Abstract
This update of early stage B-cell chronic lymphocytic leukemia (B-CLL) embraces current information on the diagnosis, biology, and intervention required to more fully develop algorithms for management of this disease. Emphasis on early stage is based on the rapid advancement in our understanding of the disease parameters and our increasing ability to predict for a given early stage patient whether there is a need for more aggressive management.
In Section I, Dr. Terry Hamblin addresses the nature of the disease, accurate diagnostic procedures, evidence for an early “preclinical” phase, the use of newer prognostic features to distinguish who will be likely to progress or not, and whether it is best to watch or treat early stage disease.
In Section II, Dr. Neil Kay and colleagues address the biologic aspects of the disease and how they may relate to disease progression. Review of the newer insights into gene expression, recurring genetic defects, role of cytokines/autocrine pathways, and the interaction of the CLL B cell with the microenvironment are emphasized. The relationship of these events to both trigger disease progression and as opportunities for future therapeutic intervention even in early stage disease is also considered.
In Section III, Dr. John Byrd and colleagues review the historical and now current approaches to management of the previously untreated progressive B-CLL patient. They discuss what decision tree could be used in the initial decision to treat a given patient. The use of single agents versus newer combination approaches such as chemoimmunotherapy are discussed here. In addition, the place of marrow transplant and some of the newer antibodies available for treatment of B-CLL are considered. Finally, a challenge to utilize our growing knowledge of the biology of B-CLL in the early stage B-CLL is proffered.
I. The Nature and Natural History of Early-Stage B-CLL
Terry J. Hamblin, MD*
Royal Bournemouth Hospital, Castle Lane East, Bournemouth BH7 7DW, Dorest, United Kingdom
Some people think that we are getting better at treating B-cell chronic lymphocytic leukemia (B-CLL) and produce survival curves that seem to show an improved survival for more recently diagnosed cases. At least part of that improvement is artificial, caused by earlier diagnosis or even the recognition as B-CLL of conditions that would not previously have crossed the threshold of diagnosis. As we diagnose B-CLL on lower and lower lymphocyte counts, we need to be doubly sure that the diagnosis is correct. It may be that a similar distinction needs to be made between B-CLL and “monoclonal lymphocytosis of undetermined origin” as that between myeloma and monoclonal gammopathies of undetermined significance (MGUS). As the proportion of cases diagnosed in early stage increases it becomes vital for the practicing physician to know who is likely to require therapy. It may be that the “watchful waiting” paradigm for early stage B-CLL needs to be challenged.
Diagnosis of True B-CLL
As time has passed we have lowered the threshold for diagnosing B-CLL. At one time a lymphocyte count of 10,000/mm3 was required1 but today we are happy with 5000/mm3.2 This reflects a greater certainty that we can distinguish B-CLL from other types of lymphocytosis, a certainty provided by the availability of immunologic markers. One consequence has been to include within the diagnosis more patients whose B-CLL is utterly benign.
This lowered guard demands a sterner conformity to the correct profile of lymphocyte markers in order to define B-CLL and exclude other types of exfoliating lymphoma in which a mild lymphocytosis is common (Table 1 ). The characteristic B-CLL pattern (CD5 and CD23 positive, surface immunoglobulin weak, CD79b weak or absent, FMC7 negative) has been codified by the Royal Marsden group.3 A score of 4 or 5 is found in almost all true B-CLLs. Very occasionally a true B-CLL will have only a weakly positive CD5 but the use of this scoring system will almost always exclude other “spillover lymphomas.” Apart from mantle cell lymphomas these will all be CD5 negative, and mantle cell lymphoma is easily distinguished even when the cellular morphology is similar; it is CD23 negative and has strong surface immunoglobulin staining. Confirmation of mantle cell lymphoma is by detection of the t(11;14) translocation and nuclear cyclin D1 staining. These “spillover lymphomas” will be excluded from further discussion.
Monoclonal Lymphocytosis of Undetermined Significance
Every journey begins with a single step. Every tumor begins with a single cell. The NCI guidelines set a minimal threshold for the diagnosis of CLL in the certain knowledge that the tumor will have existed before it is diagnosed. Exactly how early might CLL be diagnosed? And does it emerge from a profusion of proliferations that are monoclonal but not malignant? And can we tell the difference? Rawstron et al4 have designed a simple flow cytometric assay to detect minimal residual disease in patients with B-CLL who have been treated with intensive chemotherapy. This assay, based on the staining pattern of CD5, CD19, CD20, and CD79b, can detect small numbers of B-CLL cells with a sensitivity of 1 in 105. When the researchers applied this assay to routine blood counts coming through their laboratory in Leeds 3% of individuals over 40 years of age had a detectable population of cells with an identical marker profile to that of B-CLL. These cells were monotypic with respect to immunoglobulin light chains, and in the few patients where they had enough material to sequence the immunoglobulin heavy chain genes, they were clearly monoclonal. However, we should emphasize that “monoclonal” does not necessarily mean “malignant.” A B-CLL-like population could be detected twice as commonly in individuals over the age of 60 as in those between 40 and 59. As few as 3 cells per mm3 could be reliably detected, and in most the B-CLL-like cells represented a minority of the circulating B cells. Because the samples were anonymized, it was not possible to retest on a sequential basis. It is conceivable that these were only transient events.
These findings have yet to be reproduced in other laboratories, but if they are confirmed they will suggest that there is a monoclonal lymphocytosis of undetermined significance (MLUS) that bears a relationship to B-CLL similar to that MGUS bears to myeloma and that is present in a sizable proportion of elderly individuals. As with MGUS, and indeed as with myelodysplastic syndrome and carcinoma of the prostate, it is certain that many cases of MLUS that could be diagnosed will remain latent throughout an individual’s lifetime. Undoubtedly the Leeds study will prompt others to verify this, but it is a project for a young hematologist.
This is not a new concept. Han et al5 described a benign monoclonal B cell lymphocytosis without disease progression over 6-24 years, but all of these had a lymphocyte count > 10,000/mm3. The term B-MLUS has been used extensively by Mellstedt and his colleagues6–,10 to refer to patients with an isolated monoclonal B-cell lymphocytosis in blood and bone marrow but no other signs or symptoms of B-CLL. The European classification of Binet et al11 allows for lymphadenopathy in stage A disease whereas the Swedish MLUS patients do not seem to differ substantially from Rai Stage 0 B-CLL.12 To add to the confusion, during 2002 two papers have applied the term MLUS to proliferations of CD5-negative lymphocytes, distinguishing them from early stage B-CLL.13,14 While semantically correct, this usage is unhelpful.
Montserrat et al described “smoldering” B-CLL,15 which was defined as Rai stage 0 with non-diffuse bone marrow histology, hemoglobin ≥ 13 g/dL, blood lymphocytes < 30,000/mm3, and a lymphocyte doubling time of > 12 months. An analysis of the French Cooperative Group’s trial of Stage A patients16 demonstrated that a subgroup they designated A ′, with hemoglobin > 12 g/dL, lymphocyte count < 30,000/mm3, and fewer than 80% lymphocytes on bone marrow aspiration, was less likely to progress that other stage A patients.
The French criteria require a bone marrow aspiration, and the Spanish a bone marrow trephine. In view of the utility of blood tests, it may be appropriate to question the necessity of such invasive techniques in B-CLL. I would contend that bone marrow examination does not help in the diagnosis of B-CLL. Undoubtedly it gives prognostic information; future clinical trials must decide whether it gives information unavailable from blood tests alone.
Two Types of B-CLL?
The division of B-CLL into stable and progressive disease was noted by David Galton 36 years ago.17 What has been unclear is whether there is a qualitative difference between the B-CLL that pursues a progressive course and “smoldering” or stable B-CLL, or simply a quantitative one. Are they part of a spectrum? Does one metamorphose into the other? Is it, like the transformation of myelodysplastic syndrome to acute leukemia, a matter of the acquisition of further genetic damage?
Some answers to these questions have become available from the recognition of two subsets of B-CLL based on the IgVH mutational status.18,19 These two subsets have separate and distinct natural histories. Binet stage A patients without somatic mutations have a median survival of 8 years; those with somatic mutations have a median survival of 25 years.18
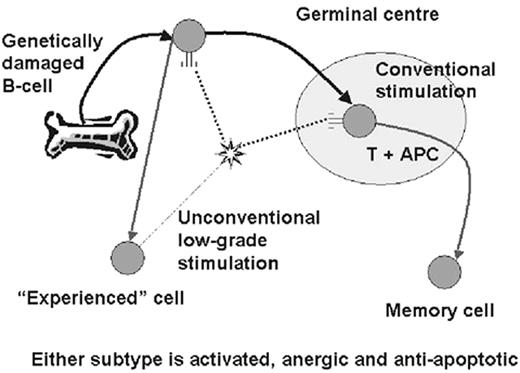
Somatic mutations occur in B cells in the germinal center under the influence of mutator enzymes generated in response to contact with antigen presented by follicular dendritic cells in the presence of T cells.20 Stimulation of the B cell receptor in any other circumstances, whether by T independent antigen or by superantigen, is thought not to induce somatic mutations (Figure 1 ).
It seems clear that B-CLL always derives from a mature cell.21 To use the term “memory” cell is controversial; perhaps to call it an “experienced” cell is more appropriate at present. Moreover, the two subsets are very similar to each other, differing from other types of lymphoma by the expression of a wide range of different genes, but from each other by the differential expression of very few.22,23 However, the mutational state is fixed. There has been no case of B-CLL in which mutations have been lost or gained by the clone during the course of the disease. There has been much debate as to whether B-CLL is one or two diseases. The fact that the mutated subset is equally common in males and females while the unmutated subset is three times as common in males suggests two separate entities, but what is clear is that the two subsets are distinct and do not metamorphose one into the other.
Natural History of B-CLL with Mutated V Genes
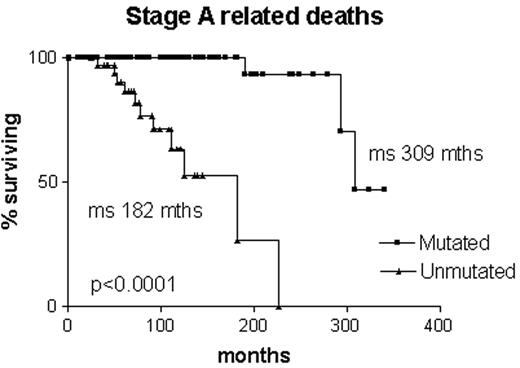
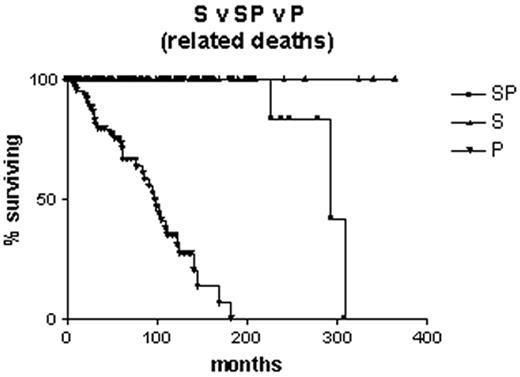
Is it correct to assume that B-CLL with mutated V genes is the same as “smoldering” B-CLL? Clearly not, since some cases progress to advanced stage disease. However, it is likely that all “smoldering” cases are contained within the mutated subset. It is interesting to note that, although the Leeds group had only limited material from their study of routine blood counts, in three cases they were able to sequence the immunoglobulin V genes. All three had somatic mutations.4 Survival curves on our own Binet stage A patients that are censored for B-CLL-unrelated deaths demonstrate that all patients with unmutated V genes are dead within 12 years of diagnosis, whereas the first death in the group with mutated V genes does not occur until 27 years after diagnosis, and only 2/70 have died from a B-CLL-related cause (Figure 2 ). In our hands, when the mutated subset progresses it does so slowly, and the survival curve resembles that of stable rather than progressive B-CLL (Figure 3 ). Nevertheless, the late complications of B-CLL, severe hypogammaglobulinemia and autoimmune hemolytic anemia, do occur in the mutated subset, and in our experience hemolytic anemia may be the presenting feature of low count stage 0 disease that is thereafter never troublesome.
Consequences for Management
A consensus has arisen that early stage B-CLL should not be treated with chemotherapy; rather, watchful waiting should be employed. The best supporting evidence for this approach is the meta-analysis of 2048 early stage patients in 7 trials randomized between immediate or deferred treatment with chlorambucil (with or without prednisolone).24 This demonstrated no benefit for either arm. The 10-year survival was slightly worse (but not statistically significantly so) for those treated early (44% versus 47%). There is, therefore, no need to treat patients with stage A disease with chlorambucil unless there is evidence of a falling hemoglobin or platelet count, progression to a later stage of disease, unsightly or uncomfortable lymphadenopathy, a lymphocyte doubling time of < 12 months, or transformation of the disease.
However, it must be admitted that all advanced B-CLL has passed through an early stage. In the large French study,25 51% of patients allocated to the deferred treatment arm eventually required treatment, and 27% eventually died of a cause related to B-CLL. Furthermore, chlorambucil is by no means the most effective treatment for B-CLL. The combination of fludarabine, cyclophosphamide, and rituximab has been reported to induce complete responses of 60% in advanced disease, with half of those tested achieving a molecular remission.26 In cancer, generally it is seldom prudent to wait until the disease progressed before applying the most effective treatment. On the other hand, it would be foolhardy to apply a potentially toxic treatment to a group of individuals who will never be bothered by their blood condition. What is needed is a foolproof way of predicting who will progress and who will not.
Prognostic Factors for Stable and Progressive Disease
Watchful waiting is clearly an appropriate management for at least half of all B-CLLs. From the foregoing it is clear that we can be more selective than applying this policy simply to all early stage disease. The French A ′16 and Montserrat’s “smoldering”15 criteria are predictive, but when observed over a 5-year period, 25% of A ′ cases and 13% of “smoldering” cases progressed, and 46% of A ′′ and 43% of “active” cases failed to progress.27
A number of serum factors have been suggested as potential prognostic indicators for early stage disease. Patients with early stage B-CLL who had serum thymidine kinase levels greater than 7.0 U/L had a significantly shorter progression-free interval than those with a lower level.28 Serum soluble CD23 segregates Binet stage B disease into more or less aggressive forms.29 Serum levels of β2-microglobulin greater than 4.0 mg/L are adverse prognostic factors in a wide range of lymphoid malignancies,30 including B-CLL.31
Chromosomal analysis is also predictive. Patients with a normal karyotype or isolated 13q14 deletions have a benign prognosis.32 On the other hand, the 10-15% of patients with 17p13 or 11q23 deletions do very badly indeed.33,34
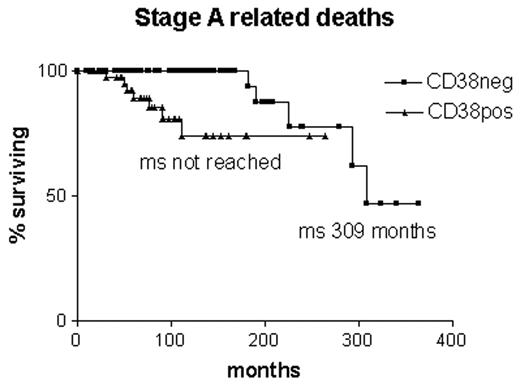
Whether any of these assays would act as indicators for early treatment of early stage disease remains to be evaluated. The worry is that they might reflect progression rather than predict it. The same may be said for surface CD38 expression. Although it was originally suggested as a surrogate assay for V gene mutations19 it appears that there is around 30% discordance between the assays.35 Furthermore, expression of CD38 changes in 25% of cases during the course of the disease, and it does not give so clear a prediction of the likelihood of B-CLL-related deaths in early stage B-CLL as V gene mutational status (Figure 4 ).35
Even V gene mutations are not a foolproof predictor. Tobin et al36 have described a new subset of B-CLL that uses the VH3-21 gene in conjunction with JH6 and a greatly abbreviated D segment, so that each case has a very similar CDR3 sequence. Despite having somatic mutations, this subset has a survival curve that resembles the unmutated subset. Time will reveal whether there are any other exceptions.
For the moment watchful waiting is still the watchword. At the same time, prospective clinical trials should evaluate whether any of the available prognostic factors can be used to successfully guide early treatment of early stage B-CLL that is destined to be fatal.
II. Progression Events in B-Chronic Lymphocytic Leukemia
Neil E. Kay, MD,1
Division of Hematology, Department of Medicine,
Mayo Clinic,
Dept. of Immunology,
Dept. of Laboratory Med. and Pathology, 200 First Street, Rochester, MN 55905 This work is in part supported by the National Cancer Institute (R01 CA95241-01, R21 CA87155-1, and R21 CA 91542-1).
This work is in part supported by the National Cancer Institute (R01 CA95241-01, R21 CA87155-1, and R21 CA 91542-1).
Advances in the understanding of biologic processes in many malignant diseases have generated unique rationale(s) for their evaluation and management. Thus, the knowledge that leukemic myeloid cells with a t(15;17) translocation have an abnormal retinoic acid receptor fusion structure has ultimately generated the successful use of retinoic acid therapies. The careful dissection of biologic events in colon cancer has uncovered several new areas for focus on both disease progression and unique preventative approaches (use of Cox-2 inhibitors). Similarly, the study of the easily available circulating blood B cells from patients with B-CLL should be just as helpful in unraveling the mysteries of this leukemic cell process. Even though the clinical staging systems of Rai and Binet have been very useful in predicting overall survival, they cannot predict an individual’s risk of disease progression in the early stage. Current helpful biologic paradigms that are linked to the CLL B cell include their low level of cell cycle activity, resistance to apoptosis, expression levels of CD38, division into germline versus somatic mutation-type clones, common recurring genetic defects, and defective antigen presenting capacity. Other less well known but relevant biologic aspects include the capacity of B-CLL cells to produce multiple cytokines that impact apoptosis/drug resistance and their ability to be induced to undergo cell signaling by these cytokines with subsequent alteration of gene expression. In addition, the presence of adhesion molecules that relate to CLL circulatory patterns and acquisition of additional genetic defects are important biologic events relevant to clinical outcome. Finally, it is necessary to consider the impact of the microenvironment on the CLL B cell. Currently, microenvironmental candidates found to affect important biologic events in the CLL B cell include circulating nurse cells, stromal cells in node and marrow, and the hypoxic atmosphere known to exist in CLL B cell tissue sites (e.g., marrow). We propose that the detailed sequential analysis of these biologic events in early stage B-CLL disease would be most instructive in predicting disease progression and in devising unique treatment strategies. This section will discuss some of these biologic events, in particular genetic, immunologic, angiogenesis, and apoptosis parameters, with an emphasis on early-stage B-CLL and discussion of early intervention points related to these parameters.
Genetic Events in B-CLL
While the exact origin of the malignant B cell in this disease is unknown and not the subject of this review, there has been a considerable amount of effort dedicated to more precise determinations of the genetic nature of the CLL B cell. Given the nature of the disease and our current understanding of the site of origin of CLL B cells, most of the cytogenetic studies have been aimed at marrow and/or blood cells. These have involved the use of conventional cytogenetics, fluorescence in situ hybridization (FISH), and most recently gene profiling assays and immunoglobulin (Ig) VH mutational status. The advances in understanding the genetic events using these approaches have initially been begrudgingly obtained but are now accelerating.
Cytogenetics
With the introduction of B-cell mitogens, it has been possible to generate metaphase cells for analysis by conventional G-banding techniques. Importantly, this approach revealed heterogeneous, clonal genetic changes in a significant proportion of patients (~40%-50%).1,2 Contributions from this period of analysis also included the more common recurring defects, including trisomy 12,1,2 11q,3 deletion of 6,4,5 and abnormalities of chromosome 13.2,6 Interestingly, these findings have been correlated with clinical outcome and/or disease course. When single cytogenetic abnormalities are examined, trisomy 12 or chromosome 6 defects are associated with shortened survival.2 However, sole abnormalities of chromosome 13q14 are linked to a more favorable clinical outcome.2 Atypical morphology is also now linked to the presence of trisomy 12.7,8 Clonal evolution in B-CLL was first demonstrated using this approach with one study showing that karyotypic evolution can be detected in around 40% of the patients over a 1-year duration.5 In addition, complex karyotypes could be found in B-CLL cells, and cohort studies showed poor clinical outcome for those patients. While this technique has helped in looking at critical genetic events, a significant percentage of CLL patients show no genetic defects likely due to the need for more extensive in vitro CLL B cell division.
