Abstract
This update provides new insights into the biology, diagnosis, prognosis, and treatment of multiple myeloma (MM) and its complications.
In Section I, Drs. John Shaughnessy, Jr., and Bart Barlogie first correlate global gene microarray expression profiling of patient MM samples with normal plasma cells to provide the basis for a developmental stage-based classification of MM. The powerful clinical utility of these analyses is illustrated in delineating mechanism of drug action, identifying novel therapeutic targets, and providing a molecular analysis not only of the tumor cell, but also of the tumor microenvironment, in MM.
In Section II, Dr. Jean-Luc Harousseau reviews the rationale and current results of high dose therapy and autologous stem cell transplantation in MM, including optimal patient selection, prognostic factors, conditioning regimens, sources of stem cells, use of tandem transplantation, and maintenance therapy. He then provides an update on the results of allotransplantation approaches in MM, focusing on proposed methods to reduce toxicity and exploit the graft-versus-MM alloimmune effect by transplantation earlier in the disease course, T cell depletion, and nonmyeloablative transplantation.
In Section III, Dr. G. David Roodman provides recent insights into the mechanisms of osteoclast activation, interactions between bone and MM cells, adhesive interactions in MM bone disease, and osteoblast suppression. These recent advances not only provide insights into pathogenesis of MM bone disease, but also form the framework for novel therapeutics.
In Section IV, Dr. Kenneth Anderson provides an up-to-date discussion of the role of the bone marrow microenvironment in promoting growth, survival, drug resistance, and migration of MM cells and the signaling cascades mediating these sequelae. These studies provide the framework for evaluation of novel therapeutics targeting the MM cell-host interaction in vivo in animal models and in derived clinical trials.
I. Global Gene Expression Profiling of Multiple Myeloma
John D. Shaughnessy, Jr, PhD,* University of Arkansas for Medical Sciences, Myeloma and Transplantation Reseach Center, 4301 West Markham St., Little Rock, AR 72205-7101 Dr. Shaughnessy has received research support from Celgene. Dr. Shaughnessy has been supported by R33 CA97513; Drs. Shaughnessy and Barlogie by P01 CA55819.
Inasmuch as all cancer mutations appear to directly or indirectly affect gene transcription, the ability to follow these changes represents a powerful way in which to classify and study the molecular biology of malignancies. Changes in gene expression can be quantitatively monitored based on complementary base pairing of nucleic acids. So-called high-density oligonucleotide microarrays (HDA) were developed by exploiting technologies adapted from the semiconductor industry using photolithography and solid-phase chemistry to produce arrays containing hundreds of thousands of oligonucleotide probes packed at extremely high densities.1 The probes are designed to maximize sensitivity, specificity, and reproducibility, allowing consistent discrimination between specific and background signals and between closely related target sequences.2 Microarray technology was first used to study cancer in 19963 and now has been used to identify disease subtypes in morphologically indistinguishable cancers4–,7 and to develop molecular predictors of response to therapeutic interventions.8,9 HDAs have now been developed such that the expression of nearly all of the estimated 35,000 human genes can be simultaneously monitored using two microarrays.
In an attempt to develop a comprehensive molecular profile of the gene expression patterns in MM, in March 2000 we began applying gene expression profiling (GEP) to newly diagnosed MM. Given the heterogeneous cellular milieu of the bone marrow, one of the first goals of our work was to develop techniques for harvesting and purifying PC. Automated anti-CD138 immunomagnetic sorting of PC from bone marrow aspirates has allowed enrichment to greater than 95% from samples containing less than 1% PC.4 We have now conducted microarray studies on more than 500 cases of MM, smoldering MM (SMM), monoclonal gammopathy of undetermined significance (MGUS), and other PC dyscrasias, as well as normal bone marrow PC, tonsil PC, and normal B-cell populations. An initial GEP study interrogating 6,800 genes in 74 newly diagnosed cases of MM, 7 MGUS, and 31 normal showed that (1) short-term longitudinal GEP reveal little intra-patient variability, suggesting that changes linked to progression, an unfortunate invariability in MM, may eventually be identified; (2) microarray derived gene expression levels and protein levels as determined by FACS analysis are tightly correlated; (3) spikes of CCND1, FGFR3, and other genes reflect the presence of 14q32 translocations involving these loci; (4) MM can be differentiated from normal PC by approximately 120 genes possibly representing fundamental genetic changes involved in, or a reflection of, neoplastic transformation; (5) MGUS, a benign PC dyscrasia, is currently indistinguishable from MM; and (6) 4 distinct molecular subgroups of MM, with clinically relevant correlations, were identified. Here, we will briefly describe some of the major observations derived from recent progress in our GEP studies of MM.
GEP Reflects Changes in Ploidy of Some but Not All Chromosomes in MM
Given the genomic chaos in MM it is important to identify which chromosomal abnormalities represent key transforming events and which represent stochastic events. Toward this end abnormal G-banding karyotypes on 212 newly diagnosed patients treated with tandem transplants from 1989 to 1998 were analyzed for gains and losses of each of the autosomes and the X chromosome (Jacobson et al, unpublished data). The most frequent gains were of chromosomes 3, 5, 7, 9, 11, 15, 19, and 21, whereas the most frequent losses were of chromosomes 13, 14, 16, and 22. To determine if gene expression patterns could be linked to changes in ploidy, we performed GEP on PC from 194 MM and 33 normal bone marrow donors, and chi-square and Wilcoxon rank sum (WRS) revealed that 1714 of ~6,800 genes were differentially expressed (P < 0.01). The chromosome map position of these genes and the number of abnormally expressed genes from individual chromosomes were determined. Although chromosome 19 harbored the largest number of altered genes, there was an almost even split of up- and down-regulated genes on this chromosome. This was in contrast to chromosome 13, in which over 90% of all altered genes were down-regulated in MM. The majority of altered genes on chromosomes 5, 15, and 21 were up-regulated, whereas genes on chromosomes 12, 13, 14, 16, and 22 were down-regulated. Importantly, adverse clinical outcome has now been linked to abnormalities in chromosomes 5, 13, 14, 15, and 21 (Jacobson et al, unpublished data 1/02). These data show that changes in ploidy affect virtually every chromosome in MM and global gene expression patterns reflect this ubiquitous phenomenon. However, some chromosomes are more frequently gained or lost in MM, and a concomitant up-regulation or down-regulation of genes mapping to a subset of these chromosomes can be observed and linked to outcome.
Co-expressed and Upregulated Cell Cycle Genes Are Linked to Abnormal Cytogenetics
Given the importance of the presence of abnormal cytogenetics as a negative prognostic variable in MM,10–,12 we sought to identify genes that distinguish this particular clinical subgroup. We performed GEP on PC from 149 newly diagnosed MM cases in which conventional G-band karyotypes were available. In total, 93 were found to have cytogenetic abnormalities (CA) and 56 to have no evidence of CA. We used chi-square, WRS, and significance analysis of microarray (SAM)13 statistical analyses to identify the most significantly differentially expressed genes in the comparison of the two groups (P < 0.01; range 0.01 to 0.000001). Pearson correlation analysis showed that all genes were up-regulated in the CA group. The most significant expression changes were of two nucleolar genes. However, genes associated with cell cycle and DNA metabolism composed, by far, the largest group. Interestingly, many of these cell cycle genes were also found to exhibit highly correlated expression as determined in a hierarchical cluster analysis (HCA). Unsupervised HCA performed on 149 MM PC using 9,220 genes revealed that the most highly correlated 23 genes contained many of the same cell cycle control genes identified above. HCA with 11 of the most highly correlated 23 genes stratified MM into two major groups. As expected a strong link between these groups and CA was observed, such that 73% of those with high expression (> 2-fold above median) of the 11 cell cycle genes had CA, whereas only 15% of those with low expression (≥ 2-fold below median) had CA (our unpublished data). Two minor groups, with intermediate levels of expression of these genes, also demonstrated CA incidences intermediate to the two extremes. Taken together, the data suggest that over-expression of a specific subset of genes associated with cell cycle and DNA metabolism is linked to presence of CA, that expression of these genes is highly correlated and possibly co-regulated.
GEP Can Be Used to Accurately Predict Deletion of Chromosome 13 and Identify Candidate Disease Genes
In a comprehensive interphase FISH analysis of chromosome 13 ploidy we have been able to demonstrate that monosomy is present in ~50-60%, 13q14 is a deletion hotspot, and FISH deletion is linked to inferior overall survival in MM.14,15 In an effort to determine if GEP can be a surrogate for FISH and also aid in the identification of a putative tumor suppressor gene, we have combined FISH deletion analysis with GEP. FISH analysis with 4 probes spanning the long arm of chromosome 13 was performed on 112 patients and 74 were determined to lack deletion (FISH13−) and 38 patients had monosomy of all 4 probes in >50% of the clonotypic PCs (FISH13+). A GEP comparison between these two groups revealed that 46 genes were differentially expressed (WRS P < 10−4). As expected from links between ploidy and GEP (see above), the top 14 differentially expressed genes mapped to chromosome 13. Although a total of 84 chromosome 13 genes were interrogated on the microarray, 34 were not expressed in PC, and 28 that were expressed showed no change across any comparison. A linear discriminate model using the expression levels of these 14 genes could predict, with 100% accuracy, the deletion status of MM (our unpublished data). Overall, these data indicate that deletion of chromosome 13 results in loss or reduced expression of specific genes on the chromosome and that profiles of these genes can be used to accurately predict chromosome 13 deletion in primary MM. We are currently determining whether loss of expression of any one or a combination of these genes is correlated with inferior outcome.
GEP Can Identify Known and Suspected MM-Associated 14q32 Translocations
Like many tumors of the B-cell lineage, MM shows recurrent rearrangements of the IGH locus at 14q32.16 We have recently shown that GEP can be used to identify these 14q32 translocations in MM.9 Briefly, genes demonstrating so-called “spiked” expression are those that are not expressed in normal bone marrow PC and most MM but that are expressed at high levels in a subset of MM cases. Spikes of FGFR3, CCND1, and CCND3 show a 100% correlation with the presence of the t(11;14)(q13;q32), t(4;14)(p16;q32), or t(6;14)(p21;q32), respectively.4,17 In 145 newly diagnosed cases of MM, CCND1 spikes were seen in 13% and FGFR3 spikes were seen in yet another 13%, MAF in 7.5%, and CCND3 in 4.1% of the cases. Although CCND1 and FGFR3 spikes never overlapped, 3 MAF spikes overlapped with a FGFR3 spike and 1 with a CCND1 spike, and 1 CCND3 spike overlapped with a CCND1 spike. Thus, 34% of primary MM exhibit spikes of 1 the 4 recurrent IGH translocation partners (our unpublished data).
As MMSET and FGFR3 are known to be concomitantly activated by the t(4;14)(p16;q32), it was surprising that a number of patients with elevated expression of MMSET lacked spikes for FGFR3. Of 180 newly diagnosed MM, 10 were found to have spikes for MMSET but not FGFR3 and 23 patients had spikes for both genes. All 33 cases had evidence of the MMSET-IGH fusion transcript, a marker of the t(4;14)(p16;q32) translocation. Thus, the t(4;14)(p16;q32) translocation appears to be present in 19% of newly diagnosed patients, with 32% of these cases lacking expression of FGFR3. Triple-color interphase FISH revealed a disassociation of MMSET from FGFR3 and VH from CH in greater than 65% of the clonotypic plasma cells in all 10 cases lacking FGFR3 spikes. Importantly, FGFR3 was deleted in greater than 90% of clonotypic PC in 7 of the 10 patient samples, suggesting that loss of FGFR3 occurred early in the disease. Thus, these data indicate that the activation of MMSET may be a critical transforming event of the t(4;14)(p16;q32). It is not yet clear whether FGFR3 is activated and crucial in the initiation of MM but not essential for, and thus lost during progression. Nevertheless, the consistent retention of the der(4) chromosome suggests that MMSET may be indispensable for both.
