Abstract
With increasing hematopoietic stem cell transplant (HSCT) activity and improvement in outcomes, there are many thousands of HSCT survivors currently being followed by non-transplant clinicians for their healthcare. Several types of late sequelae from HSCT have been noted, and awareness of these complications is important in minimizing late morbidity and mortality. Late effects can include toxicities from the treatment regimen, infections from immunodeficiency, endocrine disturbances, growth impairment, psychosocial adjustment disorders, second malignancies, and chronic graft-versus-host disease (GVHD). A variety of risk factors for these complications have been noted. The clinician should be alert to the potential for these health issues. Preventive and treatment strategies can minimize morbidity from these problems and optimize outcomes.
I. Overview of Late Complications
John R. Wingard, MD*
University of Florida, HSC, College of Medicine, 1600 SW Archer Rd., Rm. R4-116, P.O. Box 100277, Gainesville, FL 32610-3001
Hematopoietic stem cell transplantation (HSCT) provides effective therapy for patients with lymphohematopoietic, immunologic, metabolic and other disorders. Both the annual number of HSCT procedures has increased dramatically and the number of diseases for which HSCT is considered appropriate have expanded over the years. Estimates suggest that 30,000–40,000 HSCT procedures are performed annually worldwide. HSCT offers a potential for cure or long-term disease control for a number of diseases where other treatment options fail or prognostic indicators suggest that durable control is unlikely. Outcomes have also gradually improved. Data from the IBMTR, ABMTR, and EBMTR indicate gradual improvement in long-term outcomes with an average survival improvement of approximately 10% per decade. With expanding applications and increase in HSCT activity, along with technological advances in supportive care, histocompatibility testing, safer conditioning regimens, and control of graft-versus-host disease (GVHD), growth in transplant activities is likely to occur, outcomes should improve, and increasing numbers of transplant survivors will be facing life after the transplant experience.
Many patients have now been followed for two or three decades post-transplant and are presumably cured. For many HSCT survivors, cure or control of the underlying disease is not accompanied by full restoration of health.1– 7 Some patients develop long-term complications, the topic of this session.
Both long-term physical and psychosocial morbidity can occur. The types of late sequelae include toxicities from the treatment regimen, immune deficiency, autoimmune syndromes, infectious complications, endocrine disturbances, growth impairment in children, cognitive dysfunction, second malignancies, chronic GVHD, and problems with psychosocial adjustment and quality of life. These late effects can negatively affect a patient’s performance of daily activities, interpersonal and family relationships, and sense of personal well being.
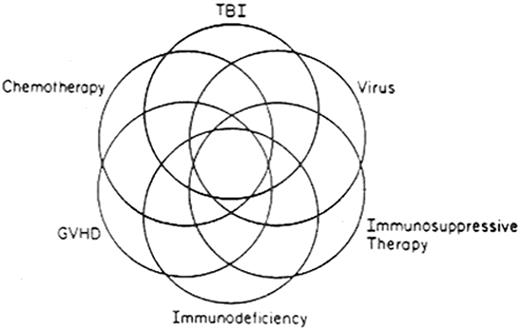
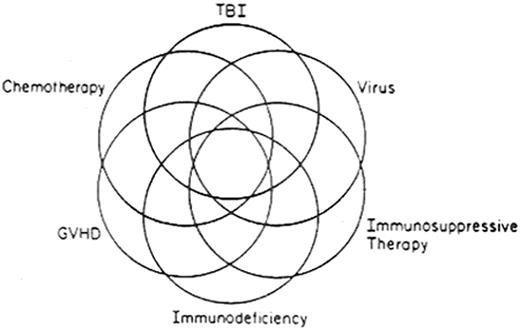
The multifactorial etiologies of posttransplant complications are illustrated in Figure 1 . Several factors act solely or in concert in causation. Some complications are due to the transplant procedure, such as GVHD, immunodeficiency, infectious complications, and autoimmune syndromes. Others are due to the conditioning regimen or prior anti-neoplastic therapy, such as sterility, alopecia, endocrine disturbances, cardiorespiratory insufficiency, renal impairment, impaired growth, and cognitive disturbances. Some are due to the underlying disease itself, such as recurrence. Some complications are multifactorial: for example, respiratory insufficiency may result from the collective effects of lung injury from the conditioning regimen, bronchiolitis obliterans from GVHD, and a superimposed infection. Table 1 lists various types of complications, with risk factors and considerations for prevention and treatment.
A variety of health behaviors, both personal and physician dependent, can improve survivors’ health and functioning and prevent some of these complications. Accordingly, it is increasingly important for non-transplant clinicians to be aware of these sequelae and be advocates for health maintenance behaviors to optimize functioning and sense of well being.
