Abstract
This review addresses the biology and the treatment of lymphomas arising from mucosa-associated lymphoid tissue (MALT). This entity, first described in 1983, represents about 8% of all non-Hodgkin's lymphomas and was recently re-classified as “extranodal marginal zone lymphomas of MALT-type.” The term marginal zone lymphoma (MZL) encompasses the three closely related lymphoma subtypes of nodal, primary splenic and extranodal lymphomas of MALT type: the latter represent the vast majority of MZL. These lymphomas arise at different anatomic sites, are composed of mature B-cells lacking expression of CD5 and CD10, often present with overlapping morphologic features, but typically quite distinct clinical behaviors. Only very recently cytogenetic/molecular genetic observations have underlined the distinctiveness of these three lymphoid neoplasms, which in both the R.E.A.L. and WHO-classifications are included in the general term of MZL. MALT lymphomas arise in numerous extranodal sites, but gastric MALT lymphoma is the most common and best studied and is, therefore, the paradigm for the group as a whole.
Dr. Isaacson describes the principal histological features of these lymphomas, including criteria to distinguish this entity from other small B-cell lymphomas. Several lines of evidence suggest that gastric lymphoma arises from MALT acquired as the result of aH. pyloriinfection. However, at least 1/3 of cases do not respond to eradication ofH. pylori. Very recent data suggest that both t(11;18) (q21;q21) and bcl10 nuclear expression are associated with failure to respond to this treatment.
Dr. Gascoyne discusses the biologic function of proteins deregulated through the different translocations, which play a role in pathogenesis of MALT lymphomas, emphasizing particularly their influence in disrupting the apoptotic pathway.
Dr. Zucca reviews findings suggesting that MALT lymphoma is an antigen driven neoplasm. He also presents specific guidelines for treatment of gastric lymphomas trying to shed some light on the amazingly inconsistent and confusing data in the literature.
Taking advantage on the more than 300 non-gastric MALT lymphomas collected by the International Extranodal Lymphoma Study Group (ILESG), Dr. Cavalli compares gastric lymphomas with those arising in many other sites.
Overall, the data presented in this session will underline the fact, that MALT lymphomas are characterized by some unique biological properties.
I. The Pathology of Gastric MALT Lymphoma and Its Response to Eradication ofHelicobacter Pylori
Peter G. Isaacson, MBChB*
University College London Medical School, Department of Histopathology, Rockefeller Building, University Street, London SC1E 6JJ, United Kingdom
The observation that the histology of certain extranodal lymphomas was related to mucosa associated lymphoid tissue (MALT) rather than that of peripheral lymph nodes was first made by Isaacson and Wright in 1983.1 These authors noted similarities between the histology of the condition known as immunoproliferative small intestinal disease (IPSID), a subtype of primary intestinal B-cell lymphoma, and primary low-grade gastric B-cell lymphoma. However, their histology differed from that of comparable low-grade nodal B-cell lymphomas in that their overall structure and cytology resembled those of MALT rather than lymph nodes. These observations were later extended to include other extranodal low-grade B-cell lymphomas, especially those of salivary gland, lung and thyroid.2 The collective histological features of these lymphomas recapitulated the features of the principal B-cell component of MALT as exemplified by the Peyer's patch.3,4 MALT lymphomas, recently re-classified as “extranodal marginal zone lymphomas of MALT-type,”5 arise in numerous extranodal sites, but gastric MALT lymphoma is the commonest and best studied and is, therefore, the paradigm for the group as a whole.
Pathology
Gastric lymphoma, like carcinoma, most often involves the antrum but may occur in any part of the stomach. The histological features of gastric MALT lymphoma, which by definition is a low grade lymphoma, closely simulate those of the Peyer's patch.6,7 The lymphoma infiltrates around reactive follicles in the region corresponding to the Peyer's patch marginal zone, spreading diffusely into the surrounding mucosa. The tumor cells may resemble germinal center centrocytes (centrocyte-like cells) or small lymphocytes, or assume a monocytoid appearance. Scattered transformed blasts are common and may cause problems in grading the lymphoma (see below). An important feature of MALT lymphomas is the presence of lymphoepithelial lesions formed by invasion of individual glands by aggregates of lymphoma cells. Certain additional histological features suggest that the cells of low-grade gastric MALT lymphoma may be participating in an immune response. These include the presence of scattered transformed blasts, plasma cell differentiation, which is maximal beneath the surface epithelium, and follicular colonization8. The latter phenomenon occurs when the lymphoma cells in the marginal zone extend centrally to replace the follicles with dispersion of the mantle zone cells resulting in a nodular appearance, or when lymphoma cells selectively colonize germinal centers, often with preservation of the mantle zone. Within the germinal centers the cells may transform, which can lead to difficulty in distinguishing MALT lymphoma from follicular lymphoma, or undergo plasma cell differentiation.
Immunophenotype and Normal Cell Counterpart
MALT lymphoma cells typically surround reactive B-cell follicles in the distribution of the marginal zone and show a tendency to involve this zone when they disseminate to lymph nodes and spleen (see below). The B-cells of MALT lymphoma share the cytological features and immunophenotype (CD20+, CD21+, CD35+, IgM+, IgD-) of marginal zone B-cells.4
Dissemination
Gastric MALT lymphoma disseminates both locally and systemically more frequently than was originally believed. Indirect evidence for this comes from the observation of recurrent MALT lymphomas in the gastric stump after partial gastrectomy in patients in whom clear resection margins were documented by histological examination.9,10 Wotherspoon et al11 used the “Swiss roll” technique to examine gastrectomy specimens of gastric MALT lymphomas and showed that numerous small tumor foci with identical immunoglobulin (Ig) light chain restriction to the main tumor mass were distributed throughout the gastric mucosa including macroscopically normal regions. Subsequently, the clonal identity of these multiple tumor foci was confirmed by sequence analysis of the rearranged Ig heavy chain genes12. Microdissection studies using clone specific PCR demonstrated that tumor cells were frequently present in reactive lymphoid tissue that showed no histological evidence of lymphoma.13
MALT lymphoma cells tend to localize in the marginal zone of regional lymph nodes without disturbing the lymph node architecture, and the incidental discovery of secondary small intestinal MALT lymphoma during gastrectomy for MALT lymphoma has been reported. Gastric MALT lymphoma has been shown preferentially to disseminate to the splenic marginal zone where it is usually undetectable by conventional histopathology.14
The tendency of gastric MALT lymphoma to disseminate to other parts of the gastrointestinal mucosa and the splenic marginal zone reflects the homing properties of MALT lymphoma cells. Dogan et al15 found that the mucosal homing receptor α4β7 integrin was strongly expressed by a secondary intestinal MALT lymphoma but not by the primary gastric lesion. However, a high level of α4β7 expression could be induced in gastric lymphoma cells following activation by a H. pylori generated T-cell response. This suggests that α4β7+ tumor cells could be similarly generated in gastric lymphoma in vivo and thus become “programmed” to home to an appropriate microenvironment where the mucosal addressin cell adhesion molecule (MAdCAM-1), the ligand for α4β7, is expressed. This includes sites such as the intestinal mucosa15,16 and splenic marginal zone.17
Differential Diagnosis
Because of differences in clinical behavior and management, it is important to differentiate MALT lymphoma from the other small B-cell lymphomas that may present in the stomach. These include mantle cell lymphoma, lymphocytic lymphoma (chronic lymphocytic leukemia) and follicular lymphoma. The cytological features of mantle cell lymphoma can closely simulate those of MALT lymphoma even to the extent that occasional lymphoepithelial lesions may be present. However, absence of transformed blasts together with expression of CD5, IgD and, importantly, intranuclear expression of cyclin D1, a consequence of t(11;14), serve to distinguish mantle cell lymphoma. Lymphocytic lymphoma is characterized by small round lymphocytes together with peripheral blood lymphocytosis. Expression of CD5, CD23 and IgD without nuclear cyclin D1 provide further distinction from MALT lymphoma. Finally, follicular lymphoma, which frequently involves the stomach, can be difficult to distinguish from MALT lymphoma with follicular colonization. The transformed MALT lymphoma cells within follicles may closely resemble centroblasts but typically are CD10 and BCL6 (nuclear) negative in contrast to the cells of follicular lymphoma, which usually express both antigens both within and between follicles.