A recent study by Avet-Loiseau and colleagues showed that 14q32 rearrangements are found in 73% (n = 669) of MM and that the translocation partners in 44% of these rearrangements are not known.18 A comparison of GEP spikes in our population and the FISH data of Avet-Loiseau et al demonstrate strong correlations with CCND1 (13% vs 16%), FGFR3 (13% vs 10%), and MAF (4.1% vs 2%). In our GEP spike analysis 21 genes, including the 4 common translocation partner genes, were spiked in 109 of 145 (75%) MM. We suspect that many of the 17 unclassified spike genes represent candidates for unknown 14q32 translocation partner genes.
A Developmental Stage-Based Classification of MM
To determine if MM could be classified based on expression similarities to cells representing distinct stages of late stage B-cell development, we analyzed global mRNA expression in CD19-enriched B cells (BC) from 7 tonsils and CD138-enriched PC from 11 tonsils, 31 normal bone marrows, and 74 MM bone marrows using microarrays interrogating 6800 genes. HCA with 3288 genes clearly segregated the four cell types and chi-square and WRS (P < 0.0005) identified 359 and 500 previously defined and novel genes that distinguish tonsil BC from tonsil PC (early differentiation genes, EDG) and tonsil PC from bone marrow PC (late differentiation genes, LDG), respectively. MM as a whole was found to have dramatically variable expression of EDG and LDG and one-way analysis of variance (ANOVA) test was used to identify the most variable EDG (vEDG) and LDG (vLDG). HCA with these genes revealed that previously defined MM gene expression subgroups (MM1 through MM4) could be linked to one of the three normal cell types. Clustering with 30 vEDG revealed that 13 of 18 MM4 clustered with tonsil BC (P = 0.00005), while 14 of 15 MM3 cases clustered with tonsil PC when using 50 vLDG (P = 0.000008), and 14 of 20 MM2 cases clustered with bone marrow PC while clustering with 50 vLDG (P = 0.00009). MM1 showed no significant linkage with normal cell types studied. Thus, genes whose expression is linked to distinct transitions in late stage B-cell differentiation can be used to classify MM.
GEP in Risk and Response Stratification
The identification of multiple gene expression–based subgroups is consistent with the variable clinical course of MM, with survival ranging from as short as 2 months to greater than 80 months after diagnosis. An important hope for GEP in this disease is that the outcome variability, which cannot be accurately accounted for with current clinical parameters, might be better understood. We are currently attempting to correlate GEP with outcome in newly diagnosed patients entering into our NCI-sponsored clinical trial Total Therapy II, which is testing the efficacy of thalidomide combined with high-dose chemotherapy and stem cell support. To date, 210 of an estimated 300 newly diagnosed patients have now joined this GEP study. In addition, we are performing serial GEP to catalog changes that take place in comparisons of PC from each patient at diagnosis and relapse. This database will be used to identify common events across patients that may point to common mechanisms of resistance. Currently there is not sufficient follow-up time to establish correlations between GEP and outcome in this population; however, we have been able to demonstrate links between GEP and initial response to VAD (vincristine, adriamycin, dexamethasone). We have identified 2 subgroups based on expression of 11 cell cycle genes (see above). A total of 143 patients in the cluster had finished 1 cycle, and response calls had been made. The data showed that whereas 70% of patients with higher than median expression of at least 8 of the 11 cell cycle genes showed a partial or complete response, only 30% of the patients with lower than median expression of 8 of 11 of these genes responded. Although initial response to VAD is not necessarily an accurate predictor of overall response, this study does bode well for the use of GEP in patient risk stratification.
To evaluate the ability of gene expression profiling (GEP) to predict response to single agent drugs, we have performed GEP on 30 patients before beginning treatment with the proteasome inhibitor PS-341. After sufficient follow-up, responders (n = 15) and non-responders (n = 15) were identified and gene expression differences in baseline samples were examined. Of the 12,000 genes surveyed WRS showed that 44 genes distinguished response from no response with P values ranging from .0095 to .00046. A multivariate step-wise discriminant analysis (MSDA) revealed that 5 of the 44 genes could be used in a response predictor model. A leave-one-out cross-validation analysis performed on a training group revealed the model was 96.7% accurate. A held-out validation group was then tested in which 10 of 14 (71.4%) cases were correctly classified. Importantly, 5 of 6 responders were accurately predicted.
GEP Before and After Short-Term Drug Treatment to Identify Potential Mechanisms of Action
All current chemotherapy regimens for MM treatment attempt to use combinations of multiple drugs affecting different, non-overlapping, and non-crossresistant space pathways. It is impossible to define, in the context of these extensive combinations, the molecular mechanisms of action of a single agent. Short-term serial gene expression studies of tumor cells after single agent drug treatment may provide insight into the mechanisms of action, especially when combined with clinical response data. This knowledge, if confirmed, can lead to the development of second-generation drugs with more effective responses and less toxicity. In addition, knowledge of pathways will allow development of drugs that target discrete points along pathways allowing use of complementary or synergistic drugs.
In an effort to gain insight into the mechanism of action of various single-agent compounds, we have performed baseline and 48-hour follow-up GEP on 56 patients before and after therapy with dexamethasone (n = 16), thalidomide (n = 12), IMiD (n = 14), or PS-341 (n = 12). A total of 60 pair-wise comparisons were performed and induced changes greater than 2-fold in at least half the patients in each drug group defined. In the studies of dexamethasone, thalidomide, IMiD, and PS-341, we identified 57, 55, 120, and 92 genes up- or down-regulated, respectively. A total of 15 genes were commonly altered after treatment with the related compounds Thal and IMiD. Thus, in vivo monitoring of gene expression following single agent drug treatment appears to be feasible. This type of study has powerful advantages over in vitro models of the same design, in that response can be taken into account in the final analysis, a variable that cannot be faithfully recapitulated in model systems.
Identification of Novel Molecular Targets Using Gene Expression Profiling
The NCI is promoting an evolution in strategy to define useful anticancer drugs, seeking to move away from empirical developmental approaches.19 The new approach requires prior definition of an agent’s molecular target, or at least a signature or predictor of useful drug effect prior to the initiation of clinical trials. Many new compounds that target cell cycle regulation are currently being tested through the NCI’s Developmental Therapeutics Program (DTP).20 The DTP has screened 70,000 compounds, one at a time and independently, for their ability to inhibit growth of a panel of 60 human tumor cell lines (http://dtp.nci.nih.gov). Recent studies have correlated GEP of the NCI 60 with drug activity.21 In this study the investigators found that gene-drug relationships for the clinical agents exemplify how variations in the transcript levels of particular genes relate to mechanisms of drug sensitivity and response. A computer program developed by the DTP, called COMPARE, has been used to identify compounds with similar growth inhibition patterns in the 60 cell line screen and has led to the successful identification of structurally unrelated compounds with similar mechanisms of action.22 We hypothesize that GEP of MM can be used to identify the presence of transcripts for known molecular targets and to identify commonalities of gene expression patterns in the NCI 60 that have been correlated with response to a particular compound and MM. GEP of a large patient population allows mining and identification of aberrantly expressed genes. Cross-referencing the candidate gene’s function with compounds that have known molecular mechanisms or molecular targets allows identification of new potential drugs. For example, the farnesyltransferase (FNTA) and RAS are overexpressed in the malignant PC of subsets of MM.4 FNTA prenylates RAS, a post-translational modification required to allow RAS to attach to the plasma membrane and function properly. Thus, it is possible that FNTA inhibitors (FTI) may have indications in MM with high expression of RAS and/or FNTA. Indeed, preclinical tests have suggested that FTI are effective in killing MM cells.23
Given the negative impact of CA and by inference the overexpression of a specific subset of genes involved in cell cycle control (see above), we hypothesize that using drugs that target specific components of the cell cycle apparatus may be a valid treatment approach for this GEP subgroup. For example, the topoisomerase 2 gene (TOP2) is overexpressed in this group, and thus it is possible that VP-16 or etoposide may show a higher efficacy in patients with TOP2 over-expression than is appreciated in MM as a whole. Elevated expression of a cyclin-ubiquitin-conjugating enzyme and 2 mitotic cyclins was also consistently observed in this subgroup. The regulated destruction of mitotic cyclins near the end of mitosis is essential for the inactivation of their partner kinase CDC2 and exit from mitosis into G1 of the next cell cycle. This inactivation requires proteolysis mediated by enzymes of the ubiquitin pathway, such as E1, E2, and E3. We suspect that interference with the ubiquitin ligation of the mitotic cyclins or prevention of their destruction by the proteasome might be able prevent these cells from entering mitosis. Recent studies have shown that a novel antitumor agent, XK469, induces mitotic arrest and is correlated with cyclin B1 ubiquination.24
Toward the Molecular Characterization of the Bone Marrow Microenvironment in MGUS and MM
It is now appreciated that MM growth, and probably growth of all cancers, partially relies on non-tumor accessory cells providing growth and survival factors as well as a sanctuary from the effects of chemotherapy. Through previous work we have identified the molecular signature of highly purified MM cells and their normal counterparts, yet, the signature of the stromal component of the MM bone marrow microenvironment and whether this signature is qualitatively altered in disease is not known. These studies are of enormous clinical relevance, as it is becoming increasingly accepted that an important adjunct to current therapies, which target the tumor cell directly, depend on the manipulation of the microenvironment. This work is probably most clearly demonstrated by the effects of thalidomide and second-generation analogs that are thought to act through multiple mechanisms, ranging from direct tumoricidal activity to anti-angiogenesis and modulation of tumor necrosis factor-alpha (TNFα) signaling through direct and/or indirect effects on the tumor microenvironment.25 The bisphosphonates are another compound that, although originally used because of an anti-osteoclast activity in reducing bone loss in MM, has also recently been shown to have a direct anti-myeloma effect.26 Changes in the microenvironment may also be linked to disease progression. GEP signatures suggest that MGUS cannot be distinguished from MM,4 yet this PC dyscrasia rarely converts to overt MM.27 Thus, it is possible that a microenvironment conducive to MM PC growth is present in de novo MM and becomes established in MGUS cases that convert.
A molecular profiling system based on biopsy material, rather than purified cells, is also envisioned to be simpler, in that it obviates the need for laborious, expensive, and technically complicated purification procedures requiring patient visits to a large medical center with facilities and expertise. In this new system, local doctors would be able to take a core biopsy, snap-freeze the samples, and ship them to a microarray core facility that would profile the case and predict class, possible treatment, and add the profile to a large archive database. Thus, this kind of a system might allow molecular profiling to become more accessible and importantly more easily utilized in inter-group trials.
Toward this end we have begun GEP of the microenvironment component of MM and MGUS bone marrow with respect to normal counterparts. We now have purified PC and biopsy pairs from over 100 MGUS, MM, and normal donors. A pilot study using pair-wise comparisons of 2 normal biopsies and 2 MM biopsies has revealed 146 genes with decreased and 86 genes with increased expression in the MM biopsies. To identify which of the 232 genes were PC specific, a pair-wise comparison of each MM biopsy with purified PCs derived from an aspirate from the same patient on the same day was performed. Subtracting overlapping significant genes revealed 75 genes that were significantly different, and these genes have been defined as microenvironment-associated genes (MAG). A total of 54 MAG showed decreased expression (range 2- to 4-fold), and 21 MAG showed increased expression (range 2- to 8-fold) in the MM biopsies.