Immunodeficiency
Antigen-specific T and B cell responses are necessary for control of many infectious pathogens. Immune responses are profoundly deficient early after HSCT; gradual restoration occurs during the first year following transplant.8– 11 Factors that impede development of protective responses include GVHD, certain viral infections, particularly those by the herpesvirus family, depletion of lymphocytes from the stem cell graft, or post-transplant immunotherapeutic maneuvers such as administration of antibodies against T and B cells (either for control of GVHD or malignancy). Donor source (peripheral blood versus bone marrow) and the degree of histocompatibility between donor and recipient also affect the pace of immune reconstitution.
The major risk factor for late infections is chronic GVHD.12,13 Chronic GVHD is associated with susceptibility for infections by encapsulated bacteria (S. pneumonaie, H. influenzae, and N. meningitidis), invasive candidiasis, aspergillosis, and herpesviruses, especially cytomegalovirus (CMV) and varicella zoster virus (VZV). Prophylaxis with an antibiotic with activity against gram positive organisms is recommended.14 It should be continued for as long as active therapy for GVHD is given. The duration of antibiotic prophylaxis beyond active therapy is unclear.
Pneumocystis carinii infection (PCP) prophylaxis with trimethaprin-sulfamethoxazole should be given for the first 6 months and continue for those receiving active therapy for chronic GVHD. Immunizations with inactivated vaccines should be given starting at 12 months.14 Live attenuated virus vaccines should be avoided during the first two years and should be avoided in those with chronic GVHD.
An infrequent but severe manifestation of VZV infection is severe abdominal pain and a clinical syndrome suggesting a perforated intra-abdominal viscus, acute pancreatitis, or severe hepatitis. This can be associated with a rapidly progressive and life threatening course. Accordingly, VZV infections should be strongly considered in the evaluation of a patient with acute abdominal pain. Acyclovir is effective therapy for VZV infection.
Autoimmune antibodies are detected in some patients especially those with chronic GVHD. Often this is without clinical consequence but autoimmune cytopenias are occasionally problematic.15,16 Recipients of ABO incompatible donor grafts also can have persistent hemolysis for months after HSCT or longer.17 This is due to persistent host isohemagglutinins, which may persist until full donor chimerism is established.
Endocrine Dysfunction
Hypothyroidism occurs in patients who have received prior cranial or mantle irradiation and in those in which total body irradiation (TBI) was part of the conditioning regimen.18– 20 Children appear to be more susceptible. Annual assessment of thyroid function is advised in all patients who have received TBI or radiotherapy to the head and neck. If hypothyroidism is noted, then replacement therapy is appropriate.
Hypoadrenalism may occur in patients on prolonged corticosteroid therapy for GVHD treatment. Gradual tapering of corticosteroids is appropriate. For patients requiring surgical procedures or with acute medical illnesses, short-term “stress” replacement is warranted.
Gonadal function is impaired lifelong in the majority of patients receiving intensive chemotherapy or TBI-containing conditioning regimens.21– 25 Lifelong infertility is typical but not universal. Several studies have suggested recovery of spermatogenesis in a minority of men years later (10-15%) and ovarian recovery has occurred in some women years later (5-10%). Accordingly, sperm banking is advisable for men in advance if possible. Similarly, once egg harvesting and cryopreservation techniques have been refined, this should also be considered in advance. Testosterone levels in men typically are normal, however estrogen levels are almost universally impaired. After transplant, annual gynecologic evaluation is advisable. Because deficiency of estrogen at an early age can lead to osteopenia, cardiovascular complications, and lipid disorders, consideration for ovarian hormone replacement should be given. Counseling should be offered as to benefits and risks and routine gynecologic evaluation is imperative. Typically, hormonal replacement is initiated 3-6 months following HSCT.
Skeletal Disorders
Osteopenia is frequent, especially in patients given TBI in the conditioning regimen, those receiving prolonged corticosteroids, patients who are inactive for prolonged periods, and women with estrogen deficiency.26,27 Bone densitometry studies are useful to monitor osteopenia over time and response to treatment. Ovarian hormonal replacement should be considered in women. Bisphosphonates may also be useful in men or in women in whom hormonal replacement is contraindicated. An exercise program is also advisable.
Avascular necrosis (AVN) of the bone occurs after corticosteroid therapy.28– 31 In one series, 8% of HSCT patients at 5 years had AVN. The hip joint is most frequently affected, but humerus and other weight bearing bones can also be involved. Multiple joints are affected in most patients. Males and adults are more susceptible. Minimization of corticosteroid therapy is paramount. Once AVN occurs, joint replacement may be necessary.