Transformation of Gastric MALT Lymphoma
Transformation of MALT lymphoma to diffuse large B-cell lymphoma (DLBCL) is heralded by the emergence of increased numbers of transformed blasts that form sheets or clusters18,19 and, finally, grow to confluence effacing the preceding MALT lymphoma. De Jong et al19 have suggested dividing gastric MALT lymphoma into four categories. Category A refers to classical low-grade MALT lymphoma in which transformed blasts comprise no more than 5% of cells and do not occur in clusters of more than ten cells. In category B, transformed cells may account for 10-20% of cells and occur in clusters of up to 20 cells. Category C is characterized by unequivocal high-grade transformation with sheets of transformed cells that may leave only small foci of low-grade disease. In category D, no MALT component is detectable and it is probably better classified as DLBCL without reference to MALT. In the hands of De Jong et al the separation into the four categories had clinical significance.
Helicobacter Pylori and Gastric Lymphoma
Several lines of evidence suggest that gastric lymphoma arises from MALT acquired as the result of this H. pylori infection. H. pylori can be demonstrated in the gastric mucosa of the majority of cases of gastric MALT lymphoma.20 In the first study in which this association was examined21 the organism was present in over 90% of cases. Subsequent studies have shown a lower incidence22 but also that the density and detectability of H. pylori decreases as lymphoma evolves from chronic gastritis.23 A case control study showed an association between previous H. pylori infection and the development of primary gastric lymphoma.24 More direct evidence confirming the importance of H. pylori in the pathogenesis of gastric lymphoma has been obtained from two studies that detected the lymphoma B-cell clone in the chronic gastritis that preceded the lymphoma and from a series of in vitro studies25 showing that lymphoma growth could be stimulated in culture by H. pylori strain specific T-cells when crude lymphoma cultures were exposed to the organism. Finally, following the initial study by Wotherspoon et al, several groups have shown that eradication of H. pylori with antibiotics together results in regression of gastric MALT lymphoma in 75% of cases.26
PCR Detection of Ig Gene Rearrangement in Gastric MALT Lymphoma
Because of the difficulty in assessing gastric biopsies both before and after eradication of H. pylori there has been a tendency to rely on molecular evidence of monoclonality detected by the polymerase chain reaction (PCR) for the diagnosis of lymphoma both before and after antibiotic therapy for H. pylori. This technique may fail to detect monoclonality in up to 30% of cases of overt lymphoma and thus produce false negative results.27 There are also reports of apparently spurious monoclonality in biopsies showing only chronic gastritis28–,30 and in biopsies following antibiotic induced regression of lymphoma where there is no histological evidence of malignancy.31 The frequency of this spurious monoclonality varies between laboratories,32 which suggests that technique may be a factor. These findings serve to emphasize that gastric MALT lymphoma should not be diagnosed in the absence of clear histological evidence.
PCR evidence of B-cell monoclonality may persist in 50% to 75% of cases that have regressed histologically following eradication of H. pylori. The persistence of monoclonality in post-treatment biopsies without histological evidence of lymphoma is consistent with the notion that eradication of H. pylori suppresses but does not necessarily eradicate the neoplastic clone in all cases. In keeping with this, in rare cases re-infection with H. pylori has been associated with relapse of the lymphoma It should be stressed, however, that persistence of the neoplastic clone is not, on its own, an indication for further treatment. There is some evidence that with time the neoplastic clone becomes undetectable.
Prediction of Response of Gastric MALT Lymphoma to Eradication ofH. pylori
The follow-up of MALT lymphoma patients following eradication of H. pylori is rather complex, requiring repeated gastroscopy, and it would be extremely useful to be able to identify the approximately 25% of cases of gastric MALT lymphoma that do not respond to eradication of H. pylori. Studies using endoscopic ultrasound have suggested that if the tumor has invaded beyond the submucosa it will not respond.33,34 More recently, two MALT lymphoma-associated translocations that have a bearing on the response to H. pylori eradication have been cloned.35,36 The first of these, t(1;14)(p22;q14), present in rare cases, has been shown to involve a novel gene, bcl10, that is strongly expressed in the nucleus of the neoplastic lymphocytes.37 The second, t(11;18)(q21;q21), is present in up to 40% of cases and is strongly associated with failure to respond to eradication of H. pylori.38 Interestingly, t(11;18) is also associated with nuclear expression of bcl10, albeit more weakly than t(1;14).39 Moreover, the frequency of both t(11;18)(q21;q21) and nuclear BCL10 expression is significantly higher in tumors that have disseminated beyond the stomach (78% and 93%, respectively) than those confined to the stomach (10% and 38%).39 These findings in part explain the results based on the use of endoscopic ultrasound33,34 and suggest that both t(11;18)(q21;q21) and BCL10 nuclear expression are associated with failure to respond to H. pylori eradication and with more advanced MALT lymphoma. This suggests that their oncogenic activities may be related.