Within the MAG up-regulated genes, the most significantly altered gene was a voltage gated K+ channel-related transcript. Two of the top 5 MAG, UMAG1 and UMAG2, code for adhesion proteins implicated in MM PC stromal cell interactions. Interestingly, the expression of UMAG1 and UMAG2 was not elevated in the MGUS biopsies. These 2 genes were expressed at >10-fold higher levels in the MM biopsy with a high percentage of PC (80% PC) as reported by clinical pathology compared to the MM sample with a relatively lower percentage of PC (20% PC) and the normal biopsies. These genes were not expressed in the purified PC from the 2 MM samples. The apparent up-regulation of 2 key adhesion molecules in cells of the microenvironment is consistent with studies suggesting that these types of molecules have a major influence on MM cell growth and drug resistance.26,28–,31UMAG3, 1 of the 5 most up-regulated MAG, is a member of the matrix metalloproteinase (MMP) family. In the MGUS biopsies, this gene showed variable expression: in the normal range in one, moderately elevated in another, and highly elevated in a third. The gene was essentially undetectable in purified PC. The over-expression of UMAG3 also has potential relevance, given the role of MMPs in various aspects of MM pathology, including angiogenesis and bone resorption.32– 34 Thus, an intensive effort is currently under way to establish a large GEP database of MM, MGUS, and normal age-matched control core biopsy samples.
Developing Molecular Diagnostic Models for MM
Global GEP of malignant and normal cells can reveal tumor-specific gene expression patterns. These unique signatures can be used to develop highly sensitive quantitative RT-PCR-based molecular diagnostic assays for patient stratification and minimal residual disease detection. In a first attempt to develop such a test, we applied a chi-square, WRS, and SAM analysis to compare the GEP of 12,000 genes in 150 cases of untreated MM and 33 normal bone morrow donors. An MSDA identified 14 genes (Table 1 ) capable of differentiating malignant from normal PCs with a high degree of accuracy in a training group of 150 MM and 26 normal PCs. When applied to a held-out validation group consisting of 78 MM cases and 3 normal bone marrow PC, the 14-gene MSDA model demonstrated an accuracy of classification of 98.8% (our unpublished data). Importantly, the model was incapable of differentiating most MGUS from MM, as 15 of 19 cases of MGUS were classified as MM (our unpublished data). An unsupervised cluster analysis with 33 normal bone marrow PCs, 19 MGUS, and 40 newly diagnosed MM using 6971 genes showed that 5 MGUS samples, including the 4 that were predicted to be normal in the MSDA model, were clustered with the normal bone marrow PC. However, 4 of the 5 MGUS were located on a separate sub-branch from normal PC and at the farthest distance from the center. This result can be interpreted in 2 ways: (1) the MGUS cells have the expected MM signature, but the ratio of MGUS cells and normal PC in these patients was skewed toward the normal so that the MGUS signature was masked; or (2) the majority of PC in the mix were MGUS cells, yet these cells had not acquired enough MM-specific GEP signatures to place these samples in the MM cluster branch. These data have potential important clinical implications in that a malignant signature can be observed in MGUS, even when PC are present at less than 10% and likely mixed with normal PC. Future studies on a larger population of MGUS cases using a whole genome microarray may allow identification of gene expression patterns unique to MGUS, with the ultimate goal of identifying those MGUS cases at highest risk of conversion to overt MM.
Potential Problems and Pitfalls
Although it is clear that GEP is closer to becoming a relevant diagnostic and prognostic tool in the clinical management of hematological malignancies and also providing new insights into the biology of cancer, it is also important to point out some of the limitations of the technology. One negative feature is that there is no universal standard microarray in use. Because “no microarray is alike” in either manufacturing standards or gene content, direct comparison of data derived from different laboratories can be difficult. While it appears that more and more centers are shifting toward the use of the commercially available Affymetrix platform, in which lot to lot variability is minimal and other standards of manufacturing are imposed, many academic centers use cDNA microarrays that are produced in-house using internal standards. Even if a consensus on the use of a universal platform were adopted, cost represents another prohibitive feature of microarray profiling. However, the expenses for HDA have been dropping over the last 3-4 years, making these kinds of studies more feasible.
A further problem with GEP involves the type of sample studied. A non-cell of interest component is present in aspirates, biopsies, or resected tissue samples from both tumor and normal samples. As we pointed out above, there is a distinct possibility that the tumor microenvironment may hold clues to tumor behavior in MM and thus its gene expression patterns may be informative; however, the ability to delineate from which cells individual signatures are derived can be virtually impossible in complex mixtures. Thus, if an experiment is designed to determine the tumor cell signature, the presence of non-tumor cells has the potential of introducing non-specific noise and must be accounted for. MM represents a good example of such a problem. The nature of MM results in dramatic intra- and inter-patient variability in the percentage of PC in a given sample. To overcome this limitation we have devised a rapid (< 2 hr from patient to array) strategy of CD138-selection of PC. The ability to purify tumor cells is unique to liquid tumors for which well-characterized antibodies to cell surface markers exist. This is in contrast to solid tumors, in which cell purification relies on laborious and technically difficult laser capture microdissection (LCM) that yields limited numbers of cells, typically requiring RNA amplification, another potential pitfall that can introduce variability and biases in RNA representation. CD138-enrichment represents a major cost as a specialized bone marrow technician is required to obtain enough aspirate to enrich the 1.5 million PC required per assay. These large volume aspirates require a pre-purification preparative stage that is both laborious and time consuming and requiring skilled technicians. The anti-CD138 microbeads used in purification also represent a major cost component. Although solving the cell contamination issue, purification presents new questions such as, does purification directly, e.g., engagement of CD138, or indirectly, e.g., removal of critical cell-cell interactions, change gene expression? And does purification eliminate tumor subpopulations, e.g., CD-138 negative PC?
Even after purification, a potential limitation of GEP is that intra-tumor heterogeneity cannot be realized. By studying populations of cells it is impossible to determine whether a detectable gene is active in all cells or in a subpopulation. This has important ramifications if the gene is associated with an aggressive sub-clone. However, recent elegant studies demonstrating the stochastic nature of gene expression44 suggest that studying mixtures of large populations, as is done in most microarray experiments, may be a more appropriate means of recognizing significant changes in patterns between two distinct sample types, e.g. normal vs. malignant. Nevertheless intratumor variability may present distinct problems in studying diseases such as Waldenström’s macroglobulinemia, which appears to be a mixture of cells existing at varying degrees of differentiation and whose expression patterns vary (our unpublished data).
Another potential problem with GEP is that unsupervised hierarchical classification schemes tend to show a low but significant degree of plasticity. That is to say that samples placed in one particular subgroup can shift to another group when additional patient samples or more genes are added to the analysis. We suspect that this problem will become less of a concern when GEP incorporates all human genes and patient outcome is taken into consideration. In this way models will be developed using only significant genes derived from training groups and validated on blinded samples. Classification schemes using these limited number of genes should also help to lower costs as MM specific micro-microarrays can be developed which will have the added benefit of requiring less numbers of cells per assay.
An added drawback is that models predicting outcome based on GEP will likely be specific for distinct therapies, such that a model predicting outcome to high dose therapy and tandem transplants will be different from one that also incorporates thalidomide or one built on standard therapy or single agents, e.g., PS-341. An additional pitfall in development of prognostic models based on the use of first and second generation microarrays is that not all human genes are represented on most arrays, possibly resulting in the absence of genes whose inclusion could increase sensitivity and specificity of models. Even though Affymetrix has now developed a two array platform that covers all 35,000 human genes, it is cost prohibitive for laboratories, like ours, who have initiated studies of clinical trails and have >100 patient samples costing hundreds of thousands of dollars on one platform, to shift microarray formats midstream. It is important to note that, although funds may not be currently available to shift formats, the samples can be archived indefinitely and used in future studies when funds are secure.
This lack of a complete genome survey also likely limits an understanding of the mechanisms of disease, as only one third of all human genes are currently being studied in our group. It is certainly possible that critical genes not studied today could hold important clues to disease biology. While GEP is revealing many new insights, changes in gene expression are liable to represent only one of many causes or effects of neoplastic development. For example, it is thought that the complexity of the human genome is magnified, possibly several fold, by alternative splicing, a phenomenon not currently recognizable by GEP. Another level of complexity in eukaryotic cell systems involves post-translational modification of proteins, e.g., glycosylation and phosphorylation, which is not appreciated directly with GEP. Furthermore, gene expression patterns do not always correlate with protein levels, and since the protein is the functional product of the mRNA blueprint, gene expression leads should be followed with complimentary and confirmatory studies. It is important to note, however, that although protein correlations are important for target validation and functional inferences, GEP patterns are sufficient for prognostic model development as long as the patterns are reproducible. A further variable not accounted for in GEP is allele variation that results in variations in functionality, e.g., drug metabolism, of gene products. Thus, even though a gene in two samples may be expressed at similar levels the two patients may have inherited different alleles that have different functional capacities.
Thus, although gene expression is providing useful new information in both clinical and basic research, it does not provide a complete picture and is best utilized in the context of broad studies using other standard laboratory techniques.
II. Role of Transplantation in Myeloma
Jean-Luc Harousseau, MD*
Hotel Dieu, 1, Place Alexis Ricordeau, BP 1005, Nantes Cedex 01 44093, France
Autologous Stem Cell Transplantation
Autologous stem cell transplantation (ASCT) has been widely used in MM in the past 15 years. While several issues have been clarified by recently completed studies, other issues are still being debated.
Comparison of conventional chemotherapy and ASCT
The Intergroupe Français du Myélome (IFM) was the first to conduct a randomized multicenter trial to address this issue in patients up to the age of 65.1 In this IFM 90 trial, autologous bone marrow transplantation (ABMT) significantly improved the response rate; 38% of patients enrolled in the ABMT arm achieved a complete remission (CR) or a very good partial remission (VGPR) versus 14% of patients enrolled in the conventional chemotherapy (CC) arm (P < 0.001). An updated analysis of this study confirms that, with a median follow-up of 7 years, ABMT significantly improves event-free survival (EFS) (median 28 months versus 18 months, 7-year EFS 16% versus 8%, P = 0.01) and overall survival (OS) (median 57 months versus 44 months, 7-year OS 43% versus 25%, P = 0.03).
Preliminary results of the British Medical Research Council (MRC) 7 trial also show a significant survival benefit in the ABMT arm (T. Child, personal communication). In the trial performed by the French group Myelome Autogreffe (MAG) 190 patients aged 55 to 65 years were randomized to receive CC or high-dose therapy (HDT) 2 Although the results of HDT appeared comparable to those achieved in the IFM 90 trial, there was no significant difference in OS between the 2 arms because of an unexpected survival in the CC arm (median 55 months with HDT versus 50 months with CC). It should be noted that 17 patients in the CC arm received ASCT at the time of relapse.
The design of the Spanish trial was different because only patients responding to initial CC were randomized.3 In this trial, the CR rate was significantly higher in the HDT arm. Although the median EFS and OS were longer in the HDT arm (42 months versus 33 months, 72 months versus 64 months), the differences were not significant.
Finally, the Italian study compared the classic combination melphalan-prednisone and 2 courses of intermediate-dose melphalan (100 mg/m2) followed by ASCT in patients up to the age of 70.4 The CR rate and median EFS were significantly better with HDT. Although currently OS is not different between the 2 arms, more patients received ASCT as salvage therapy in the CC arm.
Compared to CC, therefore, ASCT clearly improves the CR rate. The median EFS is prolonged by 5 to 13 months (Table 2 ). However, the benefit in terms of OS is less striking partly because ASCT has been used as salvage treatment in a significant number of patients at the time of failure in the CC arm. The MAG group has conducted another randomized trial comparing early and late ASCT.5 In this study, early ASCT significantly improved progression-free survival (PFS), but OS was not significantly different in the 2 arms.
To conclude, considering the low toxic death rate (< 3%), ASCT has proven benefit, at least in fit younger patients.
Selection of patients
Usually, ASCT is limited to patients up to 65 years of age with a performance status 0-2 and with a normal renal function. The issue of age limits is important because the median age of MM patients is currently over 65 years and age is a well-known prognostic factor. The introduction of hematopoietic growth factors has profoundly modified the practice of ASCT. With peripheral blood progenitor cells (PBPC) collected after priming with granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF), ASCT has become safer and can be offered to older patients. The Little Rock group has compared the outcome of 49 patients 65 years and older with 49 younger pair mates selected in a cohort of 550 patients treated with HDT.6 The CR rate was higher in younger patients (43% versus 20%, P = 0.02) and the transplant-related mortality appeared to be higher in older patients (8% versus 2%). However, because the EFS and OS were comparable, the authors concluded that age is not a biologically adverse parameter for patients treated with HDT and PBPC support and should not be an exclusion criteria for participation in what appears to be superior therapy in MM.
The same group recently published the results of ASCT in 70 patients over the age of 70 years. Although ASCT appeared feasible in this age subgroup, the use of higher dose of melphalan (200 mg/m2) was too toxic (16% toxic deaths).7
Palumbo et al showed that 2-3 courses of melphalan 100 mg/m2 with PBPC support are feasible on an outpatient basis in patients up to 75 years of age. They compared 71 patients treated with this approach to 71 pair mates treated with CC and concluded that intensive therapy was superior in terms of CR rate, EFS, and OS.8
However, the issue of selection bias should be raised in all studies on HDT in elderly patients. The benefit of ASCT and the optimal conditioning regimen in this patient population need further evaluation by randomized trials.
Usually ASCT is not proposed to patients with severe renal failure because of concerns regarding excessive toxicity of HDT. A recent retrospective study of the Spanish Registry showed a high transplant-related mortality (29%) in patients with renal failure at the time of transplant.9 However, in their study of 81 MM patients with renal failure at the time of ASCT,10 Badros et al reported that renal failure had no impact on the quality of stem cell collection and did not affect engraftment. ASCT was feasible even in patients on dialysis. However, extrahematologic toxicities were more severe and more frequent after melphalan 200 mg/m2 than after melphalan 140 mg/m2. Although compromised renal function at presentation should not exclude patients from HDT programs,9– 11 the impact of renal failure at the time of ASCT requires further study.
There are no clear data demonstrating a survival benefit for ASCT among patients aged > 65 or with renal failure. The use of ASCT in these settings should involve a discussion of this information with the patient.
Conditioning regimen
The combination of high-dose melphalan (HDM) 140 mg/m2 plus total body irradiation (TBI) has long been considered the standard conditioning regimen prior to ASCT and has been used in the IFM 90 trial. However, in newly diagnosed patients, the Royal Marsden Group has reported an impressive 70% CR rate with HDM 200 mg/m2, with a low extrahematologic toxicity.12 In 1995 the IFM initiated a randomized study comparing HDM 200 mg/m2 with HDM 140 mg/m2 plus TBI in 282 patients with newly diagnosed MM.13 In this study, HDM 200 mg/m2 was significantly less toxic (shorter duration of neutropenia and thrombocytopenia, lower incidence of grade ≥ 3 mucositis, no toxic death versus 5 in the TBI group). Although the response rate and the EFS were identical, OS was superior in the HDM 200 mg/m2 group apparently because of a better salvage after relapse (Table 3 ). Therefore, HDM 200 mg/m2 should be preferred to HDM 140 mg/m2 plus TBI as the conditioning regimen for ASCT in MM.
Other innovative conditioning regimens should be compared to this effective and well-tolerated procedure.
Source of stem cells
As in other malignancies, PBPC have replaced bone marrow as the source of stem cells in ASCT for MM because of easier accessibility and faster hematopoietic recovery. However, although tumor cell contamination is lower in PBPC harvests, the superiority of PBPC as regards the clinical outcome has never been demonstrated.
Selection of CD34+ PBPC has been compared to unselected PBPC in three multicenter randomized trials.14–,16 These trials failed to show any benefit of CD34+ selected PBPC. Moreover, in two studies the incidence of opportunistic infections appeared to be higher in the CD34+ than in the unselected PBSC arm.15,16
While sophisticated procedures to obtain tumor-free grafts have failed,17 encouraging results have been obtained with negative selection using monoclonal magnetic microbeads against CD19, CD56, and CD138.18 The benefits of this approach warrant further evaluation. Currently, unselected PBPC are the standard source of stem cells for ASCT in MM.
Variables affecting PBPC mobilization and speed of engraftment have been analyzed.19 There is a significant correlation between the number of CD34+ cells infused and hematopoietic reconstitution, and 2 × 106/kg CD34+ cells is the minimal dose to ensure safe engraftment. Prior exposure to chemotherapy, especially to alkylating agents, significantly affects stem cell collection and hematopoietic recovery. Thus, PBPC should be collected before using alkylating agents, and VAD-derived regimens should be preferred prior to ASCT. Other dexamethasone-based regimens can be used as well and a nationwide study is ongoing in the US comparing dexamethasone with thalidomide plus dexamethasone as induction treatment. The optimal regimen for mobilizing PBPC is unclear. While some investigators use cyclophosphamide plus G-CSF to increase the number of PBPC and possibly to reduce graft contamination and tumor cell mass, others use G-CSF alone to reduce morbidity and cost.20 The combination of SCF and G-CSF is an attractive alternative.21
Tandem transplantation
Almost 10 years ago, the Arkansas group showed the feasibility of 2 consecutive ASCTs thanks to the use of growth factors and of PBPC instead of bone marrow.22 In their first publication on a large number of patients, they reported that up to 73% of patients received 2 ASCTs and that the CR rate increased from 24% after the first transplant to 43% after 2 transplants.23 This experience has now been extended to more than 1000 patients.24
However, the actual impact of such an aggressive strategy on EFS and OS needed further evaluation. In 1994, the IFM initiated a randomized trial (IFM 94) comparing 1 versus 2 transplants. From October 1994 to March 1997, 403 untreated patients under the age of 60 years were enrolled by 45 centers. At diagnosis they were randomized to receive either a single ASCT prepared with melphalan (140 mg/m²) and TBI (8 Gy) or a double ASCT: the first prepared with melphalan (140 mg/m²), and the second prepared with melphalan (140 mg/m2) and TBI (8 Gy). Overall, 399 patients were evaluable. Of 199 patients assigned to the single ASCT arm, 177 (89%) actually received the planned transplant, and there were 3 toxic deaths. Of 200 patients randomized to the double transplant arm, 156 (78%) actually received 2 transplants, and there were 5 toxic deaths.
The results are summarized in Table 4 . There is no significant difference in the CR rate between single and double transplantation. However, with a median follow-up of 5 years, the median EFS and the OS are superior in the double ASCT arm (Figures 1 and 2 ).
Three other studies have also addressed the issue of further intensification (Table 5 ). Currently, none of these studies show a significant advantage for the more intensive treatment arm.16,25,26 However, it should be noted that in the IFM 94 trial, the EFS and OS curves separate after only 3 years. Since the median follow-up time of these 3 studies is short, a longer observation period is needed before drawing any definite conclusions. The Royal Marsden Group recently evaluated the outcome of 96 patients treated with a second ASCT for relapse after the first. They stated that OS of those patients was comparable to that achieved with tandem ASCT.27 However, in this study there was an obvious selection bias because the median interval between the 2 ASCT was 3.2 years. Prospective randomized studies comparing 2 ASCT up front and 1 early ASCT followed by a second at the time of progression are needed to address this issue.
Maintenance therapy
In a retrospective analysis of the European Blood and Marrow Transplantation (EBMT) registry, post-ASCT maintenance with alpha-interferon was associated with improved PFS and OS in patients responding to HDT.28 Only one randomized study of maintenance therapy post-ASCT has been completed to date.29 This trial compared alpha-interferon versus no further therapy following recovery from HDT. With a median follow-up of 77 months, the median PFS was significantly longer (42 months versus 27 months for the control arm), but the PFS and OS curves were not significantly different. Therefore, although interferon delays relapse, most if not all patients ultimately relapse. However, because this study involved only 85 patients, these results should be interpreted cautiously. In the IFM 90 trial, alpha-interferon was to be administered to all patients after HDT, and there is no plateau of the EFS curve.1 Therefore, curing patients with MM with a single course of HDT followed by alpha-interferon is unlikely. New strategies to control minimal residual disease after ASCT are necessary. They include the use of maintenance chemotherapy, thalidomide, bisphosphonates, and immune therapy (vaccination with idiotype, DNA, or tumor-associated antigens, immunotherapy with idiotype pulsed dendritic cells). These strategies are currently under evaluation.
Prognostic factors
In 231 patients with newly diagnosed MM treated with tandem transplants, multivariate analysis showed superior EFS and OS in the absence of unfavorable karyotypes (11q breakpoints and/or partial or complete deletion of chromosome 13) and with low β2 microglobulin level at diagnosis (≤ 4 mg/L).30 When combining these factors, a subgroup of patients with a very poor prognosis was identified: patients with unfavorable cytogenetics and β2 microglobulin level > 4 mg/L had a median survival of only 2.1 years, compared to 7 years for the remaining patients. New therapeutics are clearly needed for these patients.
Using a larger cohort of 1000 consecutive patients, including previously treated patients, the same authors confirmed that independent favorable features were mainly absence of chromosome 13 deletion (using conventional cytogenetics), low β2 microglobulin level, plus low C-reactive protein (CRP) level and less than 12 months of prior CC.24 Plateaus of the EFS and OS curves were noted in 45% and 60% of patients with all these favorable characteristics.
In a recent retrospective analysis of 110 patients treated with HDT, the IFM showed that the detection of chromosome 13 abnormalities (13, 13q) by fluorescence in situ hybridization (FISH) was the most powerful adverse prognostic factor.31 The combination of FISH analysis, β2 microglobulin, and IgA isotype produced a very powerful staging system in the context of HDT. Again, patients with a high β2 microglobulin level and chromosome 13 abnormalities had a very poor prognosis (Figure 3 ). The respective value of conventional cytogenetics and FISH in detecting chromosome 13 abnormalities has been debated. While FISH detects more abnormalities than karyotype (45-50% versus approximately 15%), conventional cytogenetics defines a subgroup of patients with an even poorer prognosis. As karyotype is informative in only one third of the patients, detection of any cytogenetic abnormality probably corresponds to a more aggressive behavior of the tumor and could be partly correlated to high-proliferative activity also evaluated by plasma cell labeling index.32 While cytogenetics provides additional information and confirms that hypodiploidy is a major prognosis factor,33 FISH analysis with selected probes may also help in defining prognostic subgroups: for example, patients with t(4;14) (including those with chromosome 13 abnormalities) have a very poor prognosis, and conversely, patients with t(11;14) have a prolonged survival.34 We conclude that detection of cytogenetic abnormalities, either by karyotype or by FISH, is useful to predict the outcome and define therapeutic strategies.
Allogeneic Bone Marrow Transplantation
The use of allogeneic BMT has two important drawbacks in MM. Because it is usually performed in patients under 50 years of age who have an HLA-identical sibling, it is feasible for only a small minority of MM patients. As the transplant-related mortality is very high, mainly as a consequence of infection and graft-versus-host disease (GVHD), a retrospective analysis of EBMT data showed no advantage of allogeneic BMT compared to ASCT.38 For the same reason, the allogeneic BMT arm in the US intergroup trial was closed prematurely. However, there are arguments in favor of pursuing clinical trials on allogeneic BMT in MM.
If given early in the course of the disease, allogeneic BMT yields molecular remissions39 and about one third of the patients remain free of disease 6 years later.40 As a result of the antitumor effect of the graft, allogeneic BMT is possibly the only genuinely curative therapy in MM. Reports of CR achieved after infusion of donor mononuclear cells in patients relapsing after allogeneic BMT are further evidence of the so-called graft-versus-myeloma effect.41,42 Therefore the objective of current studies on allogeneic BMT in MM is to reduce transplant-related mortality (TRM) while harnessing the graft-versus-myeloma effect.
The first way to attain this objective could be to propose allogeneic BMT early in the course of the disease. A recent survey of the EBMT registry shows a significant improvement in survival for transplants performed between 1994 and 1998, compared to previous experience.43 This improvement is the result of a significant reduction in TRM, mostly related to deaths due to bacterial and fungal infections. This encouraging result corresponds to a better selection of patients, with earlier transplantation in less heavily pretreated patients. In this study, the use of PBPC instead of bone marrow was associated with earlier engraftment but yielded no significant survival benefit.
Another way to decrease TRM is to reduce the incidence and the severity of GVHD by CD34+ selection or other modalities of T-cell depletion. T-cell depletion of donor bone marrow has been shown to reduce the incidence of GVHD but is associated with a higher relapse rate because of abrogation of the immunologic effect of the graft. Attempts to maintain graft-versus-myeloma effect with prophylactic CD4+ donor lymphocyte infusions after CD6 T-cell depleted allogeneic BMT have yielded promising short-term results.44
Finally, the use of nonmyeloablative conditioning regimens (so-called mini-allotransplants) is an attractive alternative that can be proposed to older patients. The use of a nonmyeloablative conditioning regimen with melphalan 100 mg/m2 combined with donor lymphocyte infusions has induced good disease control at 1 year but with a significant rate of GVHD in 31 patients with high-risk MM.45 Because early TRM is reduced with a nonmyeloablative conditioning regimen, this approach is currently tested in frontline treatment. However, preliminary studies in various hematologic malignancies have clearly shown that results are related to disease status and that relapse rate is high in patients with advanced disease. Therefore, reducing tumor burden with ASCT prior to mini-allotransplantation has been proposed. The Seattle group has reported preliminary results of HDM 200 mg/m2 with ASCT followed by allogeneic transplantation prepared by low-dose TBI.46 Although GVHD remains a problem, the feasibility of this approach has been demonstrated. Preliminary results are encouraging, but the follow-up is still short (1 year at the time of presentation) and this approach should be undertaken only in the context of approved clinical trials. Ongoing multicenter studies are using “genetic randomization” to compare mini-allotransplantation and a second ASCT in patients who have received a first ASCT.
Three important questions should be addressed in the near future:
Should allogeneic BMT programs be proposed up front to all eligible patients with an HLA-identical sibling or only to patients with poor prognostic factors?
Will nonmyeloablative approaches appear superior to standard allogeneic BMT with longer follow-up time?
Should donor lymphocytes be infused in patients with incomplete chimerism and/or residual disease or only in cases of posttransplant relapse?
Conclusion
There are a number of still unsolved issues in the field of transplantation in MM. Moreover the introduction of new agents (thalidomide, immunomodulatory drugs, proteasome inhibitors) will undoubtedly modify the overall strategy in the near future. Therefore, the importance of refering patients with newly diagnosed MM to specialized centers and of participating in prospective clinical trials should be emphasized.
III. Biology of Myeloma Bone Disease
G. David Roodman, MD, PhD*
University of Pittsburgh Cancer Institute, UPMC Health Systems, Division of Hem./Onc., 200 Lothrop St., Rm. E1152, Biomed. Science Tower, Pittsburgh, PA 15261
MM is a severely debilitating, incurable, and uniformly fatal neoplastic disease of B-cell origin. Although much effort has been directed at devising effective treatments for MM patients, their prognosis and survival have been relatively unchanged over the past 30 years, except for a subgroup of patients undergoing successful autologous or allogeneic stem cell transplantation. The major source of morbidity and possible mortality associated with MM is osteolytic lesions throughout the axial skeleton. Lytic bone lesions occur in 70-80% of these patients1 and are frequently associated with severe bone pain and pathologic fractures. Approximately one third of the patients develop hypercalcemia. The bone lesions result from increased osteoclastic bone resorption that occurs adjacent to the myeloma cells and not in areas of normal bone marrow. New bone formation that normally occurs at sites of bone destruction is also absent. These data suggest that locally acting factors produced by myeloma cells induce extensive bone destruction and block new bone formation. To date, the factors produced by myeloma cells in vivo that are responsible for the increased osteoclastic activity and decreased new bone formation are just being identified. In addition, only recently have in vivo models that accurately replicate the features of human myeloma bone disease (MBD) been reported,2– 5 further complicating attempts to understand the pathogenesis of the intensive bone destruction in these patients.
Studies over the past 15 years have had important findings. First, the mechanism by which bone is destroyed in myeloma is via the osteoclast (OCL), the normal bone-resorbing cell. Second, OCLs accumulate on bone-resorbing surfaces in marrow adjacent to collections of myeloma cells. Thus, it appears that the mechanism by which OCLs are stimulated in myeloma is a local one. Third, cultures of human myeloma cells in vitro produce several OCL activating factors (OAFs), but none of these cytokines appears to be clearly responsible for the bone destruction in vivo. Fourth, the increase in OCL bone resorption in myeloma is usually associated with a marked impairment in osteoblast function. Alkaline phosphatase activity in the serum is decreased or in the normal range, unlike in patients with other types of osteolytic bone disease, and radionuclide scans do not show evidence of increased uptake in over 50% of patients, indicating impaired osteoblast responses to the increase in bone resorption.
This section will review the pathophysiology of MBD, OAFs that have been implicated in MM bone disease, and possible novel therapeutic targets to block their activity. In addition, potential mechanisms that may be involved in the decreased osteoblast activity present in the bones involved with myeloma will also be discussed.
Osteoclast Activating Factors
Many factors that induce OCL formation and bone resorption have been associated with the bone destruction in patients with myeloma. These factors include interleukin-1 (IL-1), parathyroid hormone–related protein (PTHrP), RANK ligand (RANKL), lymphotoxin, tumor necrosis factor-alpha (TNFα), hepatocytic growth factor (HGF), IL-6, and matrix metalloproteinases (MMP1, MMP2, MMP9), as well as insulinlike growth factor binding protein 4 (IGF-BP-4).6–,11 Recently, macrophage inflammatory protein-1α (MIP-1α) has also been identified as a chemokine produced by myeloma cells. MIP-1α levels in bone marrow plasma from myeloma patients correlate with their disease activity and may mediate in part the bone destruction in myeloma.12
Interleukin-6
IL-6 is a potent stimulator of OCL formation and induces growth of myeloma cells in about 30% of patients.13 IL-6 is detectable in marrow plasma samples from patients with myeloma, and IL-6 can enhance the effects of other osteoclastogenic factors such as RANKL, PTHrP, or MIP-1α. In addition, Devlin et al have shown that IL-6 can mediate the effects of inflammatory cytokines that stimulate OCL formation, such as IL-1 and TNFα.14 IL-6 levels have been correlated with the presence of bone lesions and disease severity in patients with myeloma, but this finding has not been consistent.10 More important, IL-6 has little proliferative effect on mature myeloma cells, and in 5 patients with refractory myeloma, infusion of an antibody to IL-6 decreased tumor burden in only 2 of them.10 However, use of a humanized monoclonal antibody to IL-6 is scheduled for clinical trial.
IL-6 may also play other roles in the pathogenesis of myeloma because adhesive interactions between myeloma cells and marrow stromal cells result in upregulation of IL-6 production by marrow stromal cells. In addition, IL-6 can act as an antiapoptotic factor increasing the survival of myeloma cells.15 Recently, Alsina et al have shown that IL-6 can induce expression of MIP-1α in myeloma cells and may play a role in the resistance of myeloma cells to chemotherapy.16 Thus, the role of IL-6 as an OAF in myeloma is still to be determined.
Interleukin-1β
IL-1β is a potent inducer of OCL formation and bone resorption and induces hypercalcemia when given to mice. Multiple studies have implicated IL-1β as an OAF in myeloma. Freshly isolated marrow mononuclear cells from patients with myeloma secrete IL-1 into their conditioned media, and the bone-resorbing activity from these cultures could be neutralized by antibodies to IL-1.17 Furthermore, IL-1 messenger RNA (mRNA) can be detected in highly purified myeloma cells as well as in a subgroup of patients with MGUS.17 However, only pro IL-1 mRNA, rather than mature IL-1 protein, has been consistently detected in myeloma patients, and the levels of IL-1 that are produced are extremely low.17 Furthermore, 2-color immunofluorescence in situ hybridization studies have not detected IL-1β protein but have found TNFα production by clonal plasma cells from myeloma patients and patients with monoclonal gammopathy.18 IL-1 protein has not been detected in marrow plasma samples from more than 20 patients with myeloma with extensive bone disease.12 Most recently, an IL-1 receptor antagonist failed to block the bone disease and growth of the tumor in a murine model of myeloma.19 Together these data suggest that the role of IL-1 remains to be determined.
Tumor Necrosis Factor-Alpha
TNFα is a potent stimulator of OCL formation both in vivo and in vitro20,21 and has also been identified in media conditioned by bone marrow mononuclear cells from patients with myeloma. Myeloma cell lines can produce TNFα, but marrow accessory cells rather than myeloma cells may be producing TNFα in patients. TNFα has been shown by 2-color immunofluorescence in situ hybridization techniques to be produced by clonal plasma cells from both myeloma patients and MGUS patients who do not have bone disease.18 Because MGUS patients do not have bone disease, it is unlikely that TNFα is the OAF in myeloma. Furthermore, there is no correlation between TNFα levels in patients with myeloma and the extent of their bone disease.
Lymphotoxin
Lymphotoxin was the first OAF to be identified as a possible cause of MBD.22 Lymphotoxin, also called TNFβ, binds the TNFα receptor and can induce osteoclastogenesis in vitro as well as hypercalcemia in mice when given to mice in vivo.23 Lymphotoxin is produced by primary myeloma cells in vitro and myeloma cell lines, but little lymphotoxin is present in bone marrow plasma from patients with myeloma. Furthermore, there appears to be no correlation between the amount of lymphotoxin produced in vitro and the extent of MBD in vivo. Thus, production of lymphotoxin by myeloma cells occurs in vitro rather than in vivo.
Parathyroid Hormone-Related Protein
PTHrP is the mediator of the humoral hypercalcemia of malignancy and is produced by both human breast cancer cell lines and prostate cancer cell lines. PTHrP can induce OCL formation both in vivo and in vitro.24 It appears to do this by inducing RANKL expression by marrow stromal cells and osteoblast.25 PTHrP levels were elevated in 5 of 17 patients with hypercalcemia and myeloma, and immunocytochemical studies demonstrated that the myeloma cells produced PTHrP.26 Similarly, a patient with myeloma who had hypercalcemia with high serum PTHrP levels has been reported.27 PTHrP levels decreased as the patient went into remission following chemotherapy. However, the majority of patients with myeloma do not have elevated PTHrP levels in their plasma.
Hepatocyte Growth Factor
Several laboratories have demonstrated that myeloma cells produced HGF, and HGF levels correlate with disease activity.28 However, HGF is not consistently produced by myeloma cell lines and is not detectable in all patient samples tested. HGF induces IL-11 secretion by human osteoblast-like cells, and IL-11 is a potent inducer of OCL formation both in vitro and in vivo.28 IL-11 is an IL-6-like molecule that has been implicated in the increased osteoclastogenesis caused by estrogen deficiency. However, the correlation between HGF production and MBD is not clear.
RANK Ligand
RANKL is a potent osteoclastogenic factor that in combination with macrophage colony-stimulating factor (M-CSF) induces OCL formation in vitro (Figure 4 ).25 RANKL is a member of the tumor necrosis family and is expressed as a membrane-bound protein on the surface of osteoblasts, osteocytes, and marrow stromal cells. In addition, activated T cells secrete RANKL as a soluble molecule.29 RANKL appears to be cleared from the surface of cells by a TNFα-converting enzyme-like activity and is active as a soluble molecule.29 Mice that have been genetically engineered to overexpress RANKL have severe osteoporosis, while mice lacking RANKL production, or its receptor RANK, have severe osteopetrosis.25 Thus, RANKL appears to be an important factor in normal OCL formation. Furthermore, most factors that induce OCL formation, such as IL-1, IL-11, prostaglandin E2, and 1,25-(OH)2D3, induce OCL formation by acting indirectly on marrow stromal cells and upregulating RANKL production.
The relative levels of RANKL and osteoprotegerin (OPG), a decoy receptor for RANKL produced by many different cell types, including marrow stromal cells and osteoblasts, determine the level of OCL formation. Giuliani et al have clearly demonstrated that there is an imbalance between OPG and RANKL levels in the bone marrow environment of patients with MM.30 They examined myeloma cells from 26 patients and 10 myeloma cell lines and demonstrated that myeloma cells failed to express RANKL and produced low amounts of OPG. However, in coculture systems of human myeloma cells with marrow stromal cells, RANKL expression was upregulated and OPG production strongly downregulated at both the protein and mRNA levels. In addition, Pearse et al have examined marrow biopsy specimens from patients with myeloma and found that RANKL expression was markedly upregulated in bone marrow biopsies from patients with myeloma while OPG was expressed at very low levels compared to normal bone marrow biopsy specimens.31 Taken together, the sets of data suggested that there is a marked imbalance between RANKL expression and OPG levels that favor osteoclastogenesis and OCL activation. In support of these studies is a recent report by Seidel et al from the Nordic Myeloma Group, which showed that OPG levels measured in the serum of patients with MM were significantly lower than those levels in healthy age- and sex-matched controls.32 OPG levels were decreased to a greater extent in myeloma patients with osteolytic disease compared to patients who did not have bone disease. Interestingly, OPG levels did not correlate with clinical stage or survival of the patients. These findings have suggested that OPG may be a reasonable therapeutic agent to treat MBD. Several groups have suggested that myeloma cells themselves produce RANKL and directly induce OCL formation, but this has not been a consistent finding. Croucher et al have demonstrated that the murine myeloma cell line, 5T2, expressed RANKL.33 However, Pearse et al31 and Giuliani et al30 failed to demonstrate RANKL expression by human myeloma cells.
In addition to OPG, another antagonist of RANKL is RANK-Fc, a molecule made by fusing the Fc portion of immunoglobulin to a soluble form of the RANK receptor. RANK-Fc has a similar mechanism of action as OPG, but in contrast to OPG, has not been shown to bind TRAIL, a member of the TNF gene family that induces tumor cell apoptosis.34 Studies with RANK-Fc in a murine model of humeral hypercalcemia showed that both tumor burden and bone destruction were decreased in these animals when they are treated with RANK-Fc.35 Croucher et al have demonstrated that OPG also will inhibit the development of osteolytic bone disease in this model of myeloma.33 Recently, Yaccoby et al36 have also examined the potential use of RANK-Fc and bisphosphonates, such as pamidronate or zoledronate, to block bone destruction in the severe combined immunodeficient (SCID)-Hu model of myeloma. In this model, primary myeloma cells are injected into a human fetal bone rudiment implanted into SCID mice. Both agents decreased bone resorption and tumor burden. Yaccoby et al failed to find any effect on growth of extramedullary myeloma in this model. Taken together, these studies suggest that blocking bone resorption induced by RANKL or other OCL stimulatory factors may decrease tumor burden as well as bone destruction in patients with myeloma. However, to date, no convincing studies in patients have demonstrated that using very potent bisphosphonates, such as pamidronate, results in a decreased tumor burden in these patients.
MIP-1α
Chemokines in general and MIP-1α in particular are attractive candidates for an OAF in myeloma for six reasons:
MIP-1α is produced by myeloma cells in vivo.
Chemokines are active locally in anatomically restricted sites but are not active systemically even in high concentrations, consistent with MBD being a local rather than a systemic process.
Chemokines enhance adhesive interactions between cells or matrix by upregulating integrin expression.37 Adhesive interactions between myeloma cells and marrow stromal cells play an important role in MBD. For example, adhesive interactions between the α4β1 integrin on myeloma cells and the vascular cell adhesion molecule-1 (VCAM-1) on marrow stromal cells induce IL-6 secretion by stromal cells and increased expression of RANKL.38 This enhanced RANKL expression would further increase OCL formation in areas of myeloma.
MIP-1α is a chemoattractant for OCLs39 and induces OCL formation in rat marrow cultures.40
MIP-1α enhances the growth of colony forming unit-granulocyte macrophage (CFU-GM) stimulated by granulocyte-macrophage colony-stimulating factor (GM-CSF) but suppresses more primitive hematopoietic precursors.41 CFU-GM are the earliest identifiable OCL precursors.
MIP-1α has been implicated in the anemia of myeloma patients.42
The following results from our recent studies support an important role for MIP-1α as an OAF in the majority of myeloma patients. Patients with myeloma have an OAF activity in their marrow plasma that correlated with their tumor burden, as assessed by β2 microglobulin levels.12 RNAse protection and enzyme-linked immunosorbent assays (ELISA) showed that elevated levels of MIP-1α mRNA, but not IL-1α, TNFβ, or IL-6 mRNA, were present in freshly isolated bone marrow from MM patients compared to normals. Furthermore, recombinant hMIP-1α induced OCL formation in human bone marrow cultures.43 Interestingly, MIP-1α increased OCL formation induced by IL-6 in human marrow cultures and also enhanced OCL formation induced by PTHrP and RANKL, factors that have been implicated in MBD. Importantly, addition of a neutralizing antibody to MIP-1α to human bone marrow cultures treated with freshly isolated marrow plasma from myeloma patients blocked the increased OCL formation induced by these marrow plasma samples. Anti-MIP-1α had no effect on control levels of OCL formation.12 Furthermore, marrow plasma samples from normals did not induce OCL formation. In addition, we have recently reported that blocking MIP-1α activity in a model of human MBD, in which ARH-77 cells are infused into sublethally irradiated SCID mice, demonstrated that both tumor burden and bone destruction were blocked when an antisense construct to MIP-1α was transfected into the ARH-77 cells prior to transplantation.37 These data suggest that MIP-1α plays a major role in both the growth and homing of myeloma cells to bone marrow and has a direct effect on OCL formation.
Matrix Metalloproteinase-2, -7, and -9
MMPs can degrade bone matrix and expose bone mineral to OCLs for subsequent bone resorption. Myeloma cells express MMP9, although this expression of MMP9 is not regulated by either IL-6 or other cytokines that have been associated with MBD. Bone marrow stromal cells, in addition, expressed MMP1 and MMP2, which are both regulated by cytokines, are produced by myeloma cells, and may play a role in the bone destruction process. Barille et al9 have reported that pro-MMP2 is activated by a soluble metalloproteinase, MMP7, which is produced by myeloma cells, and MMP2 may play a role in degrading bone matrix.
Vascular Endothelial Growth Factor
Vascular endothelial growth factor (VEGF) is a potent inducer of neovascularization. VEGF is produced by both myeloma cells and marrow stromal cells when myeloma cells bind to marrow stromal cells. VEGF can act as a growth factor for myeloma cells and can enhance OCL formation. Studies in the osteopetrotic op/op mouse have shown that VEGF can replace M-CSF and induce OCL formation in these animals.44 The op/op mouse has a point mutation in the M-CSF gene and develops severe osteopetrosis, which improves with age. Factors such as VEGF have been implicated as part of the mechanism for the spontaneous cure of osteopetrosis in these animals over time. Thus, adhesive interactions between myeloma cells and marrow stromal cells play an important role in MBD, as discussed below.
Interactions Between Bone and Myeloma Cells
Bone is a major storehouse of growth factors that can affect the growth of tumor cells. Most of the growth factors present in the bone matrix are in latent form, such as transforming growth factor β (TGFβ), IGFs, and fibroblast growth factors. During the bone resorptive process, these growth factors are released in active form and can affect the growth of tumor cells. In addition, the OCL itself is a prodigious producer of IL-6,45 a factor that enhances the growth of myeloma cells and blocks apoptosis in myeloma cells. These findings have led to the concept that there is a symbiotic relationship between the growth of myeloma cells and the bone destructive process. Recent work in several laboratories supports this notion. For example, Yaccoby et al reported using the SCID-hu model of myeloma that blocked resorption with either RANK-Fc or zolendronic acid, both of which block bone resorption but have no direct effect on myeloma cells; decreased the bone destructive process; and decreased tumor burden.36 Moreover, we have shown that blocking MIP-1α in the ARH-77 cell line, which does not directly stimulate the growth of myeloma cells, decreases tumor growth in an in vivo model of MBD.37 However, Croucher et al, using the 5T2 murine model of myeloma, found that treating these animals with OPG failed to decrease tumor burden.33 In patients who have received bisphosphonates, there has not been a consistent decrease in tumor burden. Thus, although in animal models it appears that blocking bone destruction results in decreased tumor growth, this has not yet been found in patients. However, with the advent of more potent bisphosphonates and availability of OPG and RANK-Fc for clinical trials, there is the potential for decreasing tumor burden in myeloma patients by blocking bone destruction.
Adhesive Interactions in Myeloma Bone Disease
As noted above, adhesive interactions between marrow stromal cells and myeloma cells appear to play a critical role in the bone destructive process ongoing in myeloma as well as to affect tumor cell growth and chemoresistance of myeloma cells to agents such as doxorubicin and melphalan. When myeloma cells bind marrow stromal cells, VEGF, IL-6, and RANKL are induced. In a murine model of MBD, β1 integrins mediate the adhesive interactions between myeloma cells and marrow stromal cells. Michigami et al have shown that α4β1 integrin plays an important role in inducing a bone-resorbing activity, possibly MIP-1α, when the 5TGM1 murine myeloma cell line binds to the ST2 murine marrow stromal cell line.46 Similarly, Giuliani et al noted that upregulation of RANKL appears to involve in part the integrin α4β1.30 Thus, blocking α4β1 binding of myeloma cells to VCAM-1 on stromal cells may decrease the release of bone-resorbing factors by the marrow stromal cells. Similarly, we have shown that blocking MIP-1α expression in ARH-77 cells decreases expression of α5β1 integrin on the cells and decreases binding of ARH-77 cells to marrow stromal cells, thereby impairing tumor growth and bone destruction in an in vivo model of MBD.37
Suppression of Osteoblast Activity in MBD
In patients with myeloma, once marrow tumor burden exceeds 50%, osteoblast activity is markedly suppressed.47 At lower tumor burden, bone resorption is still coupled to new bone formation, as occurs normally. Even after patients go into complete remission with intensive chemotherapy, bone lesions usually do not heal, although Clark and Fraser have suggested that osteoblast activity may increase following high-dose chemotherapy with autologous stem cell transplant.48 These data suggest that myeloma cells induce apoptosis of osteoblasts or block osteoblast differentiation from precursors. Recently, Feliers et al reported that the myeloma-derived cell line ARH-77 produces IGF-BP-4, which can inhibit the effects of IGF1 on osteoblast growth.49 Furthermore, they found that ARH-77 cells do not release the proteolytic activity that is responsible for the degradation of IGF-BP-4, allowing it to accumulate in the bone microenvironment. This increased production of IGF-BP-4 would inhibit IGF1 activity, which is a potent stimulator of osteoblast growth. IGF1 as well as TGFβ are important growth factors that can stimulate the growth and differentiation of osteoblast. To date, no one has identified a substance produced by myeloma cells that directly suppresses osteoblast activity or induces osteoblast apoptosis. However, it appears that osteoblast and osteoblast precursors are suppressed or destroyed by the myeloma cells.
Summary
MBD is responsible for the major morbidity associated with MM and causes severe bone pain, pathologic fractures, and even life-threatening hypercalcemia in MM patients. The mechanism of bone destruction in myeloma appears to involve multiple cytokines either produced by myeloma cells or induced by myeloma cells when they bind through adhesive interactions to marrow stromal cells. Two major candidates for the mediators of MBD are RANKL and MIP-1α, although other factors have been implicated as well. The decreased bone formation that is associated with MBD is an important problem in these patients because their bone lesions normally do not heal. The mechanism responsible for the suppressed osteoblast response to the increased bone resorption is a topic of intensive investigation. In clinical trials currently are potent bisphosphonates, which can inhibit OCL formation and activity, as well as new agents such as osteoprotegerin, all of which may have a profound effect on MBD. These agents may possibly decrease tumor burden because of the importance of osteoclastic bone resorption to the growth of myeloma cells.
IV. Targeting the Myeloma Cell and Its Microenvironment
Kenneth C. Anderson, MD*
Dana-Farber Cancer Institute, 44 Binney Street, Mayer 557, Boston, MA 02115
Identification and Validation of Novel Targeted MM Therapies
MM affected 14,400 new patients in the United States in 2001, with 50,000 total patients affected, and remains incurable despite conventional and high-dose chemotherapy.1 To overcome resistance to current therapies and improve patient outcome, novel biologically based treatment approaches are needed that target mechanisms whereby MM cells grow and survive in the bone marrow (BM). In the Jerome Lipper Multiple Myeloma Center at the Dana-Farber Cancer Institute, we are attempting to derive novel therapies to improve patient outcome by targeting the MM cell as well as its BM microenvironment.2 To achieve this goal, we have developed systems for studying growth, survival, and drug resistance mechanisms intrinsic to MM cells. Importantly, we have also developed both in vitro systems and in vivo animal models to characterize mechanisms of MM cell homing to BM, as well as factors (MM cell-bone marrow stromal cell interactions, cytokines, angiogenesis) promoting MM cell growth, survival, drug resistance, and migration in the BM microenvironment.3–10 These model systems have allowed for the development of several promising biologically based therapies that can target the MM cell in its BM microenvironment and thereby overcome classical drug resistance in vitro, including thalidomide (Thal) and its more potent immunomodulatory analogs (IMiDs),11 proteasome inhibitor PS-34112 and arsenic trioxide (As2O3)13 (Figure 5, see Color Figures, page 517).
Once the promise of these novel agents was demonstrated in vitro, we rapidly tested their efficacy in murine models. Thal and the IMiDs14 3-amino-phthalimido-glutaramide15 and PS-34116 all inhibit human MM cell growth, decrease associated angiogenesis, and prolong host survival in our model where human MM cells and matrigel are injected subcutaneously into severe combine immunodeficiency (SCID) mice. We have translated our laboratory studies to Phase I and II clinical trials to evaluate their clinical utility and toxicity. Most excitingly, IMiDs17 and PS-34118 have already demonstrated marked clinical anti-MM activity even in patients with refractory relapsed MM, confirming the utility of our preclinical models to identify and validate novel therapeutics. Importantly, as in our in vitro gene array studies with conventional (dexamethasone [dex])19 and novel (PS-341)20 therapies, samples obtained from patients treated on these protocols help to identify in vivo targets and mechanisms of novel drug action on the one hand, and mechanisms of drug resistance on the other, and also aid in determining whether in vivo targets of these novel therapies correlate with their in vitro anti-MM activities. These studies have demonstrated the importance of evaluating the role of the MM cell in its BM microenvironment, both to elucidate MM pathogenesis and to identify targets for novel therapeutics.
The Role of the BM in MM Pathogenesis
We have characterized the mechanisms whereby MM cells home to the host BM and adhere to BM stromal cells (BMSCs) and extracellular matrix proteins, as well as the functional sequelae of this binding, in order to identify targets for novel therapies. Importantly, our past studies have identified those adhesion molecules mediating MM cell binding to fibronectin and BMSCs, as well as the MM cell growth and survival advantage conferred by this binding.21–,24 Our studies show that BMSCs secrete cytokines, such as IL-625 and insulin growth factor 1 (IGF-1),26 which augment growth, survival, and drug resistance of MM cells in the BM milieu. We have demonstrated that in addition to localizing tumor cells in the BM microenvironment, adhesion of MM cells to BMSCs triggers the paracrine NF-κB dependent transcription and secretion in BMSCs of IL-6, the major cytokine mediating MM cell growth, survival, and resistance to drug-induced apoptosis.22,27 Paraformaldehyde fixation of BMSCs prior to MM cell adhesion abrogates this response, confirming paracrine production of IL-6 in BMSCs. More recently, we have shown that VEGF is secreted by both MM cells and BMSCs, and that its secretion is similarly upregulated by binding of MM cells to BMSCs.4 To separate the effects of MM cell-BMSC contact and adhesion versus cytokine secretion on MM pathogenesis in the BM milieu, we have placed MM cells in Transwell insert chambers, which allows juxtaposition of MM cells to BMSCs but precludes cell-to-cell contact and adhesion. MM cells in these chambers secrete cytokines (TNFα, TGFβ, VEGF) that further upregulate IL-6 secretion in BMSCs and related sequelae.4,28,29 Importantly, our studies show that cytokines acting on MM cells in the BM milieu augment growth (IL-6, IGF-1, VEGF); protect against drug-induced apoptosis (IL-6, IGF-1); trigger migration (VEGF); augment binding of MM cells to BMSCs (TNFα); and stimulate angiogenesis (VEGF) in the BM microenvironment.4,25,26,28
In further studies of the functional significance of MM cell binding to BMSCs, we have shown that adhesion of MM cells to BMSCs itself is a dynamic process. For example, CD40 activation of MM cells both augments expression of adhesion molecules (β1 and β2 integrins)30 and induces translocation of new molecules (Ku86) to the cell surface,31,32 which augments MM cell binding to fibronectin and BMSCs. Although TNFα does not directly alter MM cell growth and survival, our recent studies show that it induces NF-κB dependent upregulation in cell surface expression of adhesion molecules (ICAM-1, VCAM-1) on both MM cells and BMSCs, resulting in increased binding and related induction of IL-6 transcription and secretion in BMSCs.28 Other studies have shown that MM cell adhesion to fibronectin confers conventional drug resistance with induction of p27 and G1 growth arrest,33 and we have demonstrated that adhesion of MM cells to paraformaldehyde-fixed BMSCs triggers MAPK activation and proliferation in MM cells,12 independent of cytokine induction in BMSCs. Homing and localization of the MM cell in the BM milieu therefore promotes tumor cell growth, survival, migration, and drug resistance, supporting our plan to target MM cell-host BMSC interactions and their sequelae in novel therapeutics. Excitingly, our recent studies show that MAPK activation and proliferation of MM cells triggered by adherence to paraformaldehyde-fixed BMSCs is completely abrogated by novel agents such as PS-341,12 validating this approach.
Molecular Targets of Novel MM Therapies
Given the constitutive secretion of cytokines in MM BM and the further induction of cytokine production triggered by MM cell localization in BM, we have characterized the signaling cascades whereby cytokines mediate proliferation, survival, drug resistance, and migration of MM cells (Figure 6, see Color Figures, page 518). For example, our studies show that cytokines (IL-6, VEGF) trigger proliferation of MM cells via MAPK signaling,3,4 whereas MM cell migration induced by cytokines (VEGF) is mediated via a PKC-dependent, ERK-independent pathway.4 We have shown that gamma irradiation (IR), Fas, and dex-induced MM cell apoptosis are mediated by distinct signaling cascades.6,7,34 Dex- (but not IR- or Fas-) triggered apoptosis is mediated via activation of related adhesion focal tyrosine kinase (RAFTK).8 Dex-mediated MM apoptosis is not associated with mitochondrial cytochrome c release7 but is mediated by Second mitochondria activator of caspase (Smac) release from mitochondria;10 cytosolic Smac disrupts the inhibitor of apoptosis XIAP/caspase 9 complex, thereby allowing activation of caspase 9, caspase 3 cleavage, and apoptosis. Whereas dex activates caspase 9,19 novel therapies (i.e., IMiD, TRAIL) activate caspase 8 and downstream cascades, suggesting the utility of coupling novel and conventional therapies.35 We have also recently begun to delineate mechanisms of drug resistance induced by cytokines. For example, we have shown that that both IL-6 and IGF-1 inhibit apoptosis triggered by dex (but not IR) via Akt signaling and NF-κB activation, with downstream induction of intracellular inhibitors of apoptosis (IAPs), including FLIP, survivin, cIAP-2 A1/BFL-1, and XIAP,26,36 as well as specific activation of SHP2 phosphatase, thereby blocking activation of RAFTK.9 These studies validate signaling cascades triggered by MM localization in the BM microenvironment mediating tumor cell growth, survival, drug resistance, and migration as novel therapeutic targets.
Antitumor Activity of Novel Agents in Animal Models
We pioneered the SCID-hu model of human MM in which human fetal bone grafts are implanted bilaterally in the flanks of SCID mice, and we showed that human MM cells implanted into these grafts proliferate, trigger IL-6 secretion in BMSCs, secrete MM idiotypic protein detectable in mouse serum, and migrate to the contralateral human BM graft but not to murine BM.37 This in vivo model of human MM therefore provides a means for identifying adhesion molecules mediating specific homing of human MM cells to the human, as opposed to murine, BM microenvironment; as well as for studying the role of microenvironmental factors (host-MM cell interactions, cytokines, angiogenesis) in MM pathogenesis. More recently, we established a beige-nude-xid mouse model and showed that Thal and the IMiDs,14 3-amino-phthalimido-glutaramide,15 and PS-34116 all inhibit human MM cell growth, decrease associated angiogenesis, and prolong host survival, providing the framework for related clinical trials.
Clinical Trials of Agents Targeting MM Cells and Their BM Microenvironment
We have begun to translate these studies of Thal/IMiDs,4,11,14,38,39 PS-341,12,24 and As2O313 from bench to bedside in related clinical trials. Specifically, increased angiogenesis in MM BM, coupled with the known antiangiogenesis activity of Thal, provided the empiric rationale for its use to treat refractory MM. We and others11,36 have shown remarkable responses even in MM refractory to all other known therapies. Our studies to date demonstrate that Thal and IMiDs act not only to inhibit angiogenesis, but also to directly induce either apoptosis or G1 growth arrest in MM cells, even those refractory to conventional therapy;11 abrogate the adhesion of MM cells to BMSCs and related protection against apoptosis;34,38 block the increased secretion of MM growth, survival, and migratory factors (IL-6, VEGF) triggered by binding of MM cells to BMSCs;38 expand patients’ natural killer (NK) cell number and function against human MM cells,39 and downregulate protectin expression in MM cells,41 thereby enhancing their susceptibility to ADCC (Figure 5, page 517). Using our SCID model, we have also shown that Thal/IMiDs mediate both anti-MM activity and antiangiogenesis in vivo.14 On the basis of this promising preclinical data, we have recently carried out a Phase I IMiD CC-5013 dose escalation (5 mg/d, 10 mg/d, 25 mg/d, and 50 mg/d) study in 27 patients (median age 57, range 40-71 years) with relapsed and refractory relapsed MM (Table 6 ). Patients received a median of 3 (range 2-6) prior regimens, including autologous stem cell transplantation and Thal in 15 and 16 patients, respectively. In 24 evaluable patients, no dose-limiting toxicity (DLT) was observed in patients treated at any dose level within the first 28 days; however, grade 3 myelosuppression developed after day 28 in all 13 patients treated with 50 mg/day CC-5013. In 12 patients, dose reduction to 25 mg/day was well tolerated and therefore considered the maximal tolerated dose (MTD). Importantly, no significant somnolence, constipation, or neuropathy has been seen in any cohort. Best responses of ≥ 25% reduction in paraprotein occurred in 17 of 24 (71%) patients (90% confidence interval [CI]: 52%, 85%), including 11 (46%) patients who had received prior Thal (Table 6). Stable disease (< 25% reduction in paraprotein) was observed in an additional 2 (8%) patients. Our study therefore provides the basis for the evaluation of CC-5013, either alone or in combination, to treat patients with MM at earlier stages of disease. Studies already ongoing or to begin soon in MM include CC-5013 as initial therapy, treatment for first relapse, and maintenance after high-dose therapy and stem cell transplantation.
Proteasome inhibitors represent a second class of therapeutics targeting the MM cell in its BM microenvironment that have recently been studied in our laboratory. Specifically, on the basis of our prior studies that found that IL-6 is the major growth and survival factor for human MM cells25 and our observation that MM cell adhesion to BMSCs triggers the transcription and secretion of IL-6 in BMSCs via an NF-κB dependent mechanism,27 we reasoned that blockade of NF-κB using the proteasome inhibitor PS-341 may mediate anti-MM activity by inhibiting paracrine IL-6 production in BMSCs. Our studies demonstrate that PS-341 acts directly on MM cells to induce apoptosis of MM cells resistant to known conventional therapies, overcomes the protective effects of IL-6, and adds to the anti-MM effects of dex12 (Figure 5, page 517). Importantly, it acts in the BM microenvironment to inhibit the binding of MM cells to BMSCs, the transcription and secretion of IL-6 triggered by MM to BMSC adhesion, and BM angiogenesis. Our recent gene microarray profiling studies demonstrate that PS-341 induces transcriptional downregulation of growth/survival signaling pathways as well as upregulation of apoptotic cascades, ubiquitin/proteasome pathways, and heat-shock proteins.20 Our exciting studies, coupled with Phase I studies of PS-341 demonstrating clinical promise in patients with refractory MM, provided the strong rationale for a recently completed multicenter Phase II trial of PS-341 in MM emanating from our Center. Two hundred two patients with relapsed and refractory MM were treated, and analysis of the first cohort of 78 patients is currently available (Table 7 ). Patients had received a mean of 5 (2-13) prior therapies, including Thal (74%) and stem cell transplant (54%). Decreases of 25% or greater in MM paraprotein were noted in 47% of patients, and no change in protein was noted in an additional 30% of patients (Table 7). Excitingly, > 90% decreases in MM paraprotein were observed in 20% of patients, including some complete responses. At a follow-up of 6.2 months, the median duration of response has not been reached. On the basis of preclinical studies showing additive anti-MM activity of dex and PS-341,12 dex was added to those 25 patients who either progressed or achieved only stabilization of disease on PS-341 alone; excitingly, all but 1 patient achieved additional anti-MM response. The drug was well tolerated, with nausea, fatigue, diarrhea, peripheral neuropathy, and thrombocytopenia as the most common adverse events. Importantly, neuropathy and thrombocytopenia were rare unless present prior to PS-341 therapy. Complete analysis of this study, including pharmacogenomics and quality-of-life data, will be available later this year. On the basis of this clinical promise, a Phase III international randomized trial of PS-341 versus dex in relapsed MM and trials of PS-341 in newly diagnosed MM patients are ongoing.
Recent studies in our laboratory have also identified As2O3 as a third agent targeting the MM cell interacting with its BM microenvironment (Figure 5, page 517). As2O3 at clinically achievable levels induces apoptosis of drug-resistant MM cell lines and patient cells via caspase 9 activation, adds to dex, and can overcome the antiapoptotic effects of IL-6.13 It also decreases MM cell binding to BMSCs, inhibits IL-6 and VEGF secretion in BMSCs induced by MM cell adhesion, and blocks proliferation even of those MM cells adherent to BMSCs. As for Thal/IMiD and PS-341, clinical trials of As2O3 are ongoing.
Evolution of a New Treatment Paradigm for MM
Our in vitro and animal model studies have demonstrated the importance of the BM in promoting MM cell growth, survival, drug resistance, and migration in the BM microenvironment, and we have already derived very promising therapies based on targeting the MM cell in its BM milieu. These studies provide the framework for development of a new MM treatment paradigm targeting both the tumor cell and its microenvironment, which is urgently needed because MM remains incurable despite all available therapies.
Intergroupe Français du Myélome (IFM) 94 trial: Comparison of single versus double autotransplantation (intention-to-treat analysis): event-free survival.
—— Double transplant arm (n = 200)
___ Single transplant arm (n = 199)
Intergroupe Français du Myélome (IFM) 94 trial: Comparison of single versus double autotransplantation (intention-to-treat analysis): event-free survival.
—— Double transplant arm (n = 200)
___ Single transplant arm (n = 199)
Intergroupe Français du Myélome (IFM) 94 trial: Comparison of single versus double autotransplantation (intention to treat analysis): overall survival.
—— Double transplant arm (n = 200)
___ Single transplant arm (n = 199)
Intergroupe Français du Myélome (IFM) 94 trial: Comparison of single versus double autotransplantation (intention to treat analysis): overall survival.
—— Double transplant arm (n = 200)
___ Single transplant arm (n = 199)
Prognostic impact of β2 microglobulin level and chromosome 13 abnormalities (detected by fluorescence in situ hybridization [FISH] analysis) in 110 patients treated with high-dose therapy: progression-free survival.
Abbreviations: O/N, number of deaths without progression and progressions/number of patients; SE, standard error
Prognostic impact of β2 microglobulin level and chromosome 13 abnormalities (detected by fluorescence in situ hybridization [FISH] analysis) in 110 patients treated with high-dose therapy: progression-free survival.
Abbreviations: O/N, number of deaths without progression and progressions/number of patients; SE, standard error
Regulation of osteoclast formation in myeloma.
Marrow stromal cells bind myeloma cells and induce production of IL-6, RANK ligand, and TNF-α. In addition, myeloma cells produce MIP-1α, IL-1, and TNF-α, which can induce osteoclastogenesis.
Regulation of osteoclast formation in myeloma.
Marrow stromal cells bind myeloma cells and induce production of IL-6, RANK ligand, and TNF-α. In addition, myeloma cells produce MIP-1α, IL-1, and TNF-α, which can induce osteoclastogenesis.
Dr. Shaughnessy has received research support from Celgene.
Dr. Shaughnessy has been supported by R33 CA97513; Drs. Shaughnessy and Barlogie by P01 CA55819.
Dr. Roodman is on the Novartis speakers bureau.
Supported by NIH grants RO-1 50947 and PO-1 78378, the Doris Duke Distinguished Clinical Research Scientist Award, a Multiple Myeloma Research Foundation Program Grant, and the Myeloma Research Fund.
Dr. Anderson receives research support from Cellgene and Millennium.

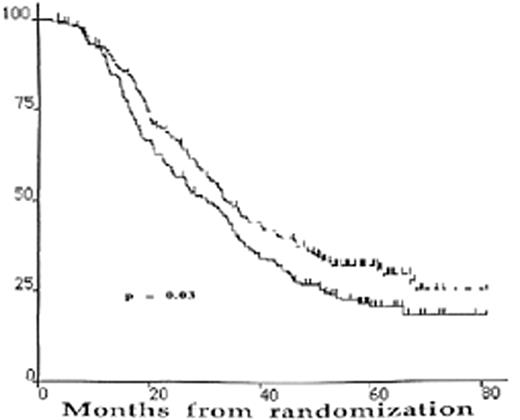
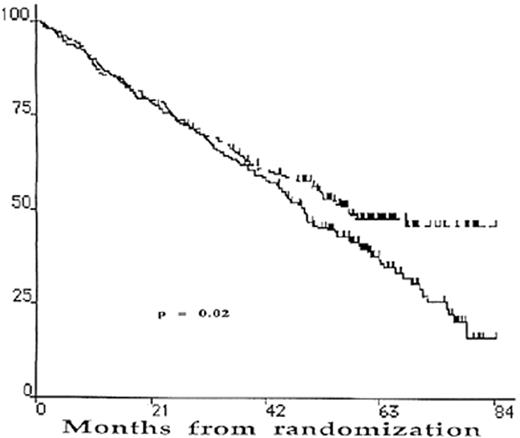
![Figure 3. Prognostic impact of β2 microglobulin level and chromosome 13 abnormalities (detected by fluorescence in situ hybridization [FISH] analysis) in 110 patients treated with high-dose therapy: progression-free survival. / Abbreviations: O/N, number of deaths without progression and progressions/number of patients; SE, standard error](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2002/1/10.1182_asheducation-2002.1.214/3/m_anderson_fig3.jpeg?Expires=1769240037&Signature=ByG3cPR-fcviA9tK5I9mKjZMkYQeHCI9h-gF8Ffl69bV3SMWbgX3lsrHFj6wIJuPjPH8BtRWf1tmlmiP~tKdHPqt6XxdYLmzOZ8lLpFZ~MJ38qZTqbJo~2KIUUkh2S0~YfHF6uBRN3zQK~THCkjVxgm4zUvAp14u~zrUbOUoTbGnzSHYnkhaRCHQlaPIi38LIeov2UNMUUuO9FU09SYJwbAIa4m7qOGpSRSIKwcFe2yLGy8qvOomOAxvdi5SYB1YWmOB~ZLXd0-aXO~blyTzthdRTwXBpnMVfGI51SfCRpjo26pG2P526MwwTT-jusZr~UEYlHAr3ciYrfhaPMmH9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