Liver
Hepatic involvement by chronic GVHD is the most common cause of late hepatopathy. However, chronic hepatitis from hepatitis B or C virus can occur due to transmission via the graft or from transfusion support. Hepatic injury often coincides with tapering of immunosuppressive therapy.32,33 Cirrhosis can result33,34 and may not become manifest for ten or more years. Iron overload resulting from red cell transfusions or altered iron absorption35 and infrequently hemosiderosis have been reported.36 Evaluation should include hepatitis serologies, PCR for hepatitis C, and ferritin levels. Histopathologic examination of the liver is advisable unless contraindicated. For patients with chronic hepatitis who are seronegative to hepatitis A, immunization with the hepatitis A vaccine should be given since a superimposed hepatitis A infection can result in acute hepatic decompensation.37 For hepatitis B, lamivudine or foscarnet can reduce hepatic injury.38 For hepatitis C, therapy with interferon plus ribavirin should be considered.39 The role of chelating therapy for iron overload is uncertain.
Ophthalmologic Problems
Cataracts are frequent, particularly in those receiving TBI or those given corticosteroids.40–,44 Both corticosteroids and TBI are independent risk factors for the development of cataracts. When visual acuity is compromised, cataract extraction and implantation of an artificial lens is indicated. Keratoconjunctivitis sicca is most commonly a manifestation of chronic GVHD but can also occur occasionally in the absence of GVHD.45 Artificial tear solutions during the day and ointment at night can provide symptomatic relief and protect the cornea from abrasions or ulceration. Ligation of the canaliculi that drain the lacrimal fluid can also be performed to optimize lubrication of the eye.
Nervous System Abnormalities
Impaired memory and short attention spans have been noted in occasional patients and appear to be multifactorial, but as yet poorly elucidated.46 Learning deficits in children have also been described related to intensive cranioradiotherapy and intrathecal chemotherapy.47 Leukoencephalopathy related to intensive cranial radiation and intrathecal chemotherapy has declined over the years with attempts to minimize therapies with overlapping toxicities. However, with increasing use of fludarabine in conditioning regimens, another potential inciting agent, additional cases may be expected. Peripheral neuropathies have also been described due to chronic GVHD, or certain chemotherapeutic or immunosuppressive medications.48,49 The neuropathy, if due to GVHD, is responsive to corticosteroids.
Respiratory
Lung injury from the cytotoxic agents in the conditioning regimen can lead to pulmonary interstitial fibrosis. With modern conditioning regimens and fractionation of TBI, this has become much less frequent today. Late interstitial pneumonitis occurs infrequently but more often in patients with chronic GVHD.50 With improved control of early CMV infection with ganciclovir during the first three months, late onset CMV pneumonitis has become more frequent, especially in patients with early CMV infection and in those with delayed immune recovery and chronic GVHD. Obstructive airway disease is more common but its etiology is poorly understood.51 One manifestation, bronchiolitis obliterans, occurs as a manifestation of chronic GVHD.52 Superinfections with respiratory viruses, bacteria and fungi are frequent complications of chronic respiratory disease and acute exacerbations of pulmonary dysfunction should be aggressively investigated and when infection is found treated appropriately.
Growth in Children
Sequential assessment of growth should be performed in all children after HSCT. Several factors may impair growth.53– 56 Hypothyroidism can result in reduced growth in children and should be evaluated in the assessment of impaired growth velocity. Growth hormone (GH) deficiency can occur following central nervous system (CNS) irradiation and the risk is dose dependent. Children given TBI also are at greater risk, especially those given TBI in a single fraction. Damage to bone epiphyses may result and can dampen response to GH. The use of chronic corticosteroid therapy for chronic GVHD can also retard growth. Gonadal insuffiency may result in delayed pubescence, an impaired growth spurt, and a reduction in height. Careful endocrine assessment should be periodically performed. In children with delayed secondary sex characteristics development, consideration to sex hormonal replacement should be made. Children with GH deficiency should be considered for synthetic GH therapy.
Psychosocial Adjustment
A number of studies have characterized psychosocial adjustment and quality of life (QOL) in long-term survivors.57– 63 For many HSCT survivors, relatively “normal” physical and social functioning, perception of health, sense of well being, and perception of QOL are reported. However, deficits occur in a substantial proportion. Such deficits include low self-esteem, psychological distress, occupational disability, impaired social, marital, and family relationships, limitations of routine daily tasks and recreational activities, unemployment, sexual dysfunction, cognitive impairment, and sleep difficulties. Several factors have been associated with susceptibility for adjustment problems. Less education has been linked to poorer emotional well being, sexual dysfunction, and less ability to perform daily activities. Higher doses of TBI have been linked to poorer cognitive function. Patients with chronic GVHD report lower levels of physical function. A number of studies are underway to characterize the impact of personal and social resources, coping strategies, and identifying patients at risk for poor psychosocial adjustment. Evaluation of interventions that target at-risk patients are a high research priority.
Infrequent Late Effects
Cardiomyopathy can occur as an acute effect of high-dose cyclophosphamide; chronic cardiac insufficiency is infrequent. Likewise, coronary artery disease has not been described as a complication of HSCT. Myopathy may result from corticosteroid use and polymyocitis can be an infrequent manifestation of chronic GVHD. Maintenance of physical activity is important to optimize functional capacity. Caries are frequent in persons with poor dental hygiene and chronic GVHD complicated by salivary gland inflammation and poor salivary production. Hygienic measures, fluoride treatment, and artificial saliva are important preventive measures. Acute hemorrhagic cystitis from cyclophosphamide or infection from several viruses occurs early after HSCT but scarring of the bladder may result and lead to chronic urinary frequency. There is no specific therapy. Acute renal dysfunction is frequent early after transplant related to nephrotoxic immunosuppressive or antimicrobial agents. Chronic nephropathy is generally infrequent but patients with prior nephrotoxic chemotherapy (e.g., platinum) or those receiving TBI are at greater risk. It has been suggested that angiotensin-converting enzyme inhibitors may have protective effects but this has not been formally evaluated. Control of hypertension is important.
Guidelines for Follow-up
Because of the various complications noted above, routine follow-up of HSCT survivors should be performed on a periodic basis. Table 2 provides a schema for guidelines for follow-up assessment. In Table 1 are a number of suggested preventive and treatment suggestions to consider during the follow-up assessments.
Control of the underlying disease for which the transplant was performed should be monitored during the first year and subsequently. The type of disease and disease status at the time of HSCT provide guidance as to the types of tests required for monitoring and likelihood of recurrence. The majority of recurrences occur during the first 2 years and risk recedes subsequently. Late recurrences of chronic myelogenous leukemia can occur. Since the effectiveness of donor lymphocyte infusion (DLI) is dependent on discovery early in relapse, monitoring by PCR or FISH for the BCR-ABL rearrangement should continue beyond the first 2 years. Engraftment can generally be monitored simply by periodic blood counts. When cytopenias occur, further assessment with chimerism assays using FISH or sex chromosome determination (for donor/recipient sex disparity) or various assays for DNA polymorphisms are useful. Bone marrow assessment with cytogenetic examination is useful in the evaluation of pancytopenia to evaluate the possibility of myelodysplastic syndrome or secondary leukemia, especially in autologous HSCT recipients where the risk is greater. Immunizations should be initiated at 12 months unless there is active GVHD, in which case it should be delayed until the survivor has completed immunosuppressive therapy. PCP prophylaxis should be given during the first 6 months and in patients receiving active therapy for GVHD. Bacterial prophylaxis should be given to individuals with chronic GVHD. Patients should be made aware of the signs and symptoms of chronic GVHD so that the patient can partner with the clinician in detection of early manifestations of GVHD. A history and physical examination are the mainstay of screening for GVHD. Liver function tests and pulmonary function tests in those with respiratory symptoms are also useful screening tests. Ovarian function should be assessed in females and consideration given for hormonal replacement in females at and beyond puberty. Thyroid screening should be performed in patients who have received TBI or prior radiotherapy in the neck area. All children should have annual growth charts maintained as well as further endocrine evaluation in individuals whose height velocity decreases.
The patient should be engaged in health maintenance behaviors. Preliminary findings from one survivor study suggest that physician-independent and physician-dependent health maintenance behaviors by HSCT survivors are no worse than, but also no better than, those of the general population.64 A regular program of health screening and promotion of health maintenance behaviors will optimize transplant outcomes and improve patient recovery.
II. Presentations, Diagnostic Challenges, and Treatment Dilemmas for Chronic GVHD
Georgia B. Vogelsang, MD*
Johns Hopkins Hospital, Department of Oncology, Bunting Blaustein Cancer Research Building, 1650 Orleans St., Room 2M89, Baltimore, MD 21231 Dr. Vogelsang has received clinical trial support from Supergen, Roche, and Centocor.
Chronic GVHD is one of the most common and significant problems affecting long-term survivors of allogeneic stem cell transplantation (SCT). The incidence of chronic GVHD is increasing due to expansion of patient and donor populations and changes in transplant procedures. Many high risk patients, on the basis of age, other medical conditions, or disease status, are being offered non-myeloablative transplants followed by DLI to achieve total donor chimerism or treat residual disease.1 Relapsed patients are also treated with DLI. These lymphocyte infusions are frequently given until GVHD occurs (hoping to achieve a graft-versus-leukemia [GVL] effect), with subsequent chronic GVHD. Older patients, with a higher risk of chronic GVHD, are also undergoing full allogeneic transplantation. Alternative donors, including matched unrelated donors, unrelated donors mismatched at a single HLA allele, and haplo-identical related donors, are being used with increasing frequency. Alternative donor transplants are complicated by higher rates of GHVD. And finally, although peripheral blood stem cell (PBSC) transplant has lead to more rapid engraftment with roughly equivalent rates of acute GVHD, many trials have shown that it is associated with a higher incidence of chronic GVHD, presumably due to the growth factors used during mobilization shifting the T helper cells to a Th2 phenotype.1,2
Up to 60% of patients surviving more than 4 months after allogeneic transplantation develop chronic GVHD.3,4 Clinical risk factors for the development of chronic GVHD include older age of patient and/or donor, use of peripheral blood as stem cell source, prior acute GVHD, second transplants, busulfan preparative regimen, and a positive screening skin or oral biopsy at day 80-100 post-SCT.5– 10
Pathogenesis
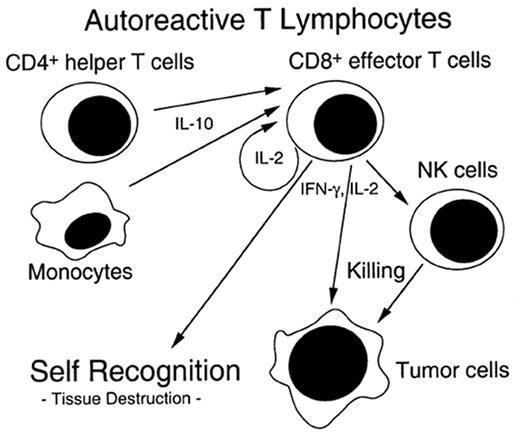
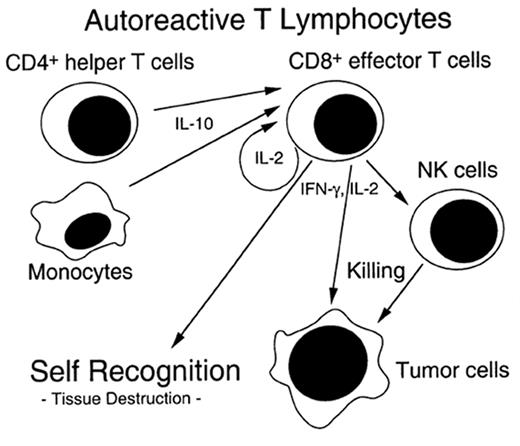
The pathophysiology of chronic GVHD remains largely a mystery. Clinical studies of chronic GVHD are difficult, in part because the disease presents months after SCT after many patients have left the direct care of the transplant center. Although animal models of chronic GVHD do exist, the most commonly used model (a parent into F1 hybrid model) produces extensive antibody-mediated damage that more closely resembles lupus than chronic GVHD.11 Another model of chronic GVHD comes from the model of cyclosporine (CSA)-induced autologous GVHD, which clinically resembles chronic GVHD.12 CSA inhibits thymic-dependent clonal deletion of autoreactive T cells, thereby paradoxically disrupting self-tolerance. Autologous GVHD is mediated ay autoreactive T cells which recognize the CLIP region of MHC class II molecules. The effector cells have broad-based recognition of tissues and the clinical manifestations when fully evolved are identical to chronic GVHD. Thymic damage is critical for expression of autologous GVHD. Chronic GVHD may stem from a immune injury similar to that of acute GVHD and SCT, which allows the development of autoreactive clones that would normally be deleted (see Figure 2 ).
Although the clinical findings in chronic GVHD may be impressive, the main effect of chronic GVHD is on immune function. Infections account for the majority of deaths in these patients. Because of decreased negative selection, reduced extrathymic generation, and/or acceleration of the normal thymic aging process with chronic GVHD, patients have an increase in peripheral autoreactive T lymphocytes.13,14 These autoreactive T lymphocytes act with interferon gamma to produce the increased collagen deposition seen histopathologically in chronic GVHD.15
Classification of Chronic GVHD
Chronic GVHD can be classified according to the type of onset, the clinical manifestations, or the extent of disease. The majority of patients with chronic GVHD have had prior acute GVHD. Their disease may evolve directly from acute GVHD (progressive), which has a grim prognosis, or may follow a period of recovery GVHD (quiescent), with an intermediate prognosis. Patients may develop chronic GVHD with no history of prior acute GVHD (de novo) and these patients have a relatively good prognosis.
Patients may also be described as having lichenoid or sclerodermatous disease based on the cutaneous manifestations. The lichenoid form occurs earlier after SCT, and may evolve into sclerodermatous GVHD. The most commonly employed staging system stratifies patients into limited or extensive disease based on the outcome of 20 patients.16 Localized skin involvement, with or without hepatic dysfunction, is classified as limited disease and does not require treatment. Patients with generalized skin involvement or with limited skin involvement in association with eye involvement, oral involvement, hepatic dysfunction with abnormal liver histology, or involvement of any other target organ are considered to have extensive disease, which needs therapy. Although this staging system is highly reproducible, it provides little information about prognosis and is of limited clinical utility.17
More recently, a grading system for chronic GVHD that stratifies patients into risk categories according to clinical characteristics at diagnosis has been reported.18 Using a database of 151 patients with chronic GVHD, three variables were found to be risk factors for shortened survival by multivariate analysis: extensive skin GVHD involving > 50% of the body surface area, platelet count of < 100,000/μL, and progressive-type onset. As reported at this meeting last year, this model was validated using data from 1108 patients from the IBMTR (n = 711), Fred Hutchinson Cancer Research Center (n = 188), University of Nebraska (n = 60), and the University of Minnesota (n = 149). Despite significant heterogeneity of the data, the proposed grading system identified three prognostic groups, each with different survival outcomes. Because this grading system is highly predictive of outcome, it may help to improve clinical management, trial design, and communication among transplant centers.
Clinical Manifestations
The diagnosis of chronic GVHD is traditionally made after day 100 post-transplant. The median time of diagnosis is day 201 after HLA-identical sibling transplant, day 159 after mismatched related transplant, and day 133 after an unrelated donor transplant.19 The clinical manifestations are summarized in Table 3 .
Immune system
Chronic GVHD causes profound immunosuppression. Most chronic GVHD deaths are attributable to infection. Functional asplenia with an increased susceptibility to encapsulated bacteria is common, and circulating Howell-Jolly bodies may be seen on peripheral blood smear. Patients are also at risk for invasive fungal infections and PCP.
Skin
Lichenoid chronic GVHD presents as an erythematous, papular rash that resembles lichen planus and has no typical distribution pattern. Scleradermatous GVHD may involve the dermis and/or the muscular fascia and clinically resembles systemic sclerosis. The skin is thickened, tight, and fragile with very poor wound-healing capacity. Alteration in pigmentation, either hypo- or hyper-pigmentation, may occur. In severe cases the skin may become blistered from poor lymphatic drainage or ulcerated from minor trauma. Because the sclerosis affects the dermis, hair loss and destruction of the sweat glands are common. Isolated fascial scleroderma presents with decreased mobility with normal appearing skin. The skin is fixed to the fascia below and careful examination of the skin is critical in making the diagnosis.
Dermal appendages
Fingernails and toenails may be affected by chronic GVHD. Nails develop vertical ridges and cracking and are very fragile. Nail problems may persist even after skin changes have resolved. Hair loss in areas of affected skin may also persist after treatment, although recovery of hair is frequently a sign of recovery. Brittle hair often precedes allopecia. Premature graying is often associated with chronic GVHD, even in children, and may effect hair and eyebrows.
Musculoskeletal system
Fascial involvement in sclerodermatous GVHD is usually associated with skin changes, but may develop with normal, but fixed overlying skin. Fasciitis causes significant limitations in range of motion if joint areas are involved. Muscle cramps are a common complaint in patients with chronic GVHD, although the pathophysiology is not understood. Myositis, with tender muscles and elevated muscle enzymes, is rare and does not explain the frequent complaints of severe cramps. Myositis, with tender muscles and elevated muscle enzymes, is rare and does not explain the frequent complaints of severe cramps. Patients with sclerodermatous GVHD and restricted range of motion may benefit from a regular program of physical therapy to help in recovery and to provide functional recommendations for limited joints.
Eyes
Ocular GVHD usually presents with irritation or dry eyes. Irreversible destruction of the lacrimal glands results in dryness, photophobia, and burning. Conjunctival GVHD is a rare manifestation of severe chronic GVHD and is associated with a poor prognosis.
Mouth
Oral mouth GVHD causes xerostomia and/or food sensitivity. More advanced disease may cause odynophagia due to extension of damage. Rarely, patients will have esophageal involvement without oral disease. Physical exam in mild disease reveals erythema with white plaques, which may be confused with thrush or herpetic infections. Lichenoid changes of more advanced disease cause more extensive plaques. Secondary infections with viruses (especially herpes simplex) and yeasts are almost universal and patients usually require treatment as long as their oral disease persists and/or immunosuppression is given. Changes in symptoms with little change in exam may occur with local infections.
Gastrointestinal tract
Many patients with chronic GVHD have GI complaints that are not necessarily related to their chronic GVHD. These symptoms are often attributable to other disease states including acute GVHD, infection, dysmotility, lactose intolerance, pancreatic insufficiency, and drug-related side effects.20 As many of these problems are very correctable, full evaluation of these symptoms is important. In a retrospective review of the intestinal biopsies of 40 patients with chronic GVHD and persistent GI symptoms, histopathologic evidence of chronic GVHD was found in only 11 patients. The majority of these patients had evidence of both acute and chronic GVHD, with only 3 patients (7%) found to have isolated chronic GVHD. Wasting in patients with chronic GVHD is common, with one recent report finding malnutrition in 43% of patients and severe malnutrition with body mass index less than 18.5 in 14%.21 The mechanisms of wasting are not fully defined but may include increased catabolic rate due to elevated resting energy expenditure and high cytokine levels, especially tumor necrosis factor (TNF). Although full nutritional evaluation and interventions are recommended, many patients with active GVHD continue to lose weight despite adequate caloric intake. Symptoms often improve with successful treatment of GVHD.
Liver
Hepatic disease typically presents as cholestasis, with laboratory evaluation revealing elevated alkaline phosphatase and elevated serum bilirubin. Isolated hepatic chronic GVHD is being seen with increased frequency with the use of DLI.22 Liver biopsy is required to confirm the diagnosis and is especially important in patients with no other symptoms of chronic GVHD, as viral infection and drug toxicity may mimic GVHD.
Respiratory tract
Bronchiolitis obliterans is a late and serious manifestation of chronic GVHD. Patients typically present with a cough or dyspnea.23 Many things may cause these symptoms (infection, reactive airway disease, fluid overload, cardiac disease, etc.); therefore, the symptoms need to be evaluated before labeling the disease as bronchiolitis obliterans. For example, patients with severe sclerotic chest wall disease may have similar symptoms but have no intrinsic pulmonary disease. Complete pulmonary function tests are needed to localize the problem. Chest CT may be normal or may show hyperinflation. Patients with bronchiolitis obliterans have minimal response to therapy and a very poor prognosis.
Patients with chronic GVHD are also at risk for chronic sinusitis, which may cause minimal symptoms. The sinuses are a frequent site of infection and should be considered in fever workups.
Hematopoietic system
Cytopenias are seen commonly in chronic GVHD patients. This may be a result of stromal damage, but autoimmune neutropenia, anemia, and/or thrombocytopenia are also seen. Thrombocytopenia at the time of chronic GVHD diagnosis has been associated with a poor prognosis.18,24– 27 Eosinophilia may be seen and may track with disease activity.
Evaluation of Suspected Chronic GVHD
The accurate and timely diagnosis of chronic GVHD is an important step in its successful treatment. Most patients have returned to the care of their primary oncologist when chronic GVHD develops. Not every rash or gastrointestinal complaint represents GVHD. In a series of 123 patients referred to Johns Hopkins for the management of refractory chronic GVHD, 9 patients had never had chronic GVHD and 26 patients had inactive disease.26 Because the therapies for chronic GVHD are highly immunosuppressive and must be continued for a prolonged time, it is important to confirm the diagnosis before initiating therapy. Conversely, subtle manifestations of chronic GVHD may go undiagnosed for months and this delay may make successful treatment and rehabilitation difficult. The diagnosis of fasciitis without skin changes, for example, may be a difficult diagnosis to establish. If a diagnosis of chronic GVHD is suspected, histologic confirmation of at least one organ system is recommended.
Treatment of Chronic GVHD
As the main manifestation of this disease is immuno-deficiency, patient education and infection prophylaxis are very important in this disease. Infection is the leading cause of death among patients with chronic GVHD. Prophylaxis against Pneumocystis carinii should be administered to all patients undergoing treatment of chronic GVHD for six months after discontinuation of immunosuppressive medications. These patients also have life-long splenic dysfunction and should therefore receive prophylaxis against encapsulated bacteria for life. The guidelines published by the American Heart Association for endocarditis prophylaxis should be followed when patients are undergoing dental or other invasive procedures. Patients receiving topical steroid therapy for oral GVHD should be treated with clotrimazole troches or nystatin swishes. Patients at risk for CMV reactivation should receive frequent monitoring with CMV surveillance cultures or antigenemia testing. A positive antigenemia test should be treated preemptively with ganciclovir, and patients with evidence of CMV disease should receive both ganciclovir and CMV-specific immunoglobulin. Some centers administer intravenous IgG to patients with hypogammaglobulinemia to keep IgG levels > 500 mg/dL. Vaccination series should be delayed until 1 year after the completion of GVHD therapy because most patients will not mount an immune response with active disease or while receiving immunosuppressive medications. Posttransplant vaccination guidelines are available on the Centers for Disease Control and Prevention web site (www.cdc.gov/mmwr/mmwr_rr.html).
The most widely employed first line therapy for treatment of chronic GVHD is CSA and prednisone, administered on alternating days. Sullivan et al reported that prednisone alone is superior to prednisone plus azathioprine for primary treatment of patients with standard-risk extensive chronic GVHD.25 However, in patients classified as high-risk on the basis of platelet counts < 100,000/μL, treatment with prednisone alone resulted in only 26% 5-year survival. When a similar group of patients was treated with alternating day CSA and prednisone, 5-year survival exceeded 50%.27 After this encouraging result, most centers adopted this regimen for initial treatment of all patients. Patients are treated initially with daily prednisone at 1 mg/kg per day and daily CSA at 10 mg/kg per day divided BID. If disease is stable or improving after two weeks, prednisone is tapered by 25% per week to a target dose of 1 mg/kg every other day. After successful completion of this steroid taper, CSA is reduced by 25% per week to alternate day dosing of 10 mg/kg per day divided BID, every other day. If the disease has completely resolved at their 9-month evaluation, patients are slowly weaned from both medications, with dose reductions approximately every two weeks. Patients with incomplete response are kept on therapy for 3 more months and then re-evaluated. If patients fail to respond by 3 months or demonstrate progressive disease, salvage regimens are warranted.28
Although this regimen of alternating CSA and prednisone is widely employed for the treatment of standard-risk (platelet count > 100,000/μL) extensive GVHD, there was no data on its effectiveness in standard risk patients. The Seattle group has reported results on a study comparing prednisone alone to prednisone plus CSA in patients without thrombocytopenia in 307 patients with extensive GVHD. Two hundred eighty-seven patients were evaluable. Paul Martin (personal communication) observed that:
The cumulative incidence of transplant-related mortality at 5 years from enrollment was 17% (95% CI, 11%-23%) in the cyclosporine plus prednisone arm and 13% (95% CI, 8%-19%) in the prednisone arm. The hazards of transplant-related mortality, overall mortality, recurrent malignancy, secondary therapy and discontinuation of all immunosuppressive therapy were not significantly different between the two arms, but survival without recurrent malignancy was lower in the two-drug arm (P = 0.03). Eighteen (13%) of the 142 patients in the CSA plus prednisone arm and 32 (22%) of the 145 patients in the prednisone arm developed avascular necrosis (P = 0.04). Treatment with CSA plus prednisone may reduce the risk of steroid-related toxicity, but results of this study do not substantiate the hypothesis that administration of CSA reduces transplant-related mortality among patients with chronic GVHD.
This uncertainty regarding the choice of front-line therapy emphasizes the importance of enrolling patients on clinical trials so that fundamental questions about the pathogenesis and treatment of chronic GVHD may be answered. Currently two large randomized trials are planned or underway for front-line therapy. One trial through COG is looking at the addition of hydroxycholoroquine to prednisone. The other multicentered trial planned by the Fred Hutchinson Cancer Research Center is examining the addition of mycophenolate mofetil (MMF) to cyclosporine or Tacrolimus (FK-506) plus prednisone in patients with extensive chronic GVHD.
Salvage therapies
Tacrolimus or FK506 has been used in steroid refractory patients.29 Six of 17 patients with extensive chronic GVHD showed response. FK506 concentrates in the liver and has a theoretical advantage over CSA for the treatment of hepatic GVHD. Although there have been a number of reports of responses to FK 506 switched from CSA, the similar mechanism of action of these drugs makes this substitution, other than in the setting of liver GVHD, a modest change. FK 506 has been combined with MMF. A review of 26 patients treated with this regimen showed a response rate of almost 50%.30 Because of the response rate to MMF and the rather disappointing results reviewed above with cyclosporine and prednisone, a multicentered trial comparing cyclosporine/FK 506 plus prednisone to the same combination with MMF has been organized through the Fred Hutchinson Cancer Research Center. This trial should answer the question of the activity of MMF in chronic GVHD. Thalidomide has been reported to have immunosuppressive properties and to be active against chronic GVHD, although most studies have found that the side effects of sedation prevent many patients from continuing on the drug.31 Etretinate is a synthetic retinoid that has been used to treat patients with systemic scleroderma. Based on reports of response in this patient population, it has been used to treat patients with sclerodermatous and fascial chronic GVHD. Of 27 patients completing a 3 month trial, 20 showed some improvement in skin lesions and/or range of motion in patients with refractory disease treated with etretinate.32 Etretinate is not currently commercially available, and acetretin, a more rapidly cleared derivative, has been used in its place. Acetretin may be added to immunosuppressive medications to increase the cutaneous response in patients with sclerodermatous GVHD. Clofazimine, an antimycobacterial drug used to treat leprosy and Mycobacterium avium complex, has anti-inflammatory activity in a number of chronic autoimmune skin disorders. Based on the success of treatment in these disorders, it was studied in 22 patients with chronic GVHD.33 Over half of the patients with sclerodermatous disease showed improvement in skin involvement, flexions contractures, or oral manifestations. Plaquenil (hydroxychloroquine) is an anti-malarial drug used in the treatment of autoimmune diseases. It interferes with antigen presentation and cytokine production, and is synergistic with CSA and tacrolimus in vitro.4 Based on promising results of 32 patients with over half responding, a large trial sponsored by COG is looking at the addition of plaquenil to a steroids plus cyclosporine for initial therapy. PUVA (8-methoxypsoralen plus ultraviolet A irradiation) has been used for treatment of steroid-resistant lichenoid chronic GVHD and in patients for whom steroids are contra-indicated.28 PUVA is very difficult to administer to sclerodermatous GVHD. Photopheresis has also been reported to be useful in chronic GVHD, although the mechanics of this therapy, including the need for a pheresis catheter and the location of the machine to deliver the therapy, have made it difficult to do trials with this approach. Pentostatin is currently being studied in several clinical trials, with early results suggesting that the drug is well tolerated, with responses seen in heavily treated patients.34 Rapamycin is also being explored. The antifibrotic properties of this drug make it particularly attractive for sclerodermatous GVHD.35,36
Future Directions
Efforts to prevent the development of chronic GVHD have been unsuccessful. