WBC).1,2 Despite almost constant progress in the treatment of this disease, there are major concerns about the predictive values of current prognostic factors and the universal intensification of therapy.3,4,5,6 The ultra low risk patients are a case in point. These patients are not identified by current prognostic factors and are treated more intensively than is necessary.
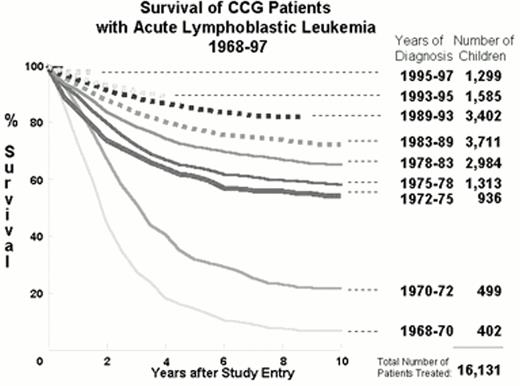
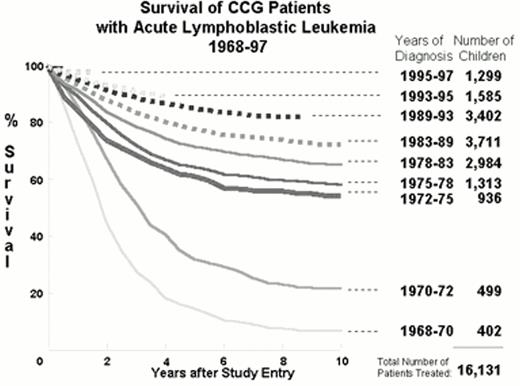
Figure 1 demonstrates that from 1960 to 1997 the implementation of the strategies listed above improved survival in over 16,000 children with ALL treated according to Children's Cancer Group (CCG) protocols.7 Other major groups treating children with ALL have had remarkably similar results.5, 8,9,10,11,12,13 Figure 1 shows an increase in 10-year survival from under 20% before 1972 to over 50% after 1972. This quantum leap resulted from the addition of CNS prophylaxis to the simple three drug induction and four drug maintenance therapy that constitute “basic ALL therapy” (Figure 2).14 All patients treated in the 1970s received cranial irradiation. Repetitive intrathecal therapy has systematically replaced cranial irradiation in the majority of patients.15, 16 Children with ALL that are curable with basic ALL therapy without cranial irradiation constitute a group of ultra low risk patients.
: Serial Kaplan-Meier actuarial survival curves for children with acute lymphoblastic leukemia treated from 1968-1997 on successive Children's Cancer Group (CCG) protocols.
The bold line indicates the era in which minimal therapy was achieving a > 50% 10-year survival in children. The broken lines represent the studies that include components of Berlin-Frankfurt-Munster (BFM) derived from the standard BFM'76 ALL study. A gift of WA Bleyer.
: Serial Kaplan-Meier actuarial survival curves for children with acute lymphoblastic leukemia treated from 1968-1997 on successive Children's Cancer Group (CCG) protocols.
The bold line indicates the era in which minimal therapy was achieving a > 50% 10-year survival in children. The broken lines represent the studies that include components of Berlin-Frankfurt-Munster (BFM) derived from the standard BFM'76 ALL study. A gift of WA Bleyer.
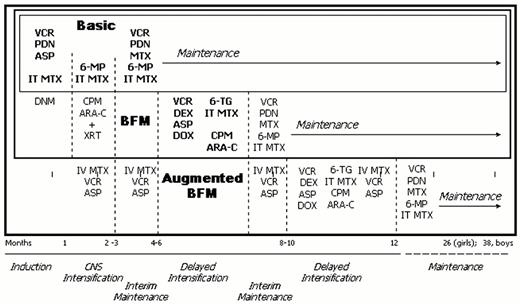
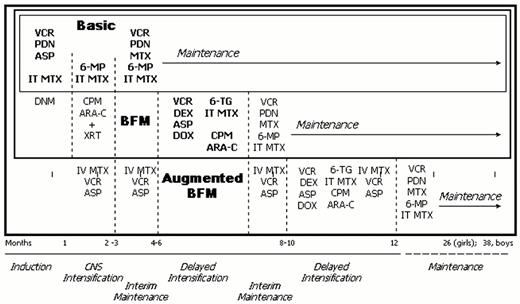
Illustration of the treatment schema for basic ALL therapy, Berlin-Frankfurt-Munster (BFM) derived therapy which includes basic therapy plus additional agents in induction, consolidation and delayed intensification, and augmented BFM therapy which includes all BFM therapy plus additional vincristine, asparaginase and intravenous methotrexate during times of potential myelosuppression plus a second delayed intensification approximately 10 months into treatment.
Abbreviations: VCR, vincristine; PDN, prednisone; ASP, L-asparaginase; IT, intrathecal; MTX, methotrexate; DNM, daunorubicin; 6MP, 6 mercaptopurine; CPM, cyclophosphamide; Ara-C, cytarabine; XRT, cranial irradiation; DEX, dexamethasone; DOX, doxorubicin.
Illustration of the treatment schema for basic ALL therapy, Berlin-Frankfurt-Munster (BFM) derived therapy which includes basic therapy plus additional agents in induction, consolidation and delayed intensification, and augmented BFM therapy which includes all BFM therapy plus additional vincristine, asparaginase and intravenous methotrexate during times of potential myelosuppression plus a second delayed intensification approximately 10 months into treatment.
Abbreviations: VCR, vincristine; PDN, prednisone; ASP, L-asparaginase; IT, intrathecal; MTX, methotrexate; DNM, daunorubicin; 6MP, 6 mercaptopurine; CPM, cyclophosphamide; Ara-C, cytarabine; XRT, cranial irradiation; DEX, dexamethasone; DOX, doxorubicin.