II. The Molecular Biology of MALT Lymphoma
Randy D. Gascoyne, MD, FRCPC*
Department of Pathology, British Columbia Cancer Agency, 600 West 10th Avenue, Vancouver BC V5Z 4E6, Canada
In the recent past, the term marginal zone lymphoma (MZL) was considered to define a specific disease entity that included the three closely related lymphoma subtypes of nodal, primary splenic and extranodal lymphomas of MALT type.1,2 These lymphomas arise at different anatomic sites, are similarly composed of mature B cells lacking expression of CD5 and CD10, often reveal overlapping morphologic features, but typically display distinct clinical behaviors.3–,6 As recently as 1996, there were developing concepts suggesting that all three lymphomas shared similar cytogenetic alterations, including whole or partial trisomy 3, trisomy 18 and structural rearrangements of chromosome 1 involving breakpoints at 1q21 and 1p34.2,7 Thus, a concept was emerging suggesting that MZL at different sites shared a common pathogenesis. Importantly, no evidence of a recurrent balanced translocation had yet been substantiated, a finding thought to play an important pathogenic role in the development of many B cell lymphomas including Burkitt's lymphoma (cmyc), follicular lymphoma (bcl2) and mantle cell lymphoma (cyclin D1).1
Since that time, several important cytogenetic/molecular genetic observations have shed light on the distinctiveness of these lymphoid neoplasms, and each is now considered a unique lymphoma subtype in both the REAL and World Health Organization (WHO) classifications.1,8 Nodal MZL and primary splenic MZL will be discussed only briefly here, principally to distinguish these disorders from extranodal marginal zone lymphoma of MALT-type. Although this latter disorder can occur in many different anatomic sites, this discussion will focus primarily on gastric MALT lymphomas as the most common extranodal site of involvement.9
Cytogenetics
Only a small number of publications have described the karyotypic abnormalities associated with MALT lymphoma, owing in part to the extranodal nature of the biopsy specimens, their small size in many cases and the difficulty in producing analyzable metaphases. Originally described in 1989, the most common structural abnormality associated with MALT lymphomas is the t(11;18)(q21;q21).10 The possibility that it might play an important role in MALT lymphoma was first suggested in 1992.11 This was confirmed by two studies published simultaneously in 1997.12,13 It is present in approximately one third of cases, has been found in most anatomic sites associated with MALT lymphomas and is often the sole cytogenetic alteration.12 The latter finding suggests a major role for this translocation as a disease initiation event, although cryptic, sub-cytogenetic changes cannot be excluded as contributing to the development of lymphoma. A second nonrandom cytogenetic alteration associated with MALT lymphoma is t(1;14)(p22;q32), which is rarely encountered.14–,16 This alteration has only been described in association with gastric and pulmonary MALT lymphoma.14,15 Thus, MALT lymphoma is unusual in comparison to most other small B cell lymphomas, having now been associated with two seemingly unrelated translocations. However, very recent data have provided insights into the mechanisms that unify these two observations (discussed in detail below).
Important negative findings in MALT lymphoma include the lack of evidence for involvement of either t(11;14)(q13;q32) or t(14;18)(q32;q21), implicating the cyclin D1 and bcl2 oncogenes, respectively. Bcl6 rearrangements involving chromosome 3q27 have been described infrequently in MALT lymphoma and are more often found in association with extranodal diffuse large B cell lymphoma (DLBCL).17,18 Moreover, there are no reported cases of nodal MZL or primary splenic MZL harboring either a t(11;18) or t(1;14), a testament to the distinct biology of these related neoplasms.19,20 A characteristic balanced translocation has not been described in nodal MZL, while splenic MZL appears to be associated with an interstitial deletion of the long arm of chromosome 7 (del 7q31-32) and trisomy 3.21– 23
The most common numerical cytogenetic abnormality in MALT lymphoma is trisomy 3.12,24 It is present in approximately 60% of cases by classical cytogenetic studies, but is not specific for this lymphoma subtype. It has been described in other lymphomas, including both nodal MZL and primary splenic MZL.23,25 The same can be said for trisomy 18 and chromosomal breakpoints involving bands 1q21 and 1p34-36, as all three alterations are common clonal evolution events in many subtypes of lymphoma.26,27 More recent studies employing fluorescence in situ hybridization (FISH) techniques have reported a much lower incidence of trisomy 3 in MALT lymphoma, ranging between 20 and 46%.28,29 The reason for this difference may reflect a combination of variable patient populations and disease definition.
Cmyc oncogene alterations may be involved as early events in the genesis of MALT lymphoma but do not involve translocation of the gene as is typical of Burkitt's lymphoma. Peng and colleagues described the presence of point mutations involving either the first exon or intron in 17% of 54 cases and suggested the role of this molecular alteration as an early event in the development of MALT lymphoma.30 The significance of genetic instability in MALT lymphomas remains controversial. Microsatellite instability (MSI; replication error phenotype) results from defects in the DNA mismatch repair pathway and is generally considered uncommon in non-Hodgkin's lymphomas (NHL).31 Peng et al reported this to be a frequent finding in MALT lymphoma, but more recent data are contradictory, finding a very low frequency of MSI in both low-grade and high-grade MALT lymphomas.32– 34
The concept of histological transformation of MALT lymphoma to DLBCL was described the Section I. Genetic events that may herald this change are similar to those recognized in nodal lymphoma and include mutations of p53 and altered methylation status or homozygous deletions of p16.35,36 Importantly, the relationship between gastric MALT lymphoma and DLBCL at the same site remains controversial, particularly as the t(11;18) is not found in gastric DLBCL.19,20,37 These data suggest two possibilities. Firstly, MALT lymphomas with a t(11;18) have a reduced frequency of transformation; secondly, some cases of de novo DLBCL involving the stomach may have a distinct histogenesis and are best considered de novo extranodal DLBCL rather than “high-grade” MALT lymphomas. Finally, the translocation breakpoint involved in t(6;14)(p21;q32) has been described in association with MALT lymphoma undergoing histological transformation and implicates deregulated expression of cyclin D3 in the pathogenesis of this event (manuscript submitted). Interestingly, this same balanced translocation involving the immunoglobulin heavy chain locus (IgH) has been described in myeloma.38
Molecular Genetics
Immunoglobulin Heavy Chain
MALT lymphoma B cells are related to normal marginal zone cells. As such, their IgH variable region gene sequence reveals a molecular signature characteristic of a post germinal center B cell, demonstrating evidence of somatic hypermutation.39,40 Intraclonal variation is also found in many cases, suggesting that expansion of the clone is occurring in the presence of antigen. Studies of both gastric and salivary gland MALT lymphomas have revealed preferential use of germline VH segments associated with autoantibody formation, in keeping with the observation that MALT lymphomas are commonly derived from a background of chronic inflammatory disorders, including autoimmune diseases.39 The limited repertoire of VH genes and conserved complementary determining region sequences in salivary gland MALT lymphomas suggests that a unique antigen may be driving the B cell proliferation.41
API2-MALT1
The t(11;18)(q21;q21) represents the most common structural abnormality in MALT lymphoma.12,13,42 In 1999, Dierlamm and colleagues showed that the t(11;18) produced a fusion of API2 on chromosome 11q21 with MLT (for MALT lymphoma-associated translocation) on chromosome 18q21.43 Two other groups simultaneously characterized the same gene on 18q21, now known as MALT1.44,45 In a subset of cases the translocation was associated with a cryptic deletion involving the 3′ portion of API2, resulting in loss of the reciprocal transcript. Thus, the 5′ API2-MALT1 3′ fusion localized to the derivative chromosome 11 was shown to be the pathogenetically important event.46
API2, also known as cIAP2, belongs to a family of inhibitors of apoptosis (IAPs) first identified in baculoviruses. They contain a BIR (baculovirus inhibitor of apoptosis repeat) motif in one to three copies, a caspase recruitment domain (CARD) and a C-terminal zinc-binding RING finger domain (absent in the IAP survivin). Several of the IAP family proteins have been shown to be potent inhibitors of activated caspases, mediated through their interaction with TNF-associated factor (TRAF) proteins, thus fulfilling a role as inhibitors of apoptosis. The BIR domain, left intact in the API2-MALT1 fusion, is thought to be important for this function.47
Until very recently, the function of the MALT1 protein was unknown. It was hypothesized that the fusion protein resulting from the t(11;18), owing to the presence of the API2 component, inhibited apoptosis and thereby conferred a survival advantage to MALT lymphomas and allowed for antigen-independent proliferation.42,43 Uren et al have now identified MALT1 as a “paracaspase,” a caspase-like protease found in several species.48 This class of proteins has altered substrate specificity in comparison to caspases. Death domains (DDs), death effector domains (DEDs) and caspase recruitment domains (CARDs) are protein modules found exclusively in proteins that mediate apoptotic signaling.49 MALT1 possesses a DD, two adjacent immunoglobulin-like domains and a caspase-like domain (but lacks a CARD domain). Similar to CARDs and DEDs, the DD of MALT1 acts as a homotypic interaction module.48 In an attempt to illuminate signaling pathways involving MALT1, yeast two-hybrid screens were performed in order to identify molecules that bind the prodomains of MALT1.48 Surprisingly, Bcl10, the protein involved in the other MALT lymphoma translocation, binds to MALT1 and appears to do so through an interaction with the two Ig-like domains (Figure 1 ). Under physiological conditions, Bcl10 and MALT1 form a tight bond and synergize to increase activation of NF-κB.50 Over-expression of full-length or mutant forms of Bcl10 has been shown to weakly activate NF-κB. Full-length MALT1 protein does not significantly activate NF-κB, nor does full-length API2. However, the fusion protein produced by the API2-MALT1 translocation significantly increases NF-κB activation.48 Truncated versions of the fusion fail to activate NF-κB, suggesting that both the BIR domains contributed by API2 and the paracaspase domain of the chimeric protein are required for complete activation of NF-κB.48
NF-κB, a member of the rel family, plays a central role in the activation of genes involved in immunity, inflammation and apoptosis.51,52 In unstimulated cells, NF-κB is sequestered in the cytoplasm through interactions with inhibitory IκB proteins. In response to a variety of stimuli, IκBα is phosphorylated and targeted for degradation by the ubiquitin pathway. The degradation of IκBα results in the translocation of NF-κB to the nucleus, where it binds to specific promoters and activates transcription. Thus, the mechanism by which Bcl10 activates NF-κB has now been elucidated. These new data demonstrate that under normal conditions, Bcl10 and MALT1 form a tight complex that serves to oligomerize and activate the caspase-like domain of MALT1, leading to induction of NF-κB.50 Unlike wild type MALT1, which appears to be dependent upon an interaction with Bcl10 as a mechanism for oligomerization and auto-activation, the API2-MALT1 fusion protein may possess a mechanism for self-oligomerization, possibly due to the three BIR domains contributed by the chimera.50 Thus, in normal cells the basal level of expression maintains the signaling pathway but can be perturbed by either a t(1;14) or a t(11;18) in MALT lymphoma that results in marked over-expression of Bcl10 or the API2-MALT1 fusion proteins, respectively. The resultant dramatic increase in NF-κB activity is likely critical to lymphoma progression.
Bcl10
The other recurrent translocation in MALT lymphomas is t(1;14)(p22;q32), which is significantly less frequent than t(11;18).14–,16 This rearrangement results in the juxtaposition of the entire coding region of bcl10 to chromosome 14 under control of the Ig enhancer element. All bcl10 breakpoints thus far characterized cluster within the 5′ promoter region of the gene.15 The human bcl10 gene encodes a protein of 233 amino acids containing an N-terminal caspase recruitment domain (CARD), characteristic of both antiapoptotic and proapoptotic proteins. In normal tissues, Bcl10 is ubiquitously expressed, but at low levels. The highest Bcl10 expression levels are found in spleen, lymph node, testis and developing central nervous system.15,53 Forced expression of wild type Bcl10 in cell lines induces both apoptosis and activates NF-κB.54,55 The translocation leads to over-expression of the Bcl10 protein but is frequently associated with frameshift mutations causing C-terminal truncations distal to the CARD.15,16 CARD-truncation mutants lose apoptosis activity and fail to induce NF-κB, whereas mutants with C-terminal truncations retain NF-κB activation but do not induce apoptosis.16 It was thus hypothesized that Bcl10 might induce tumors via two mechanisms: mutant Bcl10 would lose its pro-apoptotic function conferring a growth advantage on MALT B cells, and constitutive NF-κB activation could provide both anti-apoptotic and proliferative signals by up-regulating transcription of specific targets.16
The initial report of the cloning of the bcl10 gene indicated a high frequency of truncating bcl10 mutations independent of the presence of the t(1;14) (~45% of B and T cell lymphomas).15 These cases were analyzed using cDNA preparations from a variety of tumors. However, a comparable rate of bcl10 mutation was not detected when genomic DNA from a wide range of tumors was analyzed.56–,61 Some of these differences may be due to technical artifacts arising from the study of cDNA versus genomic DNA, resulting from “molecular misreading.”62,63 Nonetheless, subsequent reports have confirmed the presence of bcl10 mutations in a small percentage of MALT lymphomas (6.7%), follicular lymphomas (9.5%) and DLBCLs (4.3%).63 Moreover, mutations of bcl10 were slightly more common in “high-grade” MALT lymphomas, suggesting that mutations may underlie histological progression. An intriguing finding from this work was the over-expression of wild type Bcl10 in some MALT lymphoma cases with a t(1;14).63
Murine models used to study the functional role of Bcl10 have recently been described.64,65 Transgenic mice in which bcl10 is linked to an immunoglobulin enhancer construct that directs expression to lymphoid cells only develop splenomegaly due to a marked increase in splenic marginal zone B cells, similar to splenic MZL. Interestingly, these studies also revealed that mice over-expressing Bcl10 mutants in their lymphocytes have no apparent abnormalities of lymphocyte development or function, suggesting that deregulated expression of the normal Bcl10 protein by the t(1;14) rather than bcl10 mutation contributes to MALT tumorigenesis.65
Significant improvement in our understanding of the role of Bcl10 in normal intracellular signaling and tumorigenesis has resulted directly from the study of knockout mice with targeted disruption of bcl10 (bcl10-/-).53 One third of bcl10-/- embryos developed exencephaly, leading to embryonic lethality, but the mice retained their susceptibility to apoptotic stimuli. Importantly, the mice were markedly immunodeficient due to defective NF-κB activation via the antigen receptor complex of both B and T cells. These data allowed the authors to conclude that Bcl10 is unlikely to be an essential component of the mammalian cell death machinery. Truncating mutations of the bcl10 gene were postulated to be important in the pathogenesis of MALT lymphomas by conferring a relative resistance to apoptosis. The bcl10-/- data reveal that complete disruption of both bcl10 alleles fails to promote cell survival and does not result in tumor formation in the mice.53 It is well established that most MALT lymphomas originate in the setting of inflammation triggered by infection or autoimmune disorders. Moreover, gastric MALT lymphomas are in part dependent on H. pylori-specific T cells to provide the immunological stimulus for their early development and proliferation.66 Thus, translocation and up-regulated expression of either wild type or truncated Bcl10 could mimic this antigen-receptor signaling, producing constitutive activation of NF-κB, antibiotic-resistant growth and progression of the lymphoma.
Since the discovery of Bcl10, a number of proteins have been described, including a new family of proteins called the membrane-associated guanylate kinase family (MAGUK).67 Thus far, several members of this family have been described, including CARD9, CARD10, CARD11 and CARD14 and Carma 1.68–,70 Briefly, this class of proteins appears to function as upstream regulators of Bcl10. MAGUK proteins function to organize signaling complexes at plasma membranes. Each appears to bind Bcl10 at its CARD motif, resulting in NF-κB activation, but variable consequences result from this interaction including phosphorylation of Bcl10, translocation of Bcl10 from the cytoplasm to perinuclear structures and a molecular scaffolding function for the assembly of Bcl10 signaling complexes.67– 70
Bcl10 protein expression, molecular and clinical correlates
Monoclonal antibodies raised against the Bcl10 protein demonstrate expression of Bcl10 in lymphoid tissue and breast epithelium.71 In normal B cell follicles, Bcl10 is expressed in the cytoplasm of germinal center B cells and marginal zone cells, but only weakly in mantle zone cells. MALT lymphomas characterized by the t(1;14) express Bcl10 in both the nucleus and cytoplasm. Cases that lack the translocation have also been shown to express nuclear Bcl10. A preliminary study of MALT lymphomas has revealed a strong correlation between the presence of the t(11;18) and nuclear Bcl10 expression.72 These data suggest that nuclear localization of Bcl10 can occur as the result of two apparently independent cytogenetic events. Thus, these two seemingly disparate translocations that target bcl10 and MALT1 appear to affect the same signaling pathway, the result of which is the activation of NF-κB and the nuclear localization of Bcl10 protein. This latter finding may not only be important for the pathogenesis of MALT lymphomas but may serve as a surrogate marker for identifying more aggressive clinical disease and resistance to antibiotic therapy.72,73
Conclusion
In summary, our understanding of the molecular biology of MALT lymphoma has significantly improved following the recent cloning of two balanced translocations involved in this disease. Although both genes code for novel proteins with distinct protein interaction modules, suggesting involvement in the cell death pathway, the inhibition of apoptotic signaling may not be central to the pathogenesis of either bcl10 or API2-MALT1. Instead, constitutive activation of the NF-κB pathway may mimic engagement of the antigen receptor complex, driving antigen-independent growth and lymphoma progression. Curiously, two unrelated translocations appear to impact the same signal transduction pathway, giving rise to the development of a single clinicopathologic entity we recognize as MALT lymphoma. Much excitement will await the experimental data that determine the precise role of nuclear Bcl10 and how this contributes to lymphomagenesis.
III. Low Grade Gastric MALT Lymphoma
Emanuele Zucca, MD*
Oncology Institute of Southern Switzerland, Department of Medical Oncology, 6500 Bellinzona, TI, Switzerland
The term low-grade MALT lymphoma identifies a group of extranodal B-cell lymphomas, composed mostly of small cells, that share similar clinical, pathological, and molecular features; these lymphomas are defined as extranodal marginal zone B-cell lymphomas of MALT type in the Revised European-American Classification of Lymphoid Neoplasms (REAL)/World Health Organization (WHO) Classification of Lymphoid Neoplasms. The stomach is by far the most common localization.1– 2
The onset of MALT lymphoma in the stomach, where lymphocytes are not normally present, is preceded by the acquisition of a mucosa-associated lymphoid tissue (MALT) as a result of H. pylori infection.1–,2 The microorganism can be found in the gastric mucosa in nearly all instances of gastric MALT lymphoma, with several lines of evidence suggesting a link between H. pylori-chronic gastritis and the lymphoma. A close association has been reported in epidemiologic studies between H. pylori infection and gastric lymphomas of either low-grade or high-grade histology.3 In vitro experiments have demonstrated that the neoplastic cells of low-grade gastric MALT lymphoma proliferate in a strain-specific response to H. pylori and that this response is dependent on specific T-cell activation.4 The presence of the B-cell clone that would become predominant in the transformation to MALT lymphoma has been demonstrated in H. pylori gastritis specimens taken several years before development of the lymphoma.5 A regression of gastric MALT lymphoma after antibiotic eradication of H. pylori has been reported in more than half of the treated patients.6–,12 This close association of H. pylori with gastric MALT lymphoma has led to the hypothesis that the microorganism may provide the antigenic stimulus for sustaining the growth of the lymphoma in the stomach13. However, the exact mechanism of the transition from H. pylori infection to low-grade MALT lymphoma is still unclear. Most patients with H. pylori gastritis do not develop lymphoma; therefore, it is widely accepted that additional environmental and microbial or host genetic factors may play a role in gastric lymphomagenesis.1– 2
Diagnosis and Staging
The most common presenting symptoms of low-grade gastric MALT lymphomas are nonspecific dyspepsia and epigastric pain. Constitutional B symptoms are exceedingly uncommon. Endoscopy usually reveals nonspecific gastritis or peptic ulcer, with mass lesions being unusual.6,14 Few patients present with elevated lactate dehydrogenase (LDH) or β2-microglobulin levels.6
The best staging system is still controversial.15–,16 We currently use the revised version of the Blackledge staging system that was recommended for general use by an international workshop held in Lugano, Switzerland, in 1993.16 When ultrasound endoscopy is available, the TNM system (using the criteria initially proposed for gastric carcinoma by the American Joint Committee on Cancer and Union International Contre le Cancer) can also be employed, based on the echoendoscopic extent of the gastric wall involvement.10
The initial staging should comprise a gastroduodenal endoscopy with multiple biopsies from each area of the gastric map and from all the abnormal sites. Upper airway examination is required as well as all of the usual procedures performed for nodal lymphomas, including bone marrow biopsy. The presence of active H. pylori infection must be always ruled out by histology; serology studies are useful when results of histology are negative.
Unlike most low-grade B-cell lymphomas of peripheral lymph nodes, low-grade MALT lymphoma is usually a very indolent disease, often remaining localized for a prolonged period; in some cases, no progression is seen during several years without treatment. A few patients present with systemic disease, often due to the simultaneous involvement of multiple mucosal sites and it has been postulated that this pattern of dissemination may be related to specific homing properties similar to those of the normal B-cells of MALT.1 Bone marrow involvement is reported in 0-15% of cases.6,17,18 Prognosis seems particularly poor in the few cases presenting with advanced stages17–,20 or with an unfavorable IPI score.21 Patients with primary gastrointestinal presentation might have a better survival than those with nongastrointestinal MALT lymphoma.22 A deep infiltration of the gastric wall by the lymphoma has been reported to be strongly associated with spread to the regional lymph nodes, analogous to findings in gastric carcinoma. It has therefore been recommended that the depth of infiltration be included in pathology reports concerning primary gastric lymphoma of the MALT.23 Endoscopic ultrasound allows evaluation of the depth of infiltration. Moreover, it might be useful to distinguish benign lymphoid aggregate from malignant lymphoma and should be included in both the initial and the follow-up procedures whenever possible.24– 27
Treatment
Despite abundant literature on histologic, clinical, and biological features of MALT lymphoma, final results of controlled trials to define the optimal therapy have not yet been published. Published data are confusing: insufficient staging and outdated histologic classifications are a major problem of the older reports, and more recent studies often refer to retrospective series of patients not uniformly staged and treated.
Few published studies specifically report treatment outcome for localized gastric MALT lymphoma. The patients have been treated with a variety of combinations of surgery, radiotherapy, and chemotherapy, and the overall survival rates range from 80% to 95% at 5 years.6,14,17,20,29 Therefore, while prognosis of patients with MALT lymphoma seems excellent regardless of treatment, optimal therapy remains to be determined.
Increasing evidence indicates that eradication of H. pylori with antibiotics can be effectively employed as the sole initial treatment.6–,12,26–,28 In a series of 93 patients from northern Italy and southern Switzerland with low-grade gastric MALT lymphoma, no statistically significant difference was apparent in overall survival or event-free survival between patients who received different initial treatments (chemotherapy alone, surgery alone, surgery with additional chemotherapy or radiation therapy, or antibiotics against H. pylori).6 The actuarial 5-year overall survival was 82% (95% confidence interval [CI], 67%-91%) in the series as a whole. At a median follow-up of 3 years, 10 of 93 patients had died, all but 1 from a second (solid) tumor. The unexpectedly high incidence of additional neoplasms was not treatment related and has been described in other series, but its significance is controversial.30–,32 In this series, 49 patients with stage I disease were given antibiotics alone as initial treatment; eradication of H. pylori was achieved in 97% of patients (95% CI, 88.2%-99.9%), and histologic regression of the MALT lymphoma was documented in 67% of the patients (95% CI, 51%-80%) after the eradication.6 The median time required to achieve histologic regression was 5 months (range, 3 to 18 months). A German multicenter study confirms, with a median follow-up of 2 years, the efficacy of antibiotics in inducing apparently durable lymphoma remission. Moreover, this study demonstrated that patients in whom the lymphoma did not respond to H. pylori eradication may have harbored high-grade lesions that had not initially been recognized.11
An American trial of 34 patients with stage I-II disease showed that the antibiotic efficacy is higher in early lesions: 70% (95% CI, 35%-93%) of the cases with disease confined to the mucosa and submucosa achieved a complete remission (CR), whereas those with locally advanced disease infiltrating the muscularis mucosae, the serosa, or the perigastric lymph nodes had a significantly lower CR rate (38%; 95% CI, 17%-64%).10 A recent French series of 46 patients reported a CR rate of 43% with no response in the 10 H. pylori-negative cases. In this study the absence of nodal involvement was the strongest predictor of regression with a CR rate of 79% for the H. pylori-positive cases without any lymph node involvement.12 A preliminary response evaluation has been performed in the first 170 patients with localized low-grade lymphoma of the stomach enrolled in the ongoing international controlled clinical trial LY03 of chlorambucil versus observation after antibiotic therapy; it confirmed that at least half of the treated cases can achieve a histologic CR.33 A subset of patients has undergone a molecular follow-up by the PCR assay for the detection of a monoclonal rearrangement of the immunoglobulin gene: approximately half of the patients with a histologic CR also had a molecular complete response that sometimes required a long time (up to 2 years) to be demonstrated.34 Similar molecular data have been found by other authors.10–,11,28,35 These data demonstrate that PCR-detectable B-cell monoclonality may persist after the disappearance of histologic evidence of MALT lymphoma, suggesting that H. pylori eradication suppresses but does not eradicate the lymphoma clones. Whether the persistence of PCR-detected B-cell monoclonality is associated with a higher risk of lymphoma relapse remains to be determined. Thus, careful histologic examination of multiple gastric biopsies remains the cornerstone for the follow-up of gastric MALT lymphoma patients.28
In our opinion, the indolent nature of the disease in most cases of localized MALT lymphoma makes a conservative approach advisable, with antibiotic therapy as the sole initial treatment provided that strict oncohematologic and endoscopic follow-up is conducted. The use of antibiotics as first-line therapy may avert the indication for surgical resection in most patients, and we recommend eradication of H. pylori before consideration of further therapeutic options. Any of the highly effective antibiotic regimens proposed can be used.1 A strict endoscopic follow-up is recommended, with multiple biopsies taken 2 months after treatment to document H. pylori eradication and, subsequently, at least twice per year for 2 years to monitor the histologic regression of the lymphoma. In case of unsuccessful H. pylori eradication, a second-line anti-Helicobacter therapy should be attempted with alternative triple- or quadruple-therapy regimens of proton-pump inhibitor plus antibiotics. However, it is still unknown whether H. pylori eradication will definitely cure the lymphoma; therefore, long-term follow-up of antibiotic-treated patients is mandatory. Some cases of documented tumor recurrence following H. pylori reinfection have been reported, suggesting that residual dormant tumor cells can be present despite clinical and histologic remission. Relapses have also been documented in the absence of H. pylori reinfection, indicating the presence of B-cell lymphoma clones that have escaped the antigenic drive.11 The efficacy of antibiotic therapy is reduced in locally advanced disease, with bulky masses or deep infiltration of the gastric wall, and in disease associated with increased numbers of large cells. In our experience, however, eradication of H. pylori is worthwhile even in these cases but usually cannot be the unique therapeutic approach. In addition to antibiotic therapy, chemotherapy (or radiotherapy) should be given to these patients as well as to those with regional nodal involvement.1
No treatment guidelines exist for the management of patients after antibiotics failure and for the subset of cases in which no evidence of H. pylori can be found. It has been shown that the chance of a response to antibiotics is dramatically reduced in the latter group.10,12 A choice can be made between conventional oncologic modalities, including chemotherapy, radiotherapy, and surgery, alone or in combination. Unfortunately, there are no published randomized studies to help the decision.
Chemotherapy has never been adequately evaluated in gastric MALT lymphomas because it was usually not administered or given after surgery or radiotherapy. Some scanty data suggesting the efficacy of chlorambucil in low-grade gastric lymphoma can be found in the older literature,36 but only one nonrandomized trial has thus far tested the activity of chemotherapy with single alkylating agents in MALT lymphomas.37 In this study, 24 patients, 17 with stage IE and 7 with stage IV were given continuous oral administration of cyclophosphamide, 100 mg/day, or chlorambucil, 6 mg/day (median treatment duration, 18 months; range, 8-24 months). A 75% CR rate was reported. Five patients relapsed (2 with stage I and 3 with stage IV) at 12 to 96 months, all in initial sites, and 1 with large-cell transformation. The projected 5-year event-free and overall survivals were 50% and 75%, respectively.37
A preliminary report from a phase II trial of the International Extranodal Lymphoma Study Group suggest that the anti-CD20 antibody rituximab may also have a significant clinical activity in relapsing or H. pylori-negative gastric MALT lymphoma.38
Analogous to chemotherapy, the efficacy of local radiotherapy has also not been extensively studied in trials that take account of the MALT concept.1,29,39 In a small American study, 17 patients with stage I-II MALT lymphoma of the stomach without evidence of H. pylori infection or with persistent lymphoma after antibiotics were treated with radiation alone (1.5-Gy fractions in 4 weeks to the stomach and the adjacent lymph nodes, with a median total dose of 30 Gy). The results are encouraging, with 100% biopsy-confirmed CR and 100% event-free survival (at a median follow-up of 27 months).29
Surgery has been widely used in the past. Cogliatti et al20 reported a series of histologically reviewed cases of low-grade MALT lymphoma (48 patients with stage IE and 21 with stage IIE disease): 45 had surgery alone; 12 surgery and adjuvant chemotherapy; 11 surgery and irradiation; 1 surgery, chemotherapy, and radiotherapy. The 5-year overall survival was 91% (95% for stage IE and 82% for stage IIE) with no significantly different survival rates between gastrectomy alone versus additional treatment.
While the use of local treatment is evidently associated with an excellent disease control, the precise role for surgical resection must nowadays be redefined. Follow-up endoscopy may reveal the reappearance of lymphoepithelial lesions in the remaining gastric mucosa that can be responsible for local recurrence. Indeed, the fact that MALT lymphoma is often a multifocal disease suggests that clear excision margins are not necessarily a guarantee of radical resection. If surgery is chosen, a total gastrectomy may offer greater chances of cure, but this operation carries a risk of mortality and may severely impair the patient's quality of life.40– 41
IV. Non-Gastric Marginal Zone B-Cell Lymphoma of MALT Type
Franco Cavalli, MD, and Emanuele Zucca, MD
Extranodal marginal zone B-cell lymphoma is a discrete clinico-pathological entity arising in MALT. Two types of MALT can be identified in disparate organs that do not correspond to peripheral sites of the immune system. The native type consists of lymphoid tissue physiologically present in the gut (e.g., Peyer's patches), whereas acquired MALT develops in sites of inflammation in response to either infectious conditions, such as H. pylori gastritis, or autoimmune processes, such as Hashimoto's thyroiditis or myoepithelial sialadenitis (MESA) associated with Sjögren's syndrome.1–,3 In the context of these prolonged lymphoid reactive proliferations, the growth of a pathological clone can progressively replace the normal lymphoid population, giving rise to a MALT lymphoma.4– 7
Outside the stomach the role of infectious agents is less clearly defined. There is some evidence indicating that Borrelia burgdorferi may be implicated in the pathogenesis of some cutaneous lymphoma.8 The hepatitis C virus (HCV) has been linked to B-cell lymphoproliferation and autoimmunity, and has been localized in several tissues, including the gastric mucosa. Several recent studies have reported a high rate of previous HCV infection in patients with non-Hodgkin's lymphoma (NHL), suggesting a possible pathogenetic link between HCV and certain histologic lymphoma subtypes, including MALT lymphomas and splenic MZL. However, it appears that there are marked geographical differences in the prevalence of HCV among NHL patients, and its role in the development of MZLs needs to be further investigated.9– 10
The group of lymphomas classified as low-grade MALT lymphomas include a number of extranodal B-cell lymphomas defined as extranodal marginal zone B-cell lymphomas of MALT type in the Revised European-American Classification of Lymphoid neoplasms (REAL classification)11 and in the new WHO Classification of Neoplastic Diseases of the Hematopoietic and Lymphoid Tissue.12 The histologic features of low-grade B-cell lymphomas of MALT type are similar regardless of site of origin.
Far from being rare, extranodal marginal zone B-cell lymphomas account for approximately 8% of all NHL, being the third most frequent histologic subtype (after diffuse large B-cell lymphoma and follicular lymphoma).13 The stomach is by far the most common and best-studied site; however, MALT lymphomas have also been described in various non-gastrointestinal sites, such as salivary gland, thyroid, skin, conjunctiva, orbit, larynx, lung, breast, kidney, liver, prostate, and even in the intracranial dura.6–,8,14– 30 Non-gastric MALT lymphomas have been difficult to characterize because these tumors, numerous when considered together, are distributed so widely throughout the body that it is difficult to assemble adequate series of any given site. Table 1 shows the main localizations of MALT lymphomas arising outside the stomach.
Gastric MALT lymphoma usually remains localized for long periods within the tissue of origin, where it can present with multifocal lesions.1–,2 Sometimes involvement of multiple mucosal sites is present; some cases with simultaneous gastric and intestinal involvement have been reported, and thyroid and salivary gland MALT lymphomas may also disseminate to the gastrointestinal tract.31–,32 Bone marrow involvement at presentation is uncommon.1– 2
Disseminated disease appear to be more common in non-gastrointestinal MALT lymphomas, where one-quarter of cases has been reported to present with involvement of multiple mucosal sites and/or an involvement of non-mucosal sites such as bone marrow.16–,17 It has been postulated that this dissemination may be due to the specific expression of special homing receptors or adhesion on the surface of the B cells of MALT. Indeed, the integrin α4β7 has been identified as a main mucosal homing receptor that regulates the traffic of circulating lymphocytes to the mucosal tissues by binding to the addressin MAdCAM-1, a vascular recognition molecule selectively expressed on the mucosal endothelium in Peyer's patches, mesenteric lymph nodes, and sinus lining cells of the splenic marginal sinus. The α4β7 integrin is expressed by most MALT lymphoma cells and normal B-cells of MALT.33– 34
The International Extranodal Lymphoma Study Group Survey
The International Extranodal Lymphoma Study Group (IELSG) conducted an analysis of a large series of patients who were diagnosed as non-gastric MALT lymphoma with the aim of better characterizing this disease entity. Preliminary data have been presented at the 2000 meeting of the European Society for Medical Oncology (ESMO).37
The study population initially included 365 patients from 20 centers. A histological review was performed by a panel of expert pathologists; 119 of 365 patients were excluded from the study, 108 because of non-confirmed histology (20 diffuse large cell lymphomas with a low grade MALT component, 9 reactive lymphoid proliferations, 9 mantle cell lymphomas, 8 follicular lymphomas, 3 plasmacytomas, 2 peripheral T cell lymphomas, 1 lymphoplasmacytoid lymphoma, and 56 where pathological material was not available or inadequate for histologic confirmation), 2 cases because of their primary gastric localization, and 9 cases because of incomplete data or inadequate follow-up Therefore, only 246 patients were included in the analysis.
Table 2 shows the main clinical features at presentation. The median age was 60 years (range 21-92 years). One hundred and sixty-five (67%) patients presented with Ann Arbor stage I disease; 17 (7%) patients had disease involving loco-regional nodes to the primary extranodal site of disease (stage II), whereas 64 (26%) patients presented stage IV disease. B symptoms were documented in 17 patients (7%), whereas 13 patients (5%) had an impaired performance status (ECOG PS score = 2). The serum lactate dehydrogenase (LDH) level was elevated in 23% of the 213 patients who had it measured at presentation. HCV positive serology was documented in 14% of 135 tested cases. According to the International Prognostic Index (IPI) (applicable to 243 patients), 82% of patients ranked in the low or low-intermediate risk groups.
The primary site was operationally defined as the clinically dominant extranodal component, which requires diagnostic investigation and to which primary treatment must often be directed. Most of the cases (approximately 25% each) had the lymphoma primarily in the salivary glands or in the ocular adnexa. The lung was the primary site in 14% of the cases, and 12% had skin lymphoma. Upper airways and Waldeyer's ring accounted for 8% of cases, thyroid and intestinal tract for 7% each. The disease presented with concomitant involvement of multiple MALT sites in 13% of cases; 16% of patients had nodal involvement with either locoregional or disseminated adenopathy.
Two hundred and forty (98%) patients have been treated; in the remaining a wait-and see policy was adopted. Primary treatment included chemotherapy in 80 patients, radiotherapy in 54 patients or a combined modality approach (chemo-radiotherapy) in 17 patients. Eighty-three patients had a surgical resection, followed by chemotherapy (n = 20), radiotherapy (n = 24) or both (n = 6). Five patients received interferon-α therapy and one, with a skin localization, had a tumor regression after antibiotic therapy against Borrelia burgdorferi.8 Of the 123 patients who received chemotherapy, 62 had a single alkylating agent or CVP (cyclophosphamide, vincristine, prednisone), and 63 were treated with an anthracycline-containing regimen. At a median follow-up of 3.6 years there was no evidence of a survival advantage for any type of therapy. However, this finding must be taken cautiously: patients were treated according to the current policy of each institution at the time of diagnosis, and the presence of organ-specific problems presumably had also a role in the choice of treatment.
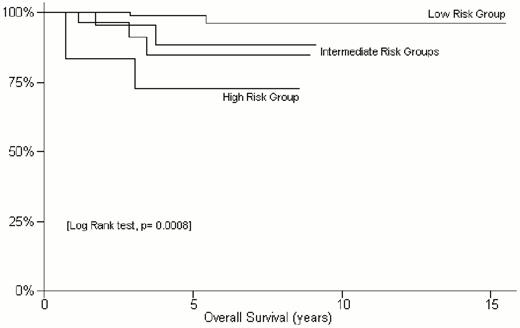
At 5 years the overall survival for the entire population (n = 246) was 93%, with a cause-specific survival of 96% and a progression-free survival of 73%. At univariate analysis, elevated LDH, advanced stage, poor IPI score were found to be significantly predictive of a poorer outcome in terms of either overall, cause-specific or progression-free survival. As shown in Figure 2 , the 5-year overall survival was 99% for the IPI low risk group, 85%-88% for the intermediate risk groups and 72% for the high risk patients (p = 0.0008). The patients without lymph node involvement at presentation had a 5-year survival of 97%, while it was 75% in those with nodal involvement (p = 0.0002). However, in agreement with the finding of Thieblemont et al,16 the involvement of multiple mucosal sites at diagnosis did not appear to change the outcome.
In conclusion, non-gastric MALT lymphomas, despite presenting with stage IV disease in approximately one quarter of cases, usually have indolent course (5-year survival of 93%). Patients at high risk according to the IPI and those with lymph node involvement at presentation, but not those with involvement of multiple MALT sites, have a worse prognosis. Localization can be an important factor because of organ-specific problems, which result in particular management strategies. However, whether different sites have a different natural history remains an open question. In the IELSG series the patients with the disease initially presenting in the upper airways appeared to have a slightly poorer outcome (5-year overall survival, 82%), but their small number prevented any definitive conclusion.
The optimal management of extranodal marginal zone B-cell lymphomas of MALT type has not yet been clearly defined. Surgery, chemotherapy, and radiotherapy alone or in combination have been employed. Based on preliminary data anti-CD20 antibodies may also have significant clinical activity.38 The treatment choice should be “patient-tailored,” taking into account the site, the stage and the clinical characteristics of the individual patient.
An improved understanding of the paracaspase MALT1 has brought clarity to the mechanisms underlying the molecular biology of MALT lymphomas.
When bcl10 expression is increased by translocation, it binds to its normal partner, MALT1 and together they increase the activity of IK kinase, resulting in the increased translocation of NF-κB into the nucleus. Alternatively, this same pathway can be activated by the novel fusion, API2-MALT1, which does not require bcl10. Therefore, both the t(1;14) resulting in deregulation of bcl10 and the t(11;18) resulting in a novel fusion API2-MALT1 appear capable of activating IK kinase, which leads to the phosphorylation and subsequent degradation of IκB and translocation of NF-κB into the nucleus. NF-κB induces the transcription of a number of different genes, including those involved in inflammation, cell viability and the immune response. Although both translocations were thought to promote lymphomagenesis by directly inhibiting apoptosis, this now seems less likely. However, this does not preclude that both translocations may affect the apoptotic signaling pathway indirectly by altering downstream apoptotic events resulting from increased nuclear NF-κB.0
An improved understanding of the paracaspase MALT1 has brought clarity to the mechanisms underlying the molecular biology of MALT lymphomas.
When bcl10 expression is increased by translocation, it binds to its normal partner, MALT1 and together they increase the activity of IK kinase, resulting in the increased translocation of NF-κB into the nucleus. Alternatively, this same pathway can be activated by the novel fusion, API2-MALT1, which does not require bcl10. Therefore, both the t(1;14) resulting in deregulation of bcl10 and the t(11;18) resulting in a novel fusion API2-MALT1 appear capable of activating IK kinase, which leads to the phosphorylation and subsequent degradation of IκB and translocation of NF-κB into the nucleus. NF-κB induces the transcription of a number of different genes, including those involved in inflammation, cell viability and the immune response. Although both translocations were thought to promote lymphomagenesis by directly inhibiting apoptosis, this now seems less likely. However, this does not preclude that both translocations may affect the apoptotic signaling pathway indirectly by altering downstream apoptotic events resulting from increased nuclear NF-κB.0
The International Extranodal Lymphoma Study Group (IELSG) study of non-gastric MALT lymphomas: Kaplan-Meier estimate of overall survival according to the International Prognostic Index risk groups (defined considering age, Ann Arbor stage, LDH levels, number of extranodal sites and performance status)
The International Extranodal Lymphoma Study Group (IELSG) study of non-gastric MALT lymphomas: Kaplan-Meier estimate of overall survival according to the International Prognostic Index risk groups (defined considering age, Ann Arbor stage, LDH levels, number of extranodal sites and performance status)