FISH
Considerable advances in definition of common recurring genetic defects have been uncovered using the FISH method, which does not require mitoses. It is now possible to find these defects in higher percentages of CLL B cells, including those of patients with early-stage disease. Indeed, the use of FISH has largely replaced conventional cytogenetics in the determination of genetic defects in B-CLL. When FISH is used, the most common gene defects are deletions involving chromosome band 13q14, trisomy 12, deletions of 11q22.3-q23.1, deletions of 6q21-q23, and, finally, mutations or deletions of 17p13.9–,11 Our studies have used a “CLL FISH” panel that detects common recurring genetic defects in B-CLL.10,11Table 2 outlines the DNA probes used for our CLL FISH panel. The findings for FISH defects in early-stage/stable disease B-CLL versus B-CLL patients with progressive disease are outlined in Table 3 . Notable aspects include FISH detection of genetic defects in around 66% of the early-stage/stable patients and a higher percentage (88%) in progressive disease CLL patients. We have found there is a large percentage within the clonal CD19+/CD5+ B cells for various patients in early-stage (Rai 0-1) disease that do not have these FISH defects (Table 4 ). This latter finding indicates that other critical initial genetic defects are likely to be involved in the events that lead to disease progression. Importantly, we did not detect obvious differences in marrow CLL B cells compared with blood CLL B cells drawn from the same patient with respect to FISH defects (data not shown). Patients with germline-type clones seem to fare poorly compared to patients with somatic mutation–type clones.12– 14 However, except for trisomy 12 being more prevalent in germline, and 13q defects more prominent in somatic mutation–type clones, we did not detect any obvious bias for specific FISH defects (Dewald et al, manuscript submitted for publication). The somatic mutation–type clones had a lower incidence of CLL FISH panel detected abnormalities (Table 5 ). Of interest, we did find that low-expressing CD38 clones (< 30% CD38 positive) had fewer FISH defects (~73%) than high-expressing CD38 clones (~93%) (Table 6 ), where more unfavorable genetic defects were found (e.g., 11q–, +12, or 17p) compared with the low-expressing CD38 clones. Finally, we found more favorable genetic defects (e.g., lower trisomy 12 and higher 13q anomalies) in the low-expressing CD38 clones compared with the high-expressing CD38 clones.
A recent cytogenetic study in B-CLL has analyzed survival in relation to the FISH defects.9 Importantly in that landmark study they were able to discern five categories via a statistical model that included 17p deletion, 11q deletion, 12q trisomy, normal karyotype, and 13q deletion as the sole genetic defect. The median survival time is clearly delineated for CLL subgroups based on their specific FISH defect, with the shortest disease duration for 17p patients (32 months) and the longest being 13q deletion status (133 months). Another high risk feature, more advanced disease, was found in the 17p deletion and 11q deletion groups. Importantly, in both our study and the latter report, early-stage disease was not exclusive of finding unfavorable genetic lesions. Thus, genetic abnormalities regardless of clinical stage can be important predictors of disease outcome. Given these findings, it is vital to know when in the course of the disease they occur, what these genetic aberrations code for, and what role these molecule(s) would play in CLL B cell biology. Unfortunately, little is known about these common genetic lesions in B-CLL except for the p53 tumor suppressor gene, which can be altered by 17p13 deletions15 or the ATM gene on 11q23.16
Continued analysis and study of these recurring genetic lesions with newer means of subdividing CLL B cell clones such as Ig variable (V) region status and/or CD38 expression should be instructive. The use of gene arrays has great promise for revealing global gene expression patterns that may be useful in discerning critical progression events.
Gene arrays
Two initial studies last year evaluated gene expression patterns in B-CLL patients.17,18 One study used a “lymphochip” containing almost 18,000 cDNAs in a cohort of 37 patients, of whom 4 had been treated. This study found that B-CLL, irrespective of the Ig mutational status, has a shared gene expression “signature,” which suggests that all B-CLL diseases contain common events in their pathogenesis.18 However, in further analysis of this cohort, it was found that many genes could be identified that separated CLL cases based on immunoglobulin (Ig) mutational status. Of immediate interest, ZAP-70 (a kinase linked to T-cell receptor activation) was found to best discriminate between cases of unmutated (germline) versus mutated (somatic mutation) Ig V gene type B-CLL clones. Finally, they also found that activation genes, detected when B cells are activated via their B cell receptor, were found more commonly in germline versus mutated B-CLL clones. Similar findings in the other study, which used a different gene profiling platform (Affymetrix chips), included a shared gene expression profile for the two types of B-cell clones defined by Ig mutational status. However, a smaller number of genes (around 30) were identified with differential expression that could distinguish Ig somatic mutation–type versus germline-type clones.17 CLL gene profile analysis showed that CLL B cells were most closely related to memory B cells. Specific genes found to be overexpressed included guanine nucleotide exchange factors with implications for the Ras pathway in the pathogenesis of this leukemia. They also found the enhanced expression of mRNA encoding for cytokine (e.g., IL-4R) or chemokine receptors (e.g., CCR7) when compared to normal memory B cells.
Our gene array approach was to compare CLL B cells purified from 38 patients of either somatic mutation–type or germline-type clones to highly purified blood B cells.19 Once again, we found the CLL gene expression signature pattern is distinct from that of normal B cells, but we were not able to clearly distinguish between germline- and somatic mutation–type clones. However, with the use of a novel software program (genes@work, IBM, New York), it was possible to discern gene expression patterns between the two Ig VH clones when factoring in if they were either CD38 high- or low-expression clones. This program also allowed us to further refine the differential gene expression pattern between control B cells and CLL clones so only 81 genes were discriminating. Some of these genes included those coding for LEF1, TGFBR3, and fibromodulin, 2 of which can be implicated in tumorigenesis. Of interest, it was not possible to discriminate between early- and late-stage B-CLL using this approach. Thus, gene array approaches are likely identifying genetic events that occur relatively early in the disease course. Collectively, this approach has many implications for investigation in B-CLL. Discovery of unique markers for B-CLL will be valuable in diagnosis, identification of events that occur in CLL B cells leading to disease progression, and eventually potential therapeutic targets. This would be particularly useful where we find clear discrimination of genetic events between normal cells and CLL B cells.
Immune Defects in B-CLL
While the primary event in B-CLL is the malignant transformation of the CLL B cell, it is likely that progression is linked to an abnormal immune system in the host. Progression of this disease could be related to dysfunctional components of the host immune cellular arm. Early work showed that while absolute T-cell numbers are elevated in untreated B-CLL, there are a large number of immune cell defects.20 These defects affect all cellular components of the immune system, including quantitative and qualitative aspects of the normal B-cell pool, T-cell subsets, natural killer (NK) cells, and dendritic cells. NK cells are deficit in function, as are dendritic cells.21,22 The latter two aspects are likely to have important roles in disease progression, since NK cells are the first line of defense in dealing with malignancy, and dendritic cells are crucial in the induction of an effective cytotoxic T-cell response to tumor cells. One additional factor in the overall dendritic cell capacity for B-CLL patients is the observation that the CLL B cell has a defective antigen-presenting capacity and can render the T cell anergic.23 Thus, blood and splenic CD4 T cells of patients with CLL failed to express surface CD154 after CD3 cross-linking. However, normal T cells exposed to increasing levels of CLL B cells will lose CD154 expression.23 These same authors have shown that with gene transfer of CD40L, the CLL B cell can become a better antigen presenting cell.24 We have shown that T cells in B-CLL have a polarized Th-2 type cytokine profile with increased spontaneous synthesis and secretion of IL-4.25 Since IL-4 is an anti-apoptosis cytokine for CLL B cells, IL-4 creates a favorable microenvironment for the leukemic CLL B cells. In addition, the polarization of Th-2 type cells can result in lesser T-cell cytotoxic capacity and could favor tumor progression.
Does the progression of B-CLL relate to these immune abnormalities? To a certain extent, this question could be answered if we knew when these arrays of immune abnormalities occurred in the course of a specific patient’s illness. Current evidence does suggest that the immune defects accrue with increasing stage.20 The impact of chemotherapy certainly can add to the deficit array of immune abnormalities seen in a given patient. Attention to correction of these immune defects, even in early-stage CLL patients, may yield additional time without morbidity and/or without the need for therapy. Consideration of cytokines such as IL-2, interferon-α/γ, IL-7, and/or IL-12 as immune adjuvants designed to enhance the host cellular resistance to clonal emergence in early-stage disease may be warranted.
Angiogenesis in B-CLL
The impact of neovascularization and angiogenic pathways in B-CLL has only recently begun to be explored. The findings to date suggest that abnormal vascularization exists in the bone marrow and nodes of B-CLL patients and that this is associated with progressive disease.26,27 The etiology of the abnormal blood vessel formation in B-CLL tissue sites is likely related to the spontaneous production by CLL B cells of the proangiogenic factor VEGF. The B-CLL cells are also capable of generating increased amounts of VEGF under hypoxic conditions.27,28 However, there are other proangiogenic factors produced by B-CLL cells, including bFGF.26,28 Since the marrow site is normally under hypoxic conditions, it is likely the in vivo spontaneous VEGF production of CLL cells is augmented by increasing tumor burden. The increased vascularization noted in marrow and nodes of B-CLL patients is highly suggestive that, like solid tumor development, the B-CLL process may depend on abnormal blood flow for disease progression. Evidence for this includes our observations that abnormal marrow angiogenesis is positively associated with increased Rai stage.26 In addition, elevated serum VEGF levels correlate with advanced disease stage.29 However when intracellular levels are measured the lower the VEGF value the poorer the clinical outcome.30 The full angiogenic potential of CLL B cells is revealed by the leukemic CLL B cell ability to produce both pro- and anti-angiogenic factors. This analysis has shown that CLL B cells produce more anti-angiogenic factor (e.g., thrombospondin-1 [TSP-1]) and less VEGF for early-stage disease compared with late stage and for somatic mutation-type versus germline-type clones.28 It would appear that CLL B cells are capable of undergoing “angiogenic switching,” which has been shown in solid tumors to be linked to disease progression. The exact mechanism(s) for this biologic maneuver in B-CLL is not clear, but exposure to hypoxic conditions can induce switching in vitro. In addition, we and others have shown that matrix metalloproteinases or gelatinases (MMP) 9 and 2 are synthesized and secreted by CLL B cells.31 These molecules are crucial in the proteolytic degradation of components of the extracellular matrix (e.g., fibronectin, collagen) leading to enhanced angiogenesis. Recent work has shown that interferons can down-regulate the function of MMP-9 secreted by CLL B cells.31 Further work designed to delineate mechanisms for angiogenic switching in B-CLL cells will surely add to our ability to either treat the disease more effectively or keep the disease in a more stable phase. Finally, analysis of the angiogenic capacity of the CLL B cell has revealed that CLL B cells may utilize the VEGF pathway as an autocrine pathway to increase their resistance to apoptosis. CLL B cells not only produce and secrete VEGF but also possess membrane receptors for VEGFR1 (Flt-1) and VEGFR2 (KDR). We have also shown that at least one of these receptors, VEGFR1, is spontaneously phosphorylated in CLL B cells. Addition of VEGF in vitro significantly increases the ability of a CLL B cell to resist undergoing spontaneous or drug-induced apoptosis. Thus, interruption of this pathway by various mediators (e.g., anti-VEGF or anti-VEGFR antibodies, receptor tyrosine kinase inhibitors) may prove useful in the management of B-CLL. bFGF has also been shown to be associated with increased CLL B cell apoptosis resistance, suggesting that vascular factors play a key role in this important feature of the CLL B cell.32 While the VEGF pathway is likely contributing to the significant resistance to apoptosis seen in CLL B cell clones, there are many redundant mechanisms that regulate this parameter.
Apoptosis
This biologic parameter is a central feature of the leukemic nonproliferating CD5+/CD19+ B cells of B-CLL patients. A full understanding of the pathways related to apoptosis resistance in these cells is critical both in understanding the disease process and in devising unique strategies. There is a growing and very significant body of literature defining the mechanisms of apoptosis in health and disease. Clearly, defects in normal apoptotic machinery contribute in many ways to tumor progression. Any consideration of the apoptosis status in B-CLL needs to consider the existing leukemic cell genetic defects and the surrounding microenvironment (e.g., stromal cells, vascular supply), which modifies these genetic events, since this interaction could lead to disease progression. The latter feature is underscored by the dramatic induction of in vitro apoptosis for the long-lived CLL B cell when taken out of the host. This section will be limited to consideration of CLL B cell apoptosis as it relates to both autocrine pathways and cellular interactions believed to be linked to disease progression in B-CLL. In addition, how the knowledge of these events can be used as new therapeutic intervention points will be considered.
Early-stage B-CLL often has expanded polyclonal T cells in blood;20 in specific, it is known that the T-helper (CD4+) cell is frequently found in infiltrated nodes and marrow.33,34 Since cytokines elaborated by T cells, such as IL-4 and interferon (α and γ), are able to increase CLL B cell apoptosis resistance, the intimacy of these 2 cells could work to the advantage of the CLL B cell. CD4+ T cells may be able to regulate disease progression by virtue of a CD40-CD40L interaction where survivin is induced in CLL B cells. Survivin is an inhibitor of apoptosis that can also modulate cell proliferation. Thus, this type of interaction can result in the increase of survivin-positive CLL B cells.35 These survivin-positive CLL B cells tend to have higher proliferative rates and increases in Bcl-2,35 and ultimately this interaction may be related to disease progression.
Other cellular components that may be contributing to apoptosis and disease progression are both the dendritic cell and the bone marrow stromal cell. Studies of marrow stromal cells in B-CLL show that the leukemic CLL B cell once bound to stromal cells is further protected from cell death.36 Of interest, patients in the early-stage phase of the CLL disease process can have CLL cells found in close association with follicular dendritic cells, suggesting that this interaction could facilitate disease progression.37 Definition of the surface membrane determinants that lead/facilitate these cellular interactions could be helpful in delaying disease progression.
More recently, a nurse like cell has been described in the blood of B-CLL patients, and this cell is able to confer significant increases in apoptosis resistance to CLL B cells.38,39 The exact mechanism of resistance appears to be linked to elaboration of SDF-1, which binds to its cognate receptor CXCR4. Given that these cellular aspects exist in early-stage disease, interruption of the cellular interactions between CLL B cells and these respective elements represents a candidate for therapeutic intervention. If this can be done with minimal toxicity, then it may be feasible to consider using these interventions in early-stage patients to prevent progression. The redundancy of CLL B cell apoptosis resistance is further underscored by our recent finding of a unique pathway mediated by a family of molecules related to tumor necrosis factors designated as April//Blys.40
Finally, a list of cytokines and their respective receptors found on CLL B cells are featured in Table 7 . Clearly, the microenvironmental influence on a CLL B cell clone will be strongly related to the cell types and the nature of the cytokines secreted by those cell types in the immediate area. Issues in the future will be to know which of these cytokines predominate in the CLL B cell involved tissue sites and which of these cytokines have the most impact on CLL B cell viability. In addition, it will be important to know if these CLL B cells acquire autocrine pathways sequentially and if these acquired pathways relate to disease progression. Importantly, each of these cytokine pathways provides an opportunity for therapeutic interventions. Thus, we have shown that a pseudomonas exotoxin-conjugated IL-4 molecule41 is able to kill a significant percentage of CLL B cells (data not shown). Since the IL-4 receptor is not as well expressed in other tissue sites, this approach has the advantage of working in a selective fashion as well.
Summary
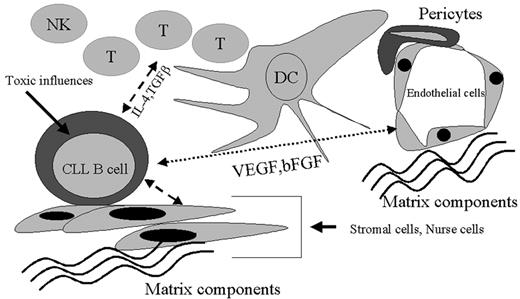
This discussion has attempted to highlight the emerging areas of biology that will help us understand disease progression in B-CLL. Clearly, this is not going to be one simple equation but will likely involve the use of complex models for developing the best formulae to help predict progression. Figure 5 outlines a schematic model of the interplay between CLL cells and their microenvironment. While genetic events in the transformation of this B cell are crucial to the disease process, this malignant cell has the potential to have multiple points of interaction with its neighbors and the secreted molecules of the resident cells. Understanding the dynamics of this leukemic cell alone and within the framework of the tissue milieu will ultimately lead us to both more accurate predictions for progression and, we hope, better therapies.
III. To Treat or Not to Treat the Patient with CLL: That Is the Question
John C. Byrd, MD,*
D. Warren Brown Professor of Leukemia Research, Director of Hematologic Malignancies, Division of Hematology-Oncology, Starling Loving Hall, Room 302, The Ohio State University, Columbus, OH 43210
Division of Hematology-Oncology, Department of Medicine, The Ohio State University
Dr. Byrd has received support for data management of trials for Genentech, IDEC, Berlex Protein Design Labs.
This work in part supported by the National Cancer Institute (P01 CA81534-02, P30 CA160058, and CA98099), The Sidney Kimmel Cancer Research Foundation, The Leukemia and Lymphoma Society of America, and The D. Warren Brown Foundation.
Therapeutic Intervention for CLL
CLL is one of the most common types of adult leukemia, with registry data suggesting that 7000 patients are diagnosed and 4500 die related to this disease each year in the United States.1 As the most common leukemia, virtually all oncologists, hematologists, and internists will care for patients with CLL. Important issues in CLL care can be divided by four junctures met during the course of each patient’s disease: (1) making the definitive diagnosis of CLL and determining individual patient prognosis; (2) determining when to treat the CLL; (3) determining how to initially treat the CLL; and (4) determining a salvage regimen suitable for an individual patient’s case in relapse. Herein we focus entirely on the initial diagnosis and the initial treatment of CLL. For further discussion of salvage therapy, the reader is referred to a recent review.2
Now That I Have CLL, What Does It Mean to Me?
At initial presentation, the great majority (80-90%) of patients with CLL will be asymptomatic. In this setting, the first question is how will this disease and its associated complications impact the patient’s well being? A major focus of research in CLL over the past 3 decades has been to examine clinical and laboratory features that predict which patients will develop symptomatic disease. (reviewed in ref. 3) As with other cancers, outcome of CLL is determined by staging. Patients with CLL are staged utilizing either the Rai or Binet system as summarized in Table 8 .4,5 Both of these discriminate CLL by the sites of disease and/or degree of cytopenias induced by leukemic marrow replacement. Patients can be categorized into three groups based on these features. According to the modified Rai criteria, patients in the low-risk group (Stage 0) have lymphocytosis without any other abnormality; patients in the intermediate-risk group have, in addition to their lymphocytosis, enlarged lymph nodes (Stage I) and/or spleen (Stage II); and patients in the high-risk group have anemia (hemoglobin < 11.0 g/dL, Stage III) and/or thrombocytopenia (platelets < 100 × 109/L, stage IV). The Binet staging system discriminates outcome based upon the number of sites involved for low and intermediate risk (Stage A patients have < 3 lymphatic regions involved, Stage B have 3 or more lymphatic regions involved) while advanced Stage C patients have anemia (hemoglobin < 10.0 g/dL) or thrombocytopenia (platelets < 100 x 109/L). Subsequent studies by several groups have further dissected therapeutic outcome of early stage CLL and have identified a group that can be considered to have smoldering CLL. While different criteria are utilized,6–,8 these patients generally have Binet Stage A disease, non-diffuse bone marrow involvement, a lymphocyte count of < 30 × 109/L, hemoglobin > 13 g/dL, and lymphocyte doubling time greater than 12 months. It is very unusual for patients to have diffuse bone marrow involvement with the other features listed (less than 2%) resulting in many practitioners (including the authors) not evaluating bone marrow morphology at diagnosis. In these patients, the risk of progression to symptomatic CLL is low (14-17%) at 5 years, and clinical outcome in this group is the same as the age-matched control population.6– 8
Nonetheless, both staging systems fail to consider factors such as age and recently identified genetic or other molecular markers as prognostic features. A better understanding of these recently identified features will allow improved decisions regarding the appropriate initial intervention. These prognostic features are outlined in Sections I and II. These newer prognostic features and our developing understanding of CLL as a disease in evolution has re-opened the question of the role of treatment in early stage disease for patients with poor prognostic features.
I’m 55 Years Old and Too Young to Have This Diagnosis, What Does It Mean to Me?
CLL is generally a disease of the elderly, with a median age at diagnosis exceeding 60 years. Only 10% of patients diagnosed with CLL are under the age of 50 and 1-2% are younger than 40. Several series have examined clinical features of younger (age < 50-55) CLL patients and have consistently noted a higher male predominance.9–,12 In some series a higher frequency of Richter’s transformation has been noted.9,10 The stage distribution of patients at diagnosis is similar between younger and elderly patients across all series reported and the Rai and Binet staging systems are effective at predicting clinical outcome. While younger patients have an improved survival when compared to older patients with CLL, their median survival was noted to be 12.3 years in one series whereas age-matched controls had a life expectancy of 31.2 years.12 In another series,9 CLL patients under age 55 had an expected 10-year survival of 45.3% versus the 96.2% survival in the control population. In this second series, only 40% of the younger patients remained without treatment whereas 49% of the elderly patients had required therapy. In each of the large reported series, only patients with smoldering CLL appear relatively unaffected by their disease, with a 94% 12-year survival in one series9 and an 85% 14-year predicted survival in another series.12 Thus, early treatment should not be considered for young patients with smoldering CLL until some evidence of progression occurs (i.e., patient no longer qualifying as smoldering CLL), but consideration of early intervention trials with the goal of cure would appear to be of most relevance to younger patients where loss of absolute life expectancy is greatest.
When to Treat CLL?
Clinicians caring for patients with CLL must focus upon the most critical clinical juncture, that being when to initiate treatment. In the absence of symptoms, observation without therapy is the current standard of care. This is based on mature data from seven different studies demonstrating no improvement in overall survival for symptomatic CLL patients receiving early therapeutic intervention with chlorambucil therapy (reviewed in 13). A meta-analysis13 that included data from six of these trials and outcomes of 2048 patients demonstrated a slightly higher death rate (42.6%) among those treated early versus those randomized to deferred therapy (41.6%), although this difference was not statistically significant. Deaths attributable to secondary malignancies were similar in both groups,13,14 despite one early report15 noting a higher risk of epithelial malignancies in patients receiving early intervention. It must be noted that in each of these studies, a large proportion of patients with smoldering or early stage disease were included. For this population of patients, therapeutic benefit would have to improve overall survival in excess of that observed in the age-matched control population without CLL. Furthermore, each of these studies employed chlorambucil, an agent that yields a low complete response rate. With more effective therapies and use of risk stratification, re-consideration of this approach in well-designed trials seems quite prudent. This is further substantiated by the now recognized genetic clonal evolution of CLL that occurs over time from initial diagnosis.16–,19 This clonal evolution coincides with the CLL cells becoming more resistant to apoptosis.20 Efforts within the German CLL Study Group and several US groups are underway to examine this question with newer CLL therapies.
Determining what constitutes sufficient symptomatology to initiate treatment in CLL is subjective and confounds cross-interpretation of clinical trials. To assist clinicians and assure uniform study entrance criteria, an NCI sponsored Working Group on CLL21 established guidelines for initiation of treatment. These indications include the presence of non-autoimmune cytopenias (Rai Stage III and IV), symptomatic lymphadenopathy or hepatosplenomegaly, disease-related B-symptoms or fatigue, extreme lymphocytosis (greater than 150-300 × 109/L), and autoimmune hemolytic anemia or thrombocytopenia not controlled with steroids. Incorporation of the patient’s individuality and associated co-morbid problems must also be considered. In this manner, it is imperative for the hematologist to critically question if the symptoms are from CLL or another unrelated diagnosis. For instance, fatigue might be reflective of underlying cardiovascular disease, sleep apnea, depression, hypothyroidism, or secondary malignancy. Careful assessment of the CLL patient as an internist first and then as a hematologist will assure treatment is not initiated for a source unrelated to the underlying disease. Indeed, the decision of when to start therapy remains one of the most challenging issues physicians caring for patients with CLL have to make.
How to Treat Symptomatic CLL?
There has been considerable debate regarding what is the best initial therapeutic approach for symptomatic CLL. CLL virtually always presents as a systemic disease and is generally treated with chemotherapy. Until recently, the goal of therapy has been one of palliation only, without hope of complete remission of the CLL. Introduction of new therapies outlined below and novel combination approaches has begun shifting the therapeutic goal of CLL to that of attaining a complete remission as defined by a recent consensus paper on response evaluation in CLL.21 A summary of different classes of therapies employed for the treatment of previously untreated, but symptomatic CLL patients follows below.
Alkylator therapy
The initial treatment of patients with symptomatic CLL has often involved therapy with chlorambucil. While this therapy is effective at palliating the majority of CLL patients, no randomized studies have established its benefit relative to overall survival as compared to observation alone. Prednisone is often included with chlorambucil despite three randomized studies not demonstrating a survival advantage to its addition (reviewed in 13). Furthermore, a meta-analysis that included 424 patients from three separate trials randomized to receive chlorambucil versus chlorambucil and prednisone could not demonstrate a survival benefit.13 Prednisone and other corticosteroids as a single agent have a minimal response rate in CLL, predispose to opportunistic infections, and can accentuate hyperleukocytosis.22 Outside of supportive therapy for autoimmune complications, there is little data to support including corticosteroids in the initial treatment of CLL. Since the introduction of chlorambucil, a variety of studies have examined different schedules and dose intensities in previously untreated CLL with varied results. The most promising regimen was a high-dose regimen (approximately 5 times the usual dosing) reported by Jaksik and colleagues.23 A fixed 15 mg daily dose of chlorambucil was administered until toxicity or complete remission. A control arm received standard dose chlorambucil. An alternative response assessment different than the NCI criteria was utilized that did not require bone marrow evaluation. After attainment of CR, patients received a twice-weekly dose of 15 mg of chlorambucil for 3 years. Even without the ability to directly compare these results to other regimens that used the NCI response criteria,21 this regimen was impressive. The treatment arm showed significantly higher CR rate (70% versus 31%, P < 0.0001) and overall survival (P < 0.005) as compared to the control arm where patients received 75 mg of chlorambucil monthly for 6 cycles followed by maintenance as outlined above. Hematological toxicity was manageable with rapid dose reduction for cytopenias. A subsequent Phase III study by this same group has demonstrated this high dose chlorambucil regimen to be superior to mini-CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone)24 and to have a similar toxicity and efficacy profile as fludarabine.25 The major limitation to building upon this regimen with other active agents is myelosuppression.23–,25 However, the lack of cellular and humoral immune suppression observed with chlorambucil as compared with the nucleoside analogs or Campath-1H make this regimen the most promising for investigations incorporating immune based therapy (i.e., vaccines). Specific details of chlorambucil dosing and use for CLL patients have been reported.26
Combination chemotherapy not including purine analogs
A variety of different combination regimens used in non-Hodgkin’s lymphoma such as mini-CHOP, CAP (cyclophosphamide, doxorubicin, and prednisone), COP (cyclophosphamide, vincristine, and prednisone), and chlorambucil + epirubicin have been examined for efficacy against chlorambucil-based regimens in previously untreated CLL (reviewed in 13). These studies have not demonstrated the superiority of combination-based approaches over chlorambucil regimens with respect to prolongation of survival. Differentiating the frequency of complete response rates in each of these studies is quite difficult because the NCI 96 criteria21 were not employed. Additionally, most studies did not require confirmatory bone marrow biopsies at completion of therapy or did not report how many CR patients had residual nodules (PR by modified NCI 96 criteria). A meta-analysis of these studies has been reported as well that corroborates the lack of benefit of these NHL therapies for CLL.13
Purine analog therapy
The introduction of the purine analogs during the mid-1980s invigorated research in CLL. These agents include the adenosine deaminase inhibitor pentostatin and fludarabine and cladribine, which are purine analogs resistant to deamination by adenosine deaminase (but not inhibitors of this enzyme). Fludarabine has been the most extensively studied agent in previously untreated CLL. Three large Phase III trials27–,29 in symptomatic untreated CLL patients have been published comparing fludarabine to chlorambucil, chlorambucil combined with fludarabine, or alternative combination therapies as outlined in Table 9 . Results from these studies have noted a higher response rate with prolongation of progression-free survival with fludarabine as compared to chlorambucil or CAP.27–,29 Myelosuppression and infections were slightly increased with fludarabine as compared to the other chemotherapy approaches. The relative benefit of fludarabine over mini-CHOP chemotherapy was similar as measured by response and response duration.29 No survival advantage was noted in any of these studies, but all three studies had provision (either on or off study) to receive fludarabine as second line therapy if the alternative treatment was not effective. For the French CLL Cooperative Group randomized study, a quality of life analysis demonstrated an advantage to fludarabine therapy.30 Based upon these studies, fludarabine remains the standard for comparison in randomized studies of newer therapy. Supportive considerations related to administering fludarabine have been reviewed.26
The use of alternative purine analogs (cladribine or pentostatin) in the treatment of CLL has been more limited. Pentostatin as a single agent had less phase II activity in a small Phase II trial performed by the CALGB.31 Phase II studies with cladribine in previously untreated CLL patients have demonstrated response rates similar to or inferior to fludarabine.32 One randomized Phase III study examined cladribine and prednisone versus chlorambucil and prednisone in symptomatic, previously untreated CLL patients.33 This study of 229 patients demonstrated a superior CR rate, overall response rate, and progression-free survival with cladribine and prednisone.33 Infections and neutropenia were more common with the cladribine and prednisone regimen. Surprisingly, opportunistic infections were not problematic when cladribine and prednisone were utilized together,33 which differs from that reported with the combination of fludarabine and prednisone.34 Other nucleoside analogs such as clofarabine are currently entering clinical trials in previously treated CLL patients and may offer advantages over those pursued to date.
Fludarabine combination therapies
A variety of combination chemotherapy studies with the purine analogs have yielded mixed results. Preclinical data35 suggest synergistic interaction between DNA damaging agents and the purine analogs. Such synergy likely occurs as a consequence of alkylator-induced DNA damage and subsequent inhibition of DNA repair by the purine analog. Combination studies with alkylating agents and each of the purine analogs have been performed.2,36 With respect to fludarabine, these studies demonstrated that myelosupression was more problematic and compromised the total administered dose of each agent. This was particularly true with chlorambucil, where significant myelosuppression was observed and increased toxicity prompted early discontinuation of this therapeutic arm in the large US intergroup trial.28 Three recent phase II reports37–,39 have demonstrated the feasibility of combining fludarabine with cyclophosphamide with or without filgrastim support as summarized in Table 9. These two therapeutic regimens are now being compared in ongoing parallel Phase III studies by the US and German CLL intergroup. A fourth study,40 also summarized in Table 9, utilized oral fludarabine combined with cyclophosphamide. Unfortunately, the oral form of fludarabine is not available in many countries, including the United States, and will therefore have limited use in clinical trials outside of Europe. Determining the benefit of cyclophosphamide as measured by improved CR rate will be important in deciding how to add antibody therapies to current CLL regimens. Combination studies incorporating cyclophosphamide or other alkylator agents with cladribine have noted significant myelosuppression and infectious morbidity. Combination of the less myelosuppressive agent pentostatin with chlorambucil and prednisone in one Phase II study of untreated CLL patients noted an overall response rate of 87%, including a 44% complete response rate.41 Opportunistic infections were problematic in this study, likely as a consequence of the corticosteroids in the absence of antimicrobial and antiviral prophylaxis.
Without new combination studies of cladribine, clofarabine, or pentostatin in previously untreated CLL patients, it is likely that future studies performed by large cooperative groups will focus on adding monoclonal antibodies to fludarabine containing regimens.