Prognostic Factors in ALL
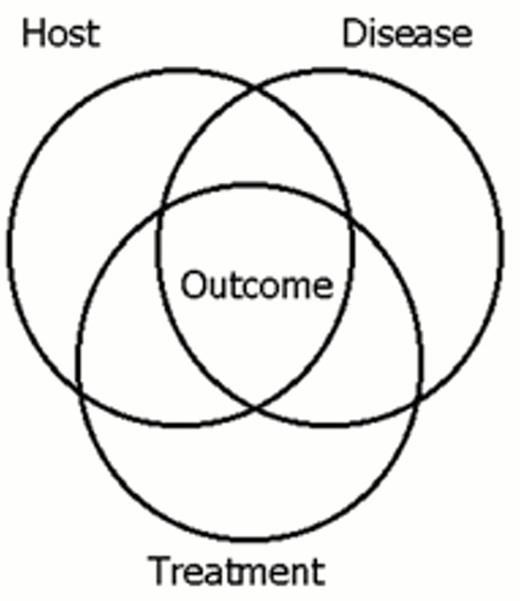
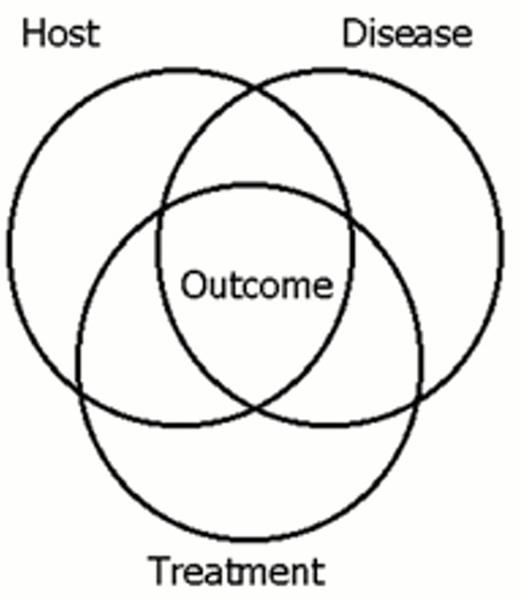
Over 60 prognostic factors have shown statistical significance in clinical trials in ALL (Table 1).5 They can be classified according to their origin: host related, disease related, and treatment related (Table 1 and Figure 3). These factors are the product of extraordinary advances in the disciplines of immunology, cell biology, cytogenetics, molecular genetics, pharmacology, infectious disease, statistics, clinical trial design and cancer therapy. In general, these factors are empirically derived rather than hypothesis driven. Some factors cannot be reproduced either in sequential studies of a single institution or cooperative group or in the contemporaneous studies of various groups, primarily because patient stratifications and treatments are changing every 3 to 5 years. Many have come and gone (Table 1). Such a wealth of empiric prognostic factors may now have become a liability.
VENN diagram showing the overlap of host related, disease-related and treatment-related factors in effecting outcome.
VENN diagram showing the overlap of host related, disease-related and treatment-related factors in effecting outcome.
In a critical analysis of prognostic factors in studies conducted between 1972 and 1994, Donadieu et al found that only the host-related factors of age and gender and disease-related factors of WBC count and karyotype sustained their significance in multivariate analyses across studies.5 However, individually and collectively, they had a very low predictive value: 1.1% for gender, 2.0% for age, 3.5% for WBC and 1.6% for karyotype.5 Their low predictive value reflects the rarity of the highly unfavorable factors and the relatively high number of patients with favorable features who die. None of these factors operates independently.17, 18
It is necessary to consider the interrelationship of prognostic factors as depicted in Figure 3. For example age is a prognostic factor because many of the children under one year of age or over nine years of age do not have common ALL. Over half of infants with ALL have t(4;11), a distinctly unfavorable clinical and molecular entity. After age 10, the incidence of Philadelphia chromosome positive (Ph+) ALL starts to rise.17,19 Ph+ ALL is also highly unfavorable. However, even within the Ph+ ALL population, outcome varies with age: age <10 years is relatively favorable.19
We have no explanation of why male gender is unfavorable is most studies except those of CCG after the late 1980s.5,9,16,20,21,22,23,24 Testicular disease or the prevalence of T-cell disease does not account for unfavorable outcome in boys. With the exception of t(9;22) and t(1:19), the study of the molecular pathogenesis of most of the karyotypic abnormalities listed in Table 1 have not yet provided coherent explanations of why they are favorable or unfavorable. Nor is it understood why the molecular fusion of t(12;21) (TEL/AML1) confers good outcome.
The low predictive value of the conventional prognostic factors of age, WBC, gender and karyotype and their lack of rationale call for the development of better prognostic categories. Dr. Pinkel has argued that treatment is really the only prognostic factor in ALL.4 At its extremes, this argument is absolutely correct: given no treatment, ALL is uniformly fatal and given the right treatment for each patient, it should never be fatal—provided, of course, the patient has access to good nutrition and to the treatment and complies with it.4,25 Unfortunately, we have not yet discovered the right treatment for every patient.
Nonetheless, treatment-related factors are the only ones than can be modified. The largest body of treatment-related prognostic factors other than the treatment itself relate to early response. In recent studies early response to treatment, no matter how the response is measured, is often the strongest prognostic factor.26 In the Berlin-Frankfurt-Münster (BFM) ALL trials reduction in peripheral blast count to <1000 blasts/mL after 7 days of prednisone and one dose of intrathecal methotrexate has emerged as the single most important prognostic factor, supplanting even t(4;11) and t(9;22).10,12 However, there are no clues about the biologic differences in patients or their leukemic cells that confer relative prednisone sensitivity or resistance. Equally compelling evidence of the weight of early response comes from the CCG-1882 trial.16 Specifically, high-risk patients who still had > 25% blasts in a day 7 marrow were randomly assigned to a standard BFM-derived therapy or an augmented BFM therapy (Figure 2). At 5 years, event-free survival (EFS) was 68% in the group receiving augmented therapy and 42% in the standard group (p < 0.001). This trial proves that intensification of treatment alters the prognosis of slow early response.
More technically sophisticated ways of defining early response or remission measure minimal residual disease (MRD). Fluorescence in situ hybridization, flow cytometry, leukemic colony assays and polymerase chain reaction (PCR)-based assays are allegedly more sensitive and more objective than peripheral blast counts or day 7 or day 14 marrow aspirates.27 It is not yet clear that they are more specific. PCR detection of MRD at the level of 10-2 is a better prognosticator of outcome of therapy than the percentage of blasts in the day 28 marrow,28 but there are no head-on comparisons of PCR-based methods to the day 7 or 14 marrow or the prednisone response. Moreover, even the most sensitive PCR assay cannot predict the off-therapy relapses that comprise more than a third of the events in standard risk ALL.29 As it is not yet proven that immunologic and molecular definitions of remission should replace the standard morphologic definition, these studies ought to be conducted in parallel with conventional studies in all patients on research protocols.
Major hidden variables in predicting response to therapy involve hereditary and acquired variability in drug disposition and metabolism and possibly diet and use of complementary and alternative medications. Although chemotherapy is the backbone of ALL treatment, formal pharmacologic investigations of the major therapeutic agents are few. The thiopurines and methotrexate (MTX) have been investigated most throughly.
Thioguanine nucleotide concentrations in erythrocytes (RBC TGNs) correlate with toxicity and outcome.30 TGNs are the metabolically active forms of 6-thioguanine and 6-mercaptopurine (6-MP). Thiopurine methyltransferase (TPMT) catalyzes the formation of inactive metabolites, the S-methylated bases.31 Erythrocytes and leukemic cells of patients deficient in TPMT accumulate high levels of toxic TGNs. About 1 in 300 children with ALL are homozygous for a TPMT polymorphism; these children have a good outcome when treated with 6 to 10% of protocol dose of thiopurine and experience severe toxicity when treated with protocol-recommended doses.31 No other polymorphisms in the numerous enzymes involved in thiopurine metabolism have been described that would explain the great intrapatient and interpatient variability in pharmacokinetics, pharmacodynamics and erythrocyte TGN levels in children with standard-risk ALL receiving oral 6-MP on CCG protocols.32 The Pediatric Oncology Group (POG) and CCG have attempted to increase the RBC TGN and systemic exposure with parenteral 6-MP. Randomized trials have shown no therapeutic benefit to IV 6-MP added to IV MTX.33 Twelve hour infusions of 6-MP combined with oral 6-MP conferred no benefit compared to 6-MP alone.24
Important variables in MTX disposition are renal clearance, intracellular transport, and accumulations of MTX polyglutamates (MTXPGs) in the leukemic cells.34, 35 Over a wide range of infusional MTX doses, median steady state concentrations vary in proportion to systemic clearance and correlate with relapse.36 St. Jude Children's Research Hospital (SJCRH) investigators randomized patients to a fixed protocol-prescribed MTX dose or MTX dosing based on renal clearance. Those receiving dosing based on clearance had a 5-year EFS of 76% compared to 66% in the others, implying that optimization of MTX disposition improved outcome.37
Factors responsible for interpatient differences in MTX metabolism involve numerous enzymes involved in folate metabolism.38 Intracellular accumulations of MTXPGs in lymphoblasts correlate with outcome in ALL, and they vary according to ploidy, lineage, and phase of the cell cycle. T-lineage lymphoblasts accumulate significantly lower levels of MTXPGs than B-lineage lymphoblasts.39 MTXPG levels may be a host-related phenomenon, a disease-related phenomenon, or both. The gene encoding reduced folate carrier (RFC) is one of several genes on chromosome 21 whose products are involved in folate metabolism. Intracellular MTXPGs correlate with copies of the RFC gene.40 RFC expression corelates with MTX transport. It is the most common mechanism of MTX resistance in vitro in ALL. Levels of polyglutamation, in turn, reflect the balance between folylpolyglutamate synthesase (FPGS) and gammaglutamylhydrolase (GGH).38 An additional chromosome 21 is the most common abnormality in hyperdiploid ALL. Patients with B-lineage ALL whose blasts have more than two copies of chromosome 21 accumulate more MTX.40
Except for recent trials at SJCRH, North American studies have consistently shown that race is a major prognostic factor in ALL.41, 42 CCG studies from 1983 to 1992 involving 8762 children and adolescents show differences in response according to race: 5-year survival was 89% for 167 Asians; 84% for 6,703 Caucasians; 78% for 1,071 Hispanics, and 74% for 506 African Americans (p < 0.001).43 Significant differences persist in multivariate analysis encomsing all other standard prognostic variables. Moreover, outcome in a limited sample did not correlate with socioeconomic status or education.
While it is conceivable that the outcomes vary by gender and race because of cultural factors and compliance issues, it is almost certain that race- and gender-associated genes affect response to treatment. N-acetyltransferase and xanthine oxidase activities show both racial and gender differences.44 The glutathione S-transferases (GSTs) detoxify environmental and endogenous toxins and may confer resistance to cytotoxic chemotherapy. Chen et al found a higher frequency of GST deletions among African Americans with ALL than among Caucasians, but GST genotype did not correlate with outcome.45 In contrast Stanulla, et al showed significant correlations with GST polymorphisms and relapse in B-precursor ALL in a predominantly Caucasian cohort of patients.46
Genetic polymorphisms of the cytochrome P450 (CYP) enzymes may be especially relevant to treatment response. CYP enzymes comprise the largest system for detoxification reactions and metabolism of xenobiotics. There are established racial variations in polymorphisms of CYP1A2, CYP2D6 and CYP3A4.44,47 The CYP2D6(B) mutation occurs in 8.5% of African Americans and 23% of Caucasians; deletion of the gene is present in 5.5% and 2.4%, respectively.47 CYP3A4 is the most abundant cytochrome in human hepatic microsomes. CYP3A4 metabolizes many antineoplastics including vincristine, vinblastine, cyclophosphamide, epipodophyllotoxins and many common supportive care agents, such as acetaminophen, erythromycin, ketoconazole, lidocaine, midazolam, nifedipine and omeprazole.48 Drugs that induce or inhibit hepatic CYP3A4 may alter vincristine deposition.49 Dexamethasone is a potent inducer of CYP3A4 activity, at least twice as potent as hydrocortisone.50 The frequency of an allelic variant in the nifedipine-specific element of CYP3A4 promoter is 0.53 in African Americans, 0.09 in Caucasians and zero in Taiwanese.51 This allelic variant is associated with increased risk of prostate cancer in men and with decreased risk of treatment-related leukemia with MLL gene translocations.51,52 CPY3A4 activity is also two times higher in females.53
Among recent CCG studies, the biggest differences in outcome by race were in CCG-1891 for standard risk ALL.23 Among 947 Caucasians, EFS at 6 years was 82±2 %; among the 47 African Americans, 54±8% (relative risks, 0.78 for Caucasians, 3.68 for African Americans).23 It is not known whether CYP3A4 genotype accounts for differences in drug effects and treatment outcomes between Caucasians and African Americans.
Patients receive many chemotherapeutic and supportive care agents. Investigations of the genetic variants of one enzyme at a time are unlikely to explain the complex host-related and treatment-related variables and outcome. Pharmacogenomics encompasses the entire spectrum of genes that determine drug disposition in an individual or a population.54 Microarray technology can display expression patterns of thousands of genes simultaneously and complex analytical programs can organize expression data into maps of biologically relevant clusters. Microarray technology can differentiate AML from ALL based solely on gene expression and can uncover heretofore unsuspected genetic pathways.55,56 The fields of pharmacogenomics and microarray technology offer new tools capable of characterizing patient disease in the context of treatment. They have the potential to yield a new powerful genus of prognostic factors. Evans and Relling have already proposed a DNA array for automated high throughput. The array includes genes involved in metabolism of chemotherapeutic agents, host-susceptibility to cancer, immune response, differentiation, proliferation, and apoptosis.54 In anticipation of this technology the cooperative groups are banking cells from all leukemia patients at all stages of therapy and designing data retrieval and distribution systems that will allow these analyses to move forward.
Intensification of Therapy
Figure 1 shows incremental gains in survival in CCG studies after 1975. Although prevention of infectious deaths and stratification of patients contributed to improved survival, changes in therapy are the major factor in improving outcome. In a series of randomized trials in CCG, basic ALL therapy evolved into complex protocols consisting of additions and subtractions of components of the BFM `76 therapy, which emphasized higher-dose prednisone or dexamethasone, intensive induction, consolidation and delayed intensification (Figure 2).15,16,22,23,57 Shortening the duration of therapy to 26 months in girls and 38 months in boys and elimination of cranial irradiation in most patients were the only reductions in therapy in 25 years. In 2000 all CCG patients with ALL receive anthracyclines, cytarabine, cyclophosphamide and dexamethasone. Half of them may not need any of these agents. In POG all patients receive moderate dose parenteral antimetabolite therapy.8,33,58 SJCRH has incorporated epipodophyllotoxins, cytarabine, intensified L-asparaginase, parenteral MTX with individualized dosing based on renal clearance, and triple intrathecal chemotherapy.9 Dana-Farber Cancer Institute has continued to use cranial irradiation in boys and those with T-cell disease and added parenteral MTX and relatively higher cumulative doses of L-asparaginase and anthracyclines.11
These intensifications result in life-shortening or debilitating complications for a minority of patients, generally less than 5% of the entire cohort of patients with ALL in a given era of studies. There are, however, subsets that are at especially high risk for specific morbidities. For example aseptic necrosis occurs in 15 to 20% of adolescents receiving augmented BFM therapy, but < 1% of the younger children.16 Obviously, the price is trivial for those patients for whom the alternatives are relapse or death. However, the price may be too high for the ultra low risk patient.
The Ultra Low Risk Patient: A Plan
Prognostic factors used in the 1970s and 1980s did not identify the ultra low risk patient. Additional prognostic factors such as karyotype and early response and detection of minimal residual disease have emerged in studies involving children who were no longer receiving basic ALL therapy. Hence, there are no cohorts of children who have received basic therapy whose leukemia is characterized for karyotype, early response, and minimal residual disease.
Since we are usually better at predicting treatment failure than success, we may not be able to identify the ultra low risk patient with improved precision. There is no guarantee that in the foreseeable future pharmacogenomics and microarray technologies will account for the observed differences in response based on age, gender, race, white count and cytogenetics. These technologies may generate hundreds of statistically significant variables of low predictive value.
On the other hand we may well be able to identify the ultra low risk patient using the tools developed over the past 30 years. To do so would require repeating some of the randomized trials of the past two decades with different sets of patients and prognostic factors. The ultra-low risk patient is female, Asian or Caucasian, age > 1 to < 10 years. Her disease is B-precursor ALL, hyperdiploid with WBC < 50,000/μL and without CNS involvement. Early response is essential: < 5% blasts on day 14 and < 10-2 PCR or immunologically detectable leukemic cells at day 28. These patients (approximately 20% of all ALL patients) can be randomly assigned to basic ALL therapy or the current standard therapy for standard-risk patients.
II. Relapsed Acute Lymphoblastic Leukemia: Bone Marrow Transplant or Conventional Treatment?
Judith Chessels, M.D.*
Molecular Haematology and Cancer Biology Unit, Institute of Child Health, University College London, 30 Guilford Street, London WC1 1EH, UK
Despite steady improvement in therapy of lymphoblastic leukemia, 20-25% of patients still experience a relapse; the majority of these relapses occur in so-called lower risk patients. The management of such patients remains unsatisfactory.1 While remission can be achieved in the majority of patients, further treatment, either with intensive chemotherapy (CT) or high-dose chemo-radiotherapy therapy and infusion of bone marrow or peripheral blood stem cells (BMT) is toxic, frequently unsuccessful and carries the risk of significant long-term morbidity.2 Despite continuing uncertainty about the best approach to treatment there have been no successful randomized trials comparing CT and BMT. Consequently, all available evidence is based on historical controls, comparative data or, at best, some form of matched pair analysis.
Factors influencing the length of second remission
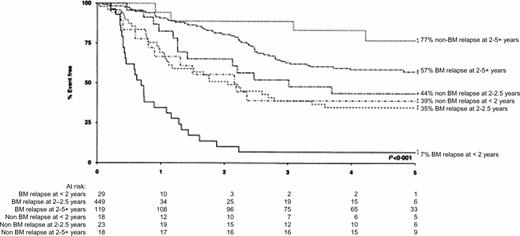
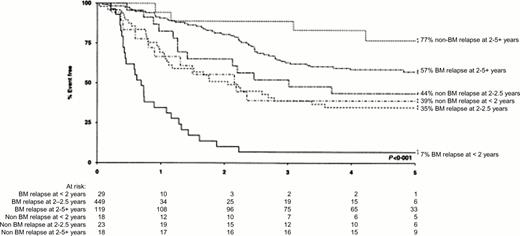
The probability of sustained second remission in ALL depends on the length of the first remission and the site of relapse. These criteria can be used as illustrated in Figure 4 to stratify patients into different risk groups. This figure shows that among children entered into the Medical Research Council (MRC) UKALLR1 study, those who had bone marrow relapse during the first 24 months from diagnosis had a very poor prognosis. The EFS for such early relapses in an unselected group of children who had received primary treatment on the MRC UKALL X trial was even worse with 3% alive in second remission at five years irrespective of the type of second treatment.3 Despite the fact that patients with apparently isolated extramedullary relapse virtually always have cryptic bone marrow involvement,4,5 those with such relapses, as shown in Figure 4, have a better prognosis. It has been reported that the outcome after combined bone marrow and extramedullary relapses is superior to that from isolated marrow relapse6 but this may be related in part to the relative length of first remission in the two groups. Other factors associated with a poor outcome after relapse are older age,3 T-ALL7 and male sex.8 The intensity of systemic treatment in first remission does not appear to have a strong influence on prognosis3,9 although it has been reported that patients who have received cranial irradiation in first remission have a worse survival than those receiving intrathecal therapy alone.9
Patients treated on the MRC UKALL R1 relapse study. Event-free sruvival in second remission according to site of relapse and duration of first remission. Reproduced from Lawson et al. British Journal of Haemology 2000;108:531-543, with permission of Blackwell Scientific Publications.
Patients treated on the MRC UKALL R1 relapse study. Event-free sruvival in second remission according to site of relapse and duration of first remission. Reproduced from Lawson et al. British Journal of Haemology 2000;108:531-543, with permission of Blackwell Scientific Publications.
Studies of leukemia biology in relapsed patients may provide additional insights into prognosis. Children with Philadelphia-positive leukaemia have a very poor prognosis.10 The TEL/AML1 fusion transcript was found in 24% of relapsed children; these had enjoyed longer first remissions and remained longer in long second remission than TEL/AML1-negative patients thus suggesting that this genetic abnormality is associated with a slower pace of disease.11 Measurements of cellular drug resistance in vitro have shown increased resistance at relapse to steroids and other drugs commonly used in first line therapy.12 Studies of MRD may be helpful in selecting treatment in second remission. Children with high levels of MRD before BMT had a high risk of subsequent relapse.13 It is possible that measurement of MRD after a period of intensified chemotherapy may identify children for whom standard BMT is likely to be unsuccessful and, alternatively, those who may be curable without recourse to high-dose therapy.
Results of chemotherapy for relapsed ALL
Remission rates in relapsed ALL of over 80% have been reported in most series with various combinations of chemotherapy.1 Following induction, most protocols include further consolidation and intensified continuing therapy for a total of about two years. Further CNS-directed treatment is essential to avoid overt CNS relapse as a second event14 and radiation therapy is normally given to sites of extramedullary relapse. However, the overall results of chemotherapy remain disappointing. Most large series of patients treated consistently with modern intensified protocols have an overall event free survival after BM relapse of 30% to 60%.7,8,15,16,17 No regimen is clearly superior, and the results mainly reflect the selection of patients. In all studies, the reported EFS after chemotherapy for early relapses involving the bone marrow (occurring within 18 months to 2 years from diagnosis) is only 5-10%. Furthermore, despite encouraging results after intensive chemotherapy for later marrow relapse, there is evidence that, even for patients with “slow pace disease” the risk of second relapse continues for many years.18
Most late extra-medullary relapses occur in the testes. These patients have a good prognosis after intensive chemotherapy and local irradiation. Management of the 5-10% of children who develop overt CNS infiltration despite CNS-directed therapy remains controversial, and most reports have very small numbers of patients. Now that few children receive cranial irradiation in first remission, intensified systemic and intrathecal chemotherapy and cranial or craniospinal irradiation should provide appropriate treatment. However, early CNS relapse in children with other adverse prognostic factors has a poor prognosis, with reported EFS in recent CCG and MRC studies of under 25%.3,9 The Pediatric Oncology Group (POG) has published relatively large trials for CNS relapse and one early study incorporating systemic intensification, triple intrathecal therapy and cranial irradiation resulted in an overall EFS of 46% at 4 years.19 More recently in a series of 83 children who received intensified chemotherapy and delayed craniospinal irradiation for CNS relapse, the 4-year EFS was 46.2% for those with initial CR of less than 18 months and 83.3% for those with longer first remissions.20
BMT for Relapsed ALL
Although successful BMT in patients with end-stage leukemia21 have been performed for 30 years, there is still no clear agreement about the indications for BMT in second remission. Furthermore, relapse rates after BMT remain high. As with chemotherapy, the chance of EFS after BMT is influenced by length of CR1, and other factors include initial WBC and previous CNS disease.22 Most studies of BMT from HLA-compatible donors have used the conventional regimen of cyclophosphamide and total body irradiation (TBI), a combination that seems superior to busulfan and cyclophosphamide in lymphoblastic leukemia.23 Uncontrolled studies using other preparative regimens (including such combinations as TBI, cytarabine and melphalan;24 hyperfractionated TBI and cyclophosphamide;25 and cytarabine and TBI26 ) have been claimed to reduce the risk of relapse.
The risks of allogeneic BMT and the paucity of HLA-compatible siblings have lead to the use of autologous BMT (ABMT) in second remission. Despite preliminary encouraging results from the USA,27,28 comparative studies from Germany,29 Italy30 and the UK3 have shown that ABMT is not superior to conventional chemotherapy. It has been suggested by the Italian group that ABMT may have a role in management of patients with early CNS relapse who lack a histocompatible sibling donor.31
Now that alternative donors are increasingly available BMT could, at least in theory, become possible for any child with relapsed leukemia. A large European review including both adults and children with ALL compared the results of ABMT and transplants from unrelated donors. Leukemia-free survival was similar in the two groups, but transplant-related mortality (TRM) was higher in the BMT group (42% vs. 17%) while relapse was lower (32% vs. 61%).32 However, TRM from selected single centers is already similar in sibling and unrelated donor transplants33,34 and further general improvements in TRM can be expected. Use of partially mismatched related donors further extends the potential access to allogeneic transplants.35
BMT versus Chemotherapy in Second Remission
There are many difficulties in comparing the outcome of BMT and chemotherapy for relapsed ALL, including lack of randomized trials, selection bias and duration of time to transplant. A summary of comparative reports of BMT and intensified chemotherapy is shown in Table 2 and has been restricted to studies which include at least 50 transplanted patients. The largest of these involved a comparison of data from the IBMTR and a cohort of patients treated with chemotherapy by the POG.36 A careful attempt was made to match chemotherapy and BMT patients Irrespective of the duration of first remission, BMT resulted in superior leukemia-free survival when compared with chemotherapy. Comparative data from Germany37 and Italy30 show similar results with a statistically significant benefit for BMT over chemotherapy in early marrow relapses. Review of the outcome for all unselected children relapsing after MRC UKALL X in the UK (who had received a variety of second-line chemotherapy protocols) demonstrated that BMT reduced the risk of a second relapse with an absolute increase in 5-year EFS of 14%.3 However, when the outcome of BMT was compared with standardized intensive chemotherapy in UKALL R1, the overall EFS rates for chemotherapy, sibling donor BMT and unrelated donor BMT were similar (Table 3). Further analysis has been performed on patients in MRC UKALL R1 and information obtained about donor availability and intention to treat. Comparison of outcome by donor availability showed no significant difference in EFS between patients with or without a histocompatible sibling donor. The patients with donors had a non significant 8-year EFS advantage of 8% (95% CI; 9%-24%) over the group without donors. Analysis by actual treatment received gave similar results with BMT patients having a 5% advantage over chemotherapy, the advantage being more apparent for patients with short first remissions. These results suggest that there may be a significant degree of selection bias in most BMT studies and that the results of BMT may be only marginally better than those of chemotherapy.38
A reasonable conclusion from the results in Table 2 is that BMT is associated with a modest increase in leukemia-free survival and that this may be most evident in children with a short early remission. The benefits may be reduced by or in some studies negated by the TRM— a factor assuming increasing importance with increasing use of unrelated donors.
BMT in Third Remission: A Viable Option?