Campath-1H
This antibody is a humanized anti-CD52 antibody whose antigen is expressed on greater than 95% of mature B and T lymphocytes. Studies with Campath-1H in untreated and heavily pre-treated CLL have yielded promising results leading to its approval in May 2001 for fludarabine-refractory CLL (reviewed in 42). Across all of the studies performed in CLL it is clearly demonstrated that Campath-1H preferentially eliminates CLL tumor cells from the blood, bone marrow, and spleen. Responses in nodal sites of disease occur but almost always are partial responses and diminish in proportion to the size of the lymph nodes. Common toxicities observed with Campath-1H include self-limited cytokine release syndrome, immunosuppression, and neutropenia. This immunosuppression can be quite severe when Campath-1H is administered at high doses or for a prolonged period, resulting in a high frequency of opportunistic infections.
One large study of Campath-1H monotherapy in previously untreated CLL patients has been reported.43 This trial administered Campath-1H subcutaneously and noted a 19% CR rate and an 87% overall response rate with effective tumor elimination in nodal sites of involvement as well. In this study subcutaneous administration of Campath-1H greatly diminished the infusion toxicity observed with this drug. However, infectious complications were still noted with 10% of patients reactivating cytomegalovirus. Three other Phase II studies ongoing or recently completed by the M.D. Anderson Cancer Center, the Cancer and Leukemia Group B (CALGB), and the CLL Research Consortium have administered Campath-1H in an abbreviated schedule following induction therapy with fludarabine-based therapies to eliminate minimal residual disease. Only one of these has been reported and noted efficacy in terms of improving final response but significant infectious morbidity in patients receiving this regimen.44 Because of the potential immunosuppression associated with Campath-1H, it is likely that future studies with this antibody will focus on elimination of minimal residual disease. A Phase III trial by the German CLL study group is currently examining the benefit of such therapy after induction therapy with fludarabine or fludarabine and cyclophosphamide.
Rituximab
This is an anti-CD20 chimeric monoclonal antibody. Rituximab administered using a NHL schedule (reviewed in 45) had little activity in previously treated CLL patients. However, two Phase II studies have subsequently demonstrated that dose escalation with the weekly NHL schedule46 or more frequent (thrice weekly) administration47 increases the clinical response significantly with minimal toxicity as compared to that observed with cytotoxic chemotherapy. In contrast, two studies48,49 have demonstrated that weekly administration of rituximab as administered to NHL patients has significant activity in previously untreated CLL patients but a low CR rate as observed with Campath-1H.43 Unlike Campath-1H, rituximab has less myelosuppression and potential for cellular immune suppression and is therefore a good candidate to combine concurrently with other active agents in CLL. In addition, it has been demonstrated that rituximab monotherapy down-regulates the anti-apoptotic proteins mcl-1 and XIAP expression in CLL cells in vivo,50 thus offering enhanced response to fludarabine-based therapy. Given the low complete response rates with rituximab monotherapy in both previously untreated and treated CLL, this antibody will likely make its greatest contribution in CLL with combination treatment strategies.
Two recent studies have combined rituximab with fludarabine-based therapies in previously untreated CLL, as summarized in Table 9.51,52 The first randomized Phase II study was undertaken by the CALGB and has recently been reported.51 Patients were randomized to receive either 6 monthly courses of fludarabine concurrent with rituximab followed 2 months later by 4 weekly doses of rituximab for consolidation therapy or sequential fludarabine monotherapy followed 2 months later by rituximab consolidation therapy. One hundred four patients were randomized to the concurrent (n = 51) or sequential (n = 53) regimens. During the induction portion of treatment, patients receiving the concurrent regimen experienced more grade 3 or 4 neutropenia (74% versus 41%) and grade 3 or 4 infusion-related toxicity (20% versus 0%) as compared to the sequential arm. The consolidation rituximab therapy was tolerated well in both arms. All other toxicities including infections were similar between the two arms. The overall response rate with the concurrent regimen was 90% (47% complete response [CR], 43% partial response [PR]; 95% confidence interval [CI] .82, .98) compared to 77% (28% CR, 49% PR; 95% CI .66, .99) with the sequential regimen. With a median follow-up time of 23 months, the median response duration and survival have not been reached for either regimen. No unexpected long-term toxicities have been observed with this regimen.
A second study undertaken by the M.D. Anderson group in previously untreated CLL patients has added rituximab (375 mg/m2 with the first cycle and 500 mg/m2 for subsequent cycles) to a slightly attenuated dose of fludarabine (25 mg/m2 IV, days 1-3) and cyclophosphamide (250 mg/m2 IV, days 1-3) for 6 cycles.52 In a preliminary report of 60 patients who completed treatment, a 66% CR rate was noted and 95% overall response was noted. Furthermore, 22 of 37 (59%) patients in CR had no evidence of minimal residual disease by PCR detection. Toxicity was quite acceptable other than a higher frequency of neutropenia as compared to previous studies by the same group with fludarabine and cyclophosphamide alone. The results of both the CALGB study and the M.D. Anderson Study are promising and emphasize the importance of both the US intergroup study (E2297) and the German study group Phase III studies examining the addition of cyclophosphamide to fludarabine in previously untreated CLL patients. If these studies are positive, it is likely that future Phase III studies incorporating rituximab will use fludarabine and cyclophosphamide as the standard therapy. However, in the absence of positive studies, omission of cyclophosphamide will likely occur.
Other antibodies that target CLL cells
Several other monoclonal antibodies are currently under clinical evaluation in the treatment of CLL and low-grade NHL as outlined in Table 10 and likely will be explored soon in previously untreated CLL.44 Hu1D10 is a humanized monoclonal antibody that targets a β-chain epitope of HLA-DR and appears to induce apoptosis via a novel pathway as compared to other monoclonal antibodies. IDEC-152 is a primatized monoclonal antibody that targets CD23 and appears to accentuate apoptosis induced by rituximab in CLL. Epratuzumab targets CD22, an antigen variably expressed on CLL. Finally studies using radioimmunotherapy using Yttrium-90 Ibritumomab Tiuxetan as consolidation therapy are currently underway at the M.D. Anderson Cancer Center. Following Phase III testing of rituximab and Campath-1H combinations, studies with these naked and radio-conjugated antibodies will likely be the next series of agents to be incorporated in Phase III studies.
Stem cell transplantation
Several Phase II studies have demonstrated the feasibility of doing autologous stem cell transplants in CLL patients, which have recently been reviewed.53 These studies have generally noted improved results when patients were transplanted in complete remission or with minimal residual clinically detectable disease and could be converted to a PCR negative status.54 A randomized Phase III trial in Europe is currently testing the efficacy of autologous bone marrow transplant following initial cytoreductive therapy. The role of standard allogeneic stem cell transplant in CLL is much less defined, except for the very young patient with refractory disease, due to the high treatment-related morbidity. Less toxic preparative regimens (i.e., mini transplants) that induce significant immunosuppression to allow engraftment, but do not produce significant regimen related morbidity, have broadened the use of allogeneic stem cell transplant in CLL.55 Patients not attaining a complete remission with initial therapy or having high risk genetic abnormalities [i.e., del(11q22-q23), del(17p13), unmutated somatic VH gene status, and p53 mutations] should be considered good candidates for early application of this modality on well designed clinical trials.
Other Approaches
The recognition of important intracellular targets that inhibit CLL cell apoptosis and other cytokines, chemokines, and angiogeneic peptides that provide a favorable environment for CLL cells to survive provides unique opportunities to eliminate residual disease following administration of initial CLL therapy. Furthermore, even in the absence of detectable minimal residual disease, such an intervention might prevent re-growth of CLL. Major advances in using sophisticated computerized modeling to predict the chemical structure of small molecules that will inhibit these intraceullar targets have occurred and these agents are now entering the clinic. In addition, significant advances in the technical aspects of developing humanized monoclonal antibodies have occurred and there are now many agents (see Table 10) that target and neutralize cytokines, chemokines, or angiogeneic peptides and that are available for clinical trials. As the next decade of CLL studies is initiated, we must now consider rational application of therapeutic approaches that either treat minimal residual disease or prevent its emergence following effective cytoreductive therapy outlined above. Such application in clinical trials applies not only to patients receiving therapy for symptomatic CLL, but also to patients with early asymptomatic disease with poor prognostic features. Such a therapeutic approach to completely eliminate detectable CLL combined with continued laboratory investigation will provide the best opportunity to alter the natural history of this disease.
Immunophenotype of B-cell chronic lymphocytic leukemia (B-CLL) and lymphomas that resemble it.
| Antigen . | B-CLL . | Mantle Cell Lymphoma . | SLVL . | Follicular Lymphoma . |
|---|---|---|---|---|
| Abbreviations: sIg , surface immunoglobulin; SLVL, splenic lymphoma with villous lymphocytes. | ||||
| sIg | Weak | ++ | ++ | ++ |
| CD5 | ++ | ++ | – | – |
| CD19 | ++ | ++ | ++ | ++ |
| CD20 | + | ++ | ++ | ++ |
| CD22 | Weak or – | ++ | ++ | ++ |
| CD23 | ++ | – | – | – |
| CD79b | Weak or – | ++ | ++ | ++ |
| FMC7 | – | ++ | ++ | ++ |
| CD10 | – | – | – | ++ |
| Antigen . | B-CLL . | Mantle Cell Lymphoma . | SLVL . | Follicular Lymphoma . |
|---|---|---|---|---|
| Abbreviations: sIg , surface immunoglobulin; SLVL, splenic lymphoma with villous lymphocytes. | ||||
| sIg | Weak | ++ | ++ | ++ |
| CD5 | ++ | ++ | – | – |
| CD19 | ++ | ++ | ++ | ++ |
| CD20 | + | ++ | ++ | ++ |
| CD22 | Weak or – | ++ | ++ | ++ |
| CD23 | ++ | – | – | – |
| CD79b | Weak or – | ++ | ++ | ++ |
| FMC7 | – | ++ | ++ | ++ |
| CD10 | – | – | – | ++ |
Summary of B-cell chronic lymphocytic leukemia (B-CLL) panel fluorescence in situ hybridization (FISH) test.
| Probes (Chromosome Locus) . | Upper Limit of Normal Ranges, %† . | ||||
|---|---|---|---|---|---|
| Genetic Anomaly . | Spectrum Orange . | Spectrum Green . | Deletion . | Trisomy . | Translocation . |
| Abbreviations: NA, not applicable. | |||||
| †Based on analysis of blood from 20 normal individuals and a one-sided 95% confidence interval for observing the maximum number of nuclei for each false-positive signal pattern seen in 200 scorable nuclei using the binomial distribution. | |||||
| 6q | C-MYB (6q23) | D6Z1 (6 centromere) | 6.5 | 1.5 | NA |
| 11q | ATM (11q23) | D11Z1 (11 centromere) | 5.0 | 1.5 | NA |
| +12 | D12Z3 (12 centromere) | MDM2 (12q15) | 7.5 | 1.5 | NA |
| 13q-x1 | D13S319 (13q14) | D13S327 (13qter) | 7.0 | 5.0 | NA |
| 13q-x2 | D13S319 (13q14) | D13S327 (13qter) | 1.5 | 5.0 | NA |
| 17p | P53 (17p13.1) | D17Z1 (17 centromere) | 8.5 | 1.5 | NA |
| t(11;14) | CCND1 (11q13) | IgH (14q32) | NA | NA | 2.5 |
| Probes (Chromosome Locus) . | Upper Limit of Normal Ranges, %† . | ||||
|---|---|---|---|---|---|
| Genetic Anomaly . | Spectrum Orange . | Spectrum Green . | Deletion . | Trisomy . | Translocation . |
| Abbreviations: NA, not applicable. | |||||
| †Based on analysis of blood from 20 normal individuals and a one-sided 95% confidence interval for observing the maximum number of nuclei for each false-positive signal pattern seen in 200 scorable nuclei using the binomial distribution. | |||||
| 6q | C-MYB (6q23) | D6Z1 (6 centromere) | 6.5 | 1.5 | NA |
| 11q | ATM (11q23) | D11Z1 (11 centromere) | 5.0 | 1.5 | NA |
| +12 | D12Z3 (12 centromere) | MDM2 (12q15) | 7.5 | 1.5 | NA |
| 13q-x1 | D13S319 (13q14) | D13S327 (13qter) | 7.0 | 5.0 | NA |
| 13q-x2 | D13S319 (13q14) | D13S327 (13qter) | 1.5 | 5.0 | NA |
| 17p | P53 (17p13.1) | D17Z1 (17 centromere) | 8.5 | 1.5 | NA |
| t(11;14) | CCND1 (11q13) | IgH (14q32) | NA | NA | 2.5 |
Fluorescence in situ hybridization (FISH) defects in stable versus progressive B-cell chronic lymphocytic leukemia (B-CLL) patients.
| CLL Status . | Total No. of Patients . | Normal FISH† . | Abnormal FISH† . | Percentage Abnormal . |
|---|---|---|---|---|
| * Stable implies any Rai stage but not requiring therapy. | ||||
| ** Progressive implies any Rai stage but requiring therapy. | ||||
| †Numbers of stable or progressive patients with normal or abnormal FISH studies. | ||||
| Stable* | 56 | 19 | 37 | 66 |
| Progressive** | 57 | 7 | 50 | 88 |
| Total | 113 | 26 | 87 | 77 |
| CLL Status . | Total No. of Patients . | Normal FISH† . | Abnormal FISH† . | Percentage Abnormal . |
|---|---|---|---|---|
| * Stable implies any Rai stage but not requiring therapy. | ||||
| ** Progressive implies any Rai stage but requiring therapy. | ||||
| †Numbers of stable or progressive patients with normal or abnormal FISH studies. | ||||
| Stable* | 56 | 19 | 37 | 66 |
| Progressive** | 57 | 7 | 50 | 88 |
| Total | 113 | 26 | 87 | 77 |
Comparison of purified clonal CD5+/CD19+ versus fluorescence in situ hybridization (FISH) anomalies in eight patients with stable disease.*
| Patient . | Genetic Anomaly† . | CD5+/CD19+, %* . | Abnormal FISH Nuclei, % . | Delta‡ . |
|---|---|---|---|---|
| * CD5+/CD19+ cells were sorted by magnetic bead methods, and their purity was confirmed by flow cytometry. | ||||
| †Two anomalies are indicated by a “/” meaning that 1 clone has both genetic defects. | ||||
| ‡Delta is difference between percentage of purified clonal CLL B cells and numbers of FISH abnormal cells. | ||||
| 1 | 11q/13q | 98 | 70/92 | 28/6 |
| 2 | 13q | 99 | 92 | 7 |
| 3 | 13q | 97 | 89 | 8 |
| 4 | 13q | 96 | 83 | 13 |
| 5 | +12/17p | 98 | 59/71 | 39/27 |
| 6 | +12 | 99 | 65 | 34 |
| 7 | 13q | 95 | 40 | 55 |
| 8 | 13q | 96 | 23 | 73 |
| Patient . | Genetic Anomaly† . | CD5+/CD19+, %* . | Abnormal FISH Nuclei, % . | Delta‡ . |
|---|---|---|---|---|
| * CD5+/CD19+ cells were sorted by magnetic bead methods, and their purity was confirmed by flow cytometry. | ||||
| †Two anomalies are indicated by a “/” meaning that 1 clone has both genetic defects. | ||||
| ‡Delta is difference between percentage of purified clonal CLL B cells and numbers of FISH abnormal cells. | ||||
| 1 | 11q/13q | 98 | 70/92 | 28/6 |
| 2 | 13q | 99 | 92 | 7 |
| 3 | 13q | 97 | 89 | 8 |
| 4 | 13q | 96 | 83 | 13 |
| 5 | +12/17p | 98 | 59/71 | 39/27 |
| 6 | +12 | 99 | 65 | 34 |
| 7 | 13q | 95 | 40 | 55 |
| 8 | 13q | 96 | 23 | 73 |
Ig V gene status and fluorescence in situ hybridization (FISH) anomalies (n = 62).
| . | Somatic Mutation . | Germline Mutation . | ||
|---|---|---|---|---|
| FISH Results . | Patients . | % . | Patients . | % . |
| Normal | 12 | 35.3 | 7 | 27 |
| Abnormal | 22 | 64.7 | 21 | 75 |
| . | Somatic Mutation . | Germline Mutation . | ||
|---|---|---|---|---|
| FISH Results . | Patients . | % . | Patients . | % . |
| Normal | 12 | 35.3 | 7 | 27 |
| Abnormal | 22 | 64.7 | 21 | 75 |
CD38 Expression and fluorescence in situ hybridization (FISH) anomalies (n = 105).
| . | CD38 Positive* . | CD38 Negative* . | ||
|---|---|---|---|---|
| FISH Results . | Patients . | % . | Patients . | % . |
| * CD38 expression is considered positive if greater than 30% of CLL B cells are CD38+. | ||||
| Normal | 2 | 7 | 21 | 27 |
| Abnormal | 26 | 93 | 56 | 73 |
| . | CD38 Positive* . | CD38 Negative* . | ||
|---|---|---|---|---|
| FISH Results . | Patients . | % . | Patients . | % . |
| * CD38 expression is considered positive if greater than 30% of CLL B cells are CD38+. | ||||
| Normal | 2 | 7 | 21 | 27 |
| Abnormal | 26 | 93 | 56 | 73 |
Cytokines and their receptors on chronic lymphocytic leukemia (CLL) B cells.
| Cytokine† . | Cytokine Receptors‡ . | Biologic Effect§ . |
|---|---|---|
| †Cytokines have been shown to be synthesized and secreted by CLL B cells. | ||
| ‡Receptors specific for the respective cytokine have been found on CLL B cell membranes. | ||
| §Functional impact of each cytokine on the clonal CLL B cell population. | ||
| Abbreviations: IL, interleukin; TNF, tumor necrosis factor; TGF, tumor growth factor; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor. | ||
| IL-2 | Present | Inhibits apoptosis |
| IL-4 | Present | Inhibits apoptosis |
| IL-8 | Present | Inhibits apoptosis |
| IL-10 | Present | Induces apoptosis |
| TNFα | ? | Inhibits apoptosis |
| TGFβ | Present | Induces apoptosis |
| Interferon α/γ | Present | Inhibits apoptosis |
| VEGF | Present (both VEGFR1 and VEGFR2) | Inhibits apoptosis |
| bFGF | ? | Induces drug resistance |
| CXCR4 | Present | Stimulates chemotaxis |
| CXCR3 | Present | Unknown |
| Cytokine† . | Cytokine Receptors‡ . | Biologic Effect§ . |
|---|---|---|
| †Cytokines have been shown to be synthesized and secreted by CLL B cells. | ||
| ‡Receptors specific for the respective cytokine have been found on CLL B cell membranes. | ||
| §Functional impact of each cytokine on the clonal CLL B cell population. | ||
| Abbreviations: IL, interleukin; TNF, tumor necrosis factor; TGF, tumor growth factor; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor. | ||
| IL-2 | Present | Inhibits apoptosis |
| IL-4 | Present | Inhibits apoptosis |
| IL-8 | Present | Inhibits apoptosis |
| IL-10 | Present | Induces apoptosis |
| TNFα | ? | Inhibits apoptosis |
| TGFβ | Present | Induces apoptosis |
| Interferon α/γ | Present | Inhibits apoptosis |
| VEGF | Present (both VEGFR1 and VEGFR2) | Inhibits apoptosis |
| bFGF | ? | Induces drug resistance |
| CXCR4 | Present | Stimulates chemotaxis |
| CXCR3 | Present | Unknown |
Staging of chronic lymphocytic leukemia.
| Rai . | Lymphocytosis . | Lymph Node Enlargement . | Spleen/Liver Enlargement . | Hemoglobin < 11 g/dL . | Platelet < 100 x 109/L . | Survival (years) . |
|---|---|---|---|---|---|---|
| 0 | Yes | No | No | No | No | > 13 |
| I | Yes | Yes | No | No | No | 8 |
| II | Yes | ± | ± | No | No | 5 |
| III | Yes | ± | ± | Yes | No | 2 |
| IV | Yes | ± | ± | ± | Yes | 1 |
| Rai . | Lymphocytosis . | Lymph Node Enlargement . | Spleen/Liver Enlargement . | Hemoglobin < 11 g/dL . | Platelet < 100 x 109/L . | Survival (years) . |
|---|---|---|---|---|---|---|
| 0 | Yes | No | No | No | No | > 13 |
| I | Yes | Yes | No | No | No | 8 |
| II | Yes | ± | ± | No | No | 5 |
| III | Yes | ± | ± | Yes | No | 2 |
| IV | Yes | ± | ± | ± | Yes | 1 |
| Binet . | Lymphocytosis . | Lymph Node Areas . | . | Hemoglobin < 11 g/dL . | Platelet < 100 x 109/L . | Survival (years) . |
|---|---|---|---|---|---|---|
| A | N/A | < 3 | No | No | 12 | |
| B | 3 or > | No | No | 5 | ||
| C | ± | Yes (or low plt) | Yes (or low Hb) | 2 |
| Binet . | Lymphocytosis . | Lymph Node Areas . | . | Hemoglobin < 11 g/dL . | Platelet < 100 x 109/L . | Survival (years) . |
|---|---|---|---|---|---|---|
| A | N/A | < 3 | No | No | 12 | |
| B | 3 or > | No | No | 5 | ||
| C | ± | Yes (or low plt) | Yes (or low Hb) | 2 |
Selected clinical trial results with fludarabine in previously untreated chronic lymphocytic leukemia (CLL) patients.
| Ref . | Regimen . | Phase . | No. Pts . | CR . | ORR . | Mean PFS (mo) . | Mean OS (mo) . |
|---|---|---|---|---|---|---|---|
| Abbreviations: Ref, reference; No, number; Flu, fludarabine; CAP, cyclophosphamide, adriamycin, prednisone; CLB, chlorambucil; CHOP—cyclophosphamide, adriamycin, vincristine, prednisone; Cy, cyclophosphamide; Ritux, rituximab; CR, complete response rate; ORR, overall response rate; PFS, progression free survival; OS, overall survival; NR, not reached; Not Rep, not reported. | |||||||
| 27 | Flu versus | III | 52 | 23 | 71 | NR | NR |
| CAP | 48 | 17 | 60 | 7 | 54 | ||
| 28 | Flu versus | III | 170 | 20 | 63 | 20 | 66 |
| CLB versus | 181 | 4 | 37 | 14 | 56 | ||
| Flu + CLB | 123 | 20 | 61 | Not Rep | 55 | ||
| 29 | Flu versus | III | 341 | 40 | 71 | 32 | 69 |
| CHOP versus | 357 | 30 | 72 | 30 | 67 | ||
| CAP | 237 | 15 | 58 | 28 | 70 | ||
| 37 | Flu + Cy | II | 17 | 51 | 92 | Not Rep | Not Rep |
| 38 | Flu + Cy | II | 34 | 35 | 88 | NR | NR |
| 39 | Flu + Cy | II | 36 | 42 | 64 | Not Rep | Not Rep |
| 40 | Flu + Cy | II | 59 | 47 | 78 | Not Rep | Not Rep |
| 51 | Flu + Ritux versus | II | 51 | 47 | 90 | NR | NR |
| Flu then Ritux | 53 | 28 | 77 | NR | NR | ||
| 52 | Flu + Cy + Ritux | II | 60 | 66 | 95 | NR | NR |
| Ref . | Regimen . | Phase . | No. Pts . | CR . | ORR . | Mean PFS (mo) . | Mean OS (mo) . |
|---|---|---|---|---|---|---|---|
| Abbreviations: Ref, reference; No, number; Flu, fludarabine; CAP, cyclophosphamide, adriamycin, prednisone; CLB, chlorambucil; CHOP—cyclophosphamide, adriamycin, vincristine, prednisone; Cy, cyclophosphamide; Ritux, rituximab; CR, complete response rate; ORR, overall response rate; PFS, progression free survival; OS, overall survival; NR, not reached; Not Rep, not reported. | |||||||
| 27 | Flu versus | III | 52 | 23 | 71 | NR | NR |
| CAP | 48 | 17 | 60 | 7 | 54 | ||
| 28 | Flu versus | III | 170 | 20 | 63 | 20 | 66 |
| CLB versus | 181 | 4 | 37 | 14 | 56 | ||
| Flu + CLB | 123 | 20 | 61 | Not Rep | 55 | ||
| 29 | Flu versus | III | 341 | 40 | 71 | 32 | 69 |
| CHOP versus | 357 | 30 | 72 | 30 | 67 | ||
| CAP | 237 | 15 | 58 | 28 | 70 | ||
| 37 | Flu + Cy | II | 17 | 51 | 92 | Not Rep | Not Rep |
| 38 | Flu + Cy | II | 34 | 35 | 88 | NR | NR |
| 39 | Flu + Cy | II | 36 | 42 | 64 | Not Rep | Not Rep |
| 40 | Flu + Cy | II | 59 | 47 | 78 | Not Rep | Not Rep |
| 51 | Flu + Ritux versus | II | 51 | 47 | 90 | NR | NR |
| Flu then Ritux | 53 | 28 | 77 | NR | NR | ||
| 52 | Flu + Cy + Ritux | II | 60 | 66 | 95 | NR | NR |
Potential targets for the treatment of chronic lymphocytic leukemia (CLL).
| I. Neutralizing Target . | Clinically Available Antibody . | Small Molecule . |
|---|---|---|
| IL-8 | Yes (human) | No |
| IL-6 | Yes (preclinical) | Yes |
| TNF-α | Yes (humanized, neutralizing receptor) | Yes |
| IFN-γ | Yes (humanized) | Yes |
| SDF | Yes (preclinical) | No |
| VEGF | Yes (humanized) | Yes |
| bFGF | No | Yes |
| CD59 or CD55 | No | No |
| IL-4 | Yes | No (preclinical) |
| ZAP-70 | N/A | No (preclinical) |
| AKT | N/A | Yes (preclinical) |
| PI3 kinase | N/A | No (preclinical) |
| DNA methyl-transferase | N/A | Yes |
| Histone deacetylase | N/A | Yes |
| Phosphodiesterase | N/A | Yes |
| I. Neutralizing Target . | Clinically Available Antibody . | Small Molecule . |
|---|---|---|
| IL-8 | Yes (human) | No |
| IL-6 | Yes (preclinical) | Yes |
| TNF-α | Yes (humanized, neutralizing receptor) | Yes |
| IFN-γ | Yes (humanized) | Yes |
| SDF | Yes (preclinical) | No |
| VEGF | Yes (humanized) | Yes |
| bFGF | No | Yes |
| CD59 or CD55 | No | No |
| IL-4 | Yes | No (preclinical) |
| ZAP-70 | N/A | No (preclinical) |
| AKT | N/A | Yes (preclinical) |
| PI3 kinase | N/A | No (preclinical) |
| DNA methyl-transferase | N/A | Yes |
| Histone deacetylase | N/A | Yes |
| Phosphodiesterase | N/A | Yes |
| II. Cell Surface Targets . | ||
|---|---|---|
| CD20 | Yes (chimeric) | N/A |
| CD52 | Yes (humanized) | N/A |
| HLA-DR | Yes (humanized) | N/A |
| CD22 | Yes (humanized) | N/A |
| CD23 | Yes (chimeric) | N/A |
| CD47 | No | N/A |
| II. Cell Surface Targets . | ||
|---|---|---|
| CD20 | Yes (chimeric) | N/A |
| CD52 | Yes (humanized) | N/A |
| HLA-DR | Yes (humanized) | N/A |
| CD22 | Yes (humanized) | N/A |
| CD23 | Yes (chimeric) | N/A |
| CD47 | No | N/A |
Diagrammatic representation of the two subsets of chronic lymphocytic leukemia (CLL).
Conventionally, B cells meet antigen in the germinal center in the context of T cells and antigen presenting cells. This stimulates somatic mutation of the immunoglobulin V genes, and a memory B cell emerges from the germinal center. The unmutated subset might be explained by envisioning unconventional stimulation of the B cell receptor outside the germinal center, either by T-independent antigens or superantigen. This does not induce somatic mutation, but does stimulate an “experienced” immunophenotype.
Diagrammatic representation of the two subsets of chronic lymphocytic leukemia (CLL).
Conventionally, B cells meet antigen in the germinal center in the context of T cells and antigen presenting cells. This stimulates somatic mutation of the immunoglobulin V genes, and a memory B cell emerges from the germinal center. The unmutated subset might be explained by envisioning unconventional stimulation of the B cell receptor outside the germinal center, either by T-independent antigens or superantigen. This does not induce somatic mutation, but does stimulate an “experienced” immunophenotype.
Actuarial survival curves of 114 stage A patients, censored for non-B-CLL deaths, comparison of patients with mutated and unmutated IgVHgenes.
Actuarial survival curves of 114 stage A patients, censored for non-B-CLL deaths, comparison of patients with mutated and unmutated IgVHgenes.
Actuarial survival curves of 191 patients with B-CLL, censored for non-B-CLL deaths. Comparison between stable (S; n = 93), progressive (P; n = 65), and slowly progressive (SP; n = 33) cases.
Actuarial survival curves of 191 patients with B-CLL, censored for non-B-CLL deaths. Comparison between stable (S; n = 93), progressive (P; n = 65), and slowly progressive (SP; n = 33) cases.
Actuarial survival curves of 114 stage A patients, censored for non-B-CLL deaths, comparison of patients with CD38 positive and CD38 negative B-CLL.
Actuarial survival curves of 114 stage A patients, censored for non-B-CLL deaths, comparison of patients with CD38 positive and CD38 negative B-CLL.
The maintenance and expansion of the malignant B-cell chronic lymphocytic leukemia (B-CLL) clone is primarily related to the genetic events induced by various inherited or environmental processes that lead to transformation. However, the overall process of leukemic B-cell clone survivorship is also linked to a complex circuitry of cytokines and chemokines that act in both autocrine and paracrine pathways. Importantly, stromal cells, dendritic cells, endothelial cells, nurse cells, and various immune cells contribute to this cytokine/chemokine array, which ultimately contributes to the behavior of the CLL B cell. Apparent physical association of the leukemic CLL B cell with these various cell types suggests that there is significant cellular crosstalk that assists in the induction of a microenvironment favorable to the leukemic CLL B cell.
The maintenance and expansion of the malignant B-cell chronic lymphocytic leukemia (B-CLL) clone is primarily related to the genetic events induced by various inherited or environmental processes that lead to transformation. However, the overall process of leukemic B-cell clone survivorship is also linked to a complex circuitry of cytokines and chemokines that act in both autocrine and paracrine pathways. Importantly, stromal cells, dendritic cells, endothelial cells, nurse cells, and various immune cells contribute to this cytokine/chemokine array, which ultimately contributes to the behavior of the CLL B cell. Apparent physical association of the leukemic CLL B cell with these various cell types suggests that there is significant cellular crosstalk that assists in the induction of a microenvironment favorable to the leukemic CLL B cell.
Dr. Byrd has received support for data management of trials for Genentech, IDEC, Berlex Protein Design Labs.
This work in part supported by the National Cancer Institute (P01 CA81534-02, P30 CA160058, and CA98099), The Sidney Kimmel Cancer Research Foundation, The Leukemia and Lymphoma Society of America, and The D. Warren Brown Foundation.