For children with “slow pace” disease, a long first remission and, perhaps, other favorable prognostic factors such as TEL-AML1 gene rearrangement, it could be argued that chemotherapy in second remission might be effective and would avoid the complications of BMT. Patients who relapsed after chemotherapy could be offered BMT in third remission. The BFM relapse group have argued for such a strategy and claimed comparable results after BMT in second and third remission.39 In view of the encouraging reports of the use of unrelated donors in third remission,40 this approach might be possible for children who lack a related donor.
New Approaches to Treatment
Unfortunately, new chemotherapeutic approaches to treatment in relapsed ALL have not been very successful. It is to be hoped that the development of more specific therapy such as immunotoxins may enhance the effects of chemotherapy.41 Measures to enhance immune modulation may play a role in decreasing the risk of relapse after transplantation.42
The Way Forward
A tentative approach to the management of relapsed ALL is illustrated in Table 3. The allocation to risk groups is arbitrary but based on large reported series of patients. For the highest risk group, with a projected EFS under 25%, approaches such as intensified pre-transplant chemotherapy, use of alternative donors and early withdrawal of immune suppression are justifiable. For the lowest risk group of patients, conventional chemotherapy with radiotherapy as indicated affords a reasonable chance of success. The largest group of patients are those in the intermediate group where the published benefits of BMT may have been exaggerated by selection bias. It should be possible in theory to compare the outcome of chemotherapy and BMT, especially in children who have achieved low or minimal levels of MRD after the first few months of treatment—ideally by randomization. The difficulties of studies in relapsed ALL should not preclude systematic attempts to improve and refine treatment.
III. Acute Lymphoblastic Leukemia in Infants
Carolyn A. Felix, M.D.*
Division of Oncology, Abramson Research Center, RM 902B, The Children's Hospital of Philadelphia, 3516 Civic Center Boulevard, Philadelphia PA 19104
C.A.F. supported by NIH Grants CA66140, CA77683, CA80175, CA85469 and Leukemia and Lymphoma Society Scholar Award
The annual incidence of ALL in infants less than one year old in the United States is 19 per million, resulting in about 126 new cases per year.1 Unlike in ALL in children, there is a predominance of girls.1 Its distinct biology and age at presentation place infant ALL at one end of the diverse spectrum of pediatric ALL. Infant ALL is often associated with presenting features that confer a poor prognosis, including white blood cell count ≥ 50 x 109/L, bulky extramedullary disease and CNS involvement.2,3,4,5,6,7,8 A TdT+, CD19+, HLA DR+, CD10 (CALLA)-early B-cell precursor immunophenotype is characteristic; lack of CD10 expression is a poor prognostic factor.2,3,4,5,6,7,8 Myeloid antigen expression, which is common, has a variable impact on prognosis.9 Translocation of the MLL (MIXED LINEAGE LEUKEMIA) gene at chromosome band 11q23, which occurs in utero, is central to pathogenesis.10,11 This distinct molecular pathogenesis is integral to the poor results obtained with current treatments.6,8,12,13,14,15,16,17 Even within the first year of life, age may be important; the younger the infant, the worse the outcome.2,3,4,6,7,8 At the millennium, curative treatments for infant ALL are far behind the therapy for childhood ALL. Use of more intensified treatment regimens has been the common theme in infant ALL as for all of ALL but, in infant ALL, success has been only marginal. The young host is distinct and the treatment morbidity is different.
Molecular Pathogenesis of Infant ALL
Translocations of a breakpoint cluster region of the MLL (ALL-1, HRX, Htrx-1) gene at chromosome band 11q23 are the hallmark of infant ALL and occur in about 80% of cases1 . MLL translocations, which are also characteristic of monoblastic and myelomonocytic AML in infants and young children, are much less common in de novo leukemias of other ages (reviewed in 18,19 ). The gene was also called HRX and Htrx-1 for its homology to the trithorax gene that controls thoracic body segmentation in Drosophila. The MLL protein regulates transcription; it is important in the differentiation of hematopoietic cells (reviewed in 18,19 ). Translocations of the MLL gene are leukemogenic (reviewed in 18,19 ).
MLL translocations involve numerous different partner genes that encode protein products of several different types (reviewed in 18,19 ). The AF-4 gene at chromosome band 4q21 is the most common partner gene of MLL in infant ALL. The ENL gene at chromosome band 19p13 is another common partner gene in ALL. Both encode transcription factors (reviewed in 18,19 ). MLL translocations are believed to cause leukemia by the mechanism of gene fusion (reviewed in 18,19,20 ). The der(11) chromosome produces an in-frame transcript that yields a fusion protein consisting of the amino terminus of MLL and the carboxy terminus of the translocation partner but the precise mechanism of action of the diverse fusion proteins is unknown. MLL fusion proteins may exert a dominant negative effect whereby the amino terminus of MLL is uncoupled from the remainder of the protein, leading to the formation of inactive MLL protein complexes and dysfunctional transcriptional regulation.20 An active functional contribution of the translocation partner in the fusion protein has also been suggested.19 In experimental systems, MLL translocations confer resistance to stress-induced cell death and specific drug resistance, which may be relevant in patients.21,22
Natural History of Infant ALL
MLL translocations occur before birth. Observations of identical, non constitutional MLL gene rearrangements in leukemias of both twins in pairs of monozygous twins established that the translocation is an in utero event.10 Backtracking leukemia with the MLL-AF-4 fusion by PCR analysis of the genomic DNA contained in neonatal Guthrie cards indicated that the translocation was an in utero event in singleton births as well.11 Not only has the t(4;11) occurred, but there is proliferation of cells with the translocation to a level of detection by the time of birth. In infant ALL, Ig heavy and light chain genes can be rearranged or germline18 and the rearrangements are of fetal type,23 indicating that the target cell for transformation is more primitive than in ALL in children. Thus, the MLL translocation occurs in proliferating B progenitor cells in utero.
Molecular Epidemiology of Infant ALL
Knowledge of the natural history of infant ALL prompted attempts to understand the MLL translocation mechanism. The cloning of genomic breakpoint junctions of several MLL translocations led to the conclusion that DNA damage and repair are components of the process.24,25,26 The sequences suggested staggered and/or multiple sites of breakage as elements of damage and joining of similar DNA ends as an element of repair. Alu repeat sequences near the breakpoint junctions suggested that Alu-directed non homologous recombination was involved in the repair.24,25,26
However, the nature of the damage and the etiologic agent(s) resulting in MLL translocations in infant ALL remain unknown. High birth weight and a history of maternal fetal loss have been associated with an increased risk of infant ALL, corroborating the importance of the period before birth.27 MLL translocations in infant leukemias resemble MLL translocations in leukemias associated with chemotherapy with DNA topoisomerase II inhibitors. Since DNA topoisomerase II inhibitors increase DNA topoisomerase II-mediated chromosomal breakage, the treatment-related leukemias suggested that DNA topoisomerase II may play a role in MLL translocations in the infant cases. Coupled with the in utero origin of the translocations, this led Ross et al to search for associations with DNA topoisomerase II inhibitors in maternal diet.28 Noteworthy also is that several dietary flavonoids, which are DNA topoisomerase II inhibitors, induce breakage within the MLL gene in experimental systems.29 Nonetheless, the epidemiological studies suggested that maternal consumption of dietary DNA topoisomerase II inhibitors was a factor in infant AML, but not in infant ALL.28
Benzoquinones are other dietary DNA topoisomerase II inhibitors. Recently, it was shown that a polymorphism in the NAD(P)H:Quinone Oxidoreductase (NQO1) gene confers genetic susceptibility in infant ALL with t(4;11) translocations.30 NQO1 detoxifies the potentially carcinogenic benzoquinones by converting them to hydroquinones.30 NQO1 genotype is the only known genetic risk factor for infant ALL. Since quinone moieties form DNA adducts and can damage DNA, it is plausible that NQO1 genotype confers susceptibility in infant ALL.
Prognostic Factors in Infant ALL
Over the years, age2,3,4,6,7,8 and WBC count2,3,5,7,8 have remained prognostic factors in infant ALL as in pediatric ALL31 but, in infant ALL, the MLL translocation is a special prognostic factor. An estimated ∼30-50% of cases of infant ALL carry the t(4;11) translocation.3,4,5,6,7,8,<15,17 Cytogenetic evidence of this translocation is associated with poor outcome2,8,16,17 and was the best predictor of a poor response in multivariate analyses.8,17 It is controversial whether translocations fusing MLL with other partner genes have the same adverse effect.7,8,14,15,16,17,32,33 Other translocations of band 11q23 were associated with ∼50% 4-year EFS in the CCG 107 and 1883 trials compared to 3% for the t(4;11).8 While still an open question, in the CCG, this led to the tentative conclusion that the t(4;11) specifically, but not other translocations of chromosome band 11q23, may be associated with a poor prognosis.8,17 In contrast, the BFM trials did not find outcome differences in infant ALL with t(4;11) compared to other translocations of band 11q23; in these studies, the respective 6-year EFS rates were 18% and 31%.7
Even within the first year, age emerges as an important prognostic factor. Diagnosis at a younger age, variably defined as < 3 months, < 6 months or < 9 months, adversely affects the outcome.2,3 ,6,7,8,34 Multivariate analysis of infants treated in the UK showed that age alone was significant; 5-year EFS rates were 40% for infants > 6 months old but < 10% for younger infants, due in part to the toxicities of intensive treatment.4 The adverse effect of the t(4;11) on prognosis may be age related. Pui et al reported significantly different EFS rates of 17% v. 63% in infants versus children with ALL with the t(4;11).35 Age also influenced the effect of the t(4;11) in the BFM 83, 86 and 90 trials, in which the 6-year EFS rates were 18% in infants and 47% in children with ALL with t(4;11).7 Similarly, in ALL with the t(11;19) involving MLL and ENL, outcome is the worst for infants.36 Table 4
Early response to treatment is also a prognostic factor in multivariate analyses. In the ALL-BFM 83, 86, and 90 trials, the in vivo response after a 7-day prednisone prephase and one dose of intrathecal methotrexate was the strongest predictor of outcome.7 Estimated 6-year EFS rates were 53% and 15%, respectively, in prednisone good- and poor-responder groups7 . Similarly, two CCG trials determined that a slow early response to therapy, defined as an M2/M3 marrow rating at day 14, was associated with a 6% 4-year DFS compared to a 43% 4-year DFS if the marrow rating was M1.8
The probability of MLL translocation is greatest in the younger infants with high WBC count and the CD10- immunophenotype. The adverse effect of CD10 negativity may be due in part to its association with the t(4;11).8 Expression of NG2 proteoglycan on the leukemia cell surface also correlates with MLL translocations and poor outcome in infant ALL.37
Conversely, age and biologic features may identify a small subset of infants who do not need the same intensive treatment. Older infants with CD10+ leukemias lacking MLL translocation may comprise this group. For example, the POG observed 57% EFS in infants diagnosed at > 9 months old.3 The CD10+, CD19+, CD34+ B progenitor immunophenotype, present in about ∼20% of cases, may be associated with more favorable EFS.8 Lack of MLL translocation is associated with better EFS in infant ALL.5,6,8,12,13 The outcome of older infants with CD10+ leukemias lacking MLL translocation in most series, nonetheless, is still inferior compared to that of children.
Treatment Strategies and Outcomes in Infant ALL
The poor response and CNS complications when standard- and high-risk childhood ALL regimens were used for infant ALL have led to specialized approaches. The CCG-160 series for childhood ALL included cranial irradiation; the 4-year EFS for infants was only 22%. The CCG-107 regimen for infants did not include cranial irradiation, but added daunorubicin in induction and very high dose methotrexate infusions (33g/m2) for systemic and extramedullary treatment in consolidation and intensification.8 This strategy resulted in a remission induction rate of 88% and increased the 4-year EFS to 33%.8 The successor protocol, CCG-1883, was intended to maximize tolerable post-induction chemotherapy with the addition of systemic cytosine arabinoside, L-asparaginase, and cyclophosphamide and intrathecal methotrexate to consolidation. The remission induction rate was 94% and the 4-year EFS was 39%.8 Four-year overall survival rates for the CCG-107 and 1883 trials were 45% and 51%, compared to 31% in the historical controls.8 The marrow was the primary site of relapse; CNS relapse rates were low without irradiation.8 The cumulative 4-year probability of an isolated or combined marrow relapse was ∼50%.8 Early relapse was the primary cause of death; ∼70% of relapses occurred within 1 year of diagnosis.
The POG used the approach of remission induction with cyclophosphamide, vincristine, cytosine arabinoside and prednisone; consolidation with teniposide and cytosine arabinoside; triple intrathecal chemotherapy for the CNS; and continuation therapy with methotrexate and 6-mercaptopurine, with alternating pulses of cyclophosphamide, vincristine, cytosine arabinoside and prednisone, or teniposide and cytosine arabinoside. The 4-year actuarial EFS was 28%.3 The therapy was associated with a high incidence of induction toxicity and marrow, extramedullary and combined relapses.3
The CCG and POG conducted a pilot study of induction intensification and suggested that continuous intensive, multi agent chemotherapy including high-dose methotrexate and high-dose cytosine arabinoside without an interim maintenance in the first year was tolerable in infants.38 In one series, 4 of 7 infants treated in first or second remission with allogeneic stem cell transplantation after TBI, cyclophosphamide, and etoposide conditioning were alive at two years.39 Another study suggested that bone marrow transplantation occasionally could be efficacious as salvage therapy for infant ALL in second remission.40 Because the outcome was only modestly improved with the high-dose chemotherapy of the CCG-1883 trial, the current combined protocol of the Children's Oncology Group includes intensive chemotherapy and stem cell transplantation if there is evidence of MLL translocation and a suitable donor can be found. The strategy of allogeneic transplantation after aggressive induction and re-induction phases builds further on the intensified treatment theme.
There are, however, problems associated with stem cell transplantation for infant ALL. Few infants have HLA-matched, sibling donors, necessitating use of alternative donor transplants, which has increased risks.41 The efficacy of cytoreductive regimens for ALL that do not contain TBI may be inferior to TBI-containing regimens42 but there is reluctance to use TBI in infants. Furthermore, the MRC of the UK observed a high relapse rate and excessive TRM among infants prospectively treated in first remission with an HLA-matched transplant.4
Nonetheless, aggressive chemotherapy and allogeneic stem cell transplantation are the only current research alternatives for infant ALL. The POG reported a pilot study using a four-drug remission induction, followed by early, intensified consolidation with 12 alternating drug pair treatments over 30 weeks.43 The drug pairs were intermediate-dose methotrexate and intermediate-dose 6-mercaptopurine, cytosine arabinoside and daunorubicin, and cytosine arabinoside and teniposide. Standard triple intrathecal chemotherapy was used for CNS prophylaxis.43 The results were disappointing; the 5-year EFS rate was 17%.43
The Dana Farber Cancer Institute Consortium added a month of intensive high-dose methotrexate and cytosine arabinoside infusions after the induction phase.44 CNS treatment included intrathecal methotrexate and cytosine arabinoside, and cranial irradiation at one year of age.44 This resulted in an increase in DFS to 54% at 50 months compared to 9% in historical controls.44
In contrast to other groups, the BFM trials have not employed specific therapy for infants except that cranial irradiation is not used. Rather, infants are assigned to standard-, medium-, and high-risk groups based on the leukemic burden and the early prednisone response.7 Intensified therapy after the steroid prephase and induction includes re-induction and intrathecal therapy. Four intermediate-dose methotrexate infusions are substituted for the cranial irradiation.7 The 6-year EFS in infants was 43%, with significant differences in the prednisone poor- and good-responder groups as described above.7 With modified BFM therapy, the EORTC-Childhood Leukaemia Cooperative Group also achieved a 4-year EFS of 41% in infants.5
Similarly, the MRC UK ALL trials were not specifically for infants. The MRC UK ALL VIII and X trials included a three- or four-drug induction, intrathecal methotrexate, cranial irradiation at 2 years and, in some cases, intensification blocks with etoposide and cytosine arabinoside; high-dose methotrexate; and mitoxantrone and cytosine arabinoside. The intensification therapy was directed at the monocytic features of infant ALL. A subsequent pilot study evaluated intensification with high-dose chemotherapy and autologous or allogeneic bone marrow transplantation. The combined results were disappointing with an EFS rate at 6 years of 24% and significant relapses and toxic deaths in the younger infants.4
SJCRH admitted infants to the Total Therapy Studies X-XIII for childhood ALL. The estimated 5-year EFS rates for infants < 6 months and > 6 months old at diagnosis were 8% and 42%, respectively, and for infants with ALL with 11q23/MLL rearrangements versus those lacking 11q23/MLL 6% and 56%, respectively.6
Treatment Complications in Infant ALL
Complications of the treatment for infant ALL are both acute and long term. The physiologic hypogammaglobulinemeia of infancy can be protracted and, with myelosuppression, bacterial and fungal infections and central venous line infections frequently occur. Sepsis, mucositis, bleeding, cardiac toxicity and interstitial pneumonitis can be serious or result in toxic deaths,3,7,8 which are more common in infants than in children.4,6,8 The few infants who survived earlier treatment regimens with cranial irradiation had significant developmental delays and learning disabilities.45 Cranial irradiation may cause cataracts and endocrine dysfunction.44,46 CNS-directed therapy with very high dose methotrexate infusions in the CCG 107 study was associated with better neurodevelopmental outcome and a low relapse rate in the CNS.2,45 The neurocognitive deficits resulting from CNS-directed therapy are more profound in younger infants.45 Short stature, cataracts, gonadal failure and hypothyroidism are late effects of TBI.42,47 Infants receiving TBI who have not had prior CNS disease or cranial irradiation may not experience the same neurodevelopmental sequelae as infants who received prior irradiation to the CNS.47
Conclusions
There are many unanswered questions about why curative treatments for infant ALL characterized by MLL gene translocations have lagged far behind the therapy for ALL in children, and about how to solve the problem. Both the host and the disease, characterized by an otherwise rare translocation, clearly are different. Younger infants, in particular, have the poorest outcome. The daunting results of recent larger trials raise questions about whether any significant progress has been made in cases with t(4;11).2,3,4,5,6,7,8 Does high dose methotrexate, which nearly doubled the EFS in the CCG2 , affect the outcome in this subset? Why does the early prednisone response outweigh the significance of the t(4;11) in trials of the BFM7 ? Will the benefit of high-dose cytosine arabinoside observed in pilot studies in cases with t(4;11)44 hold in larger series? Will hybrid regimens directed at the monocytic component of infant ALL34 and/or stem cell transplantation be associated with an improved outcome? What is the appropriate treatment for the older infant with CD10+ leukemia lacking the MLL gene translocation? Beyond better drugs and drug combinations and better stem cell transplantation, targeted therapeutics may be fresh approaches. Implementation of treatment modalities targeted to MLL chimeric oncoproteins mandates a deeper understanding of the key molecular alterations and pathways causing the disease. While better treatments and improvements in supportive care may influence prognosis,34 all future hope may not lie in better treatments. The features of MLL genomic breakpoint junction sequences have led to a DNA damage-repair model for the translocations.24,25,26 Additional hope may lie in preventing translocations by resolving which exposure(s) are most relevant to the damage and refining who is most at risk. Whether the critical DNA damaging agent(s) is exogenous or endogenous still remains unanswered. Future molecular epidemiological studies may reveal whether specific maternal chemical exposures or endogenous quinones detoxified by NQO1 are relevant in infant ALL.30 Whether there are necessary, rate-limiting events besides the translocations48 also is unknown. The required hit(s) are likely to be few because the time to development of disease is short27 , but molecular epidemiological studies during the prenatal period and early infancy may lead to prevention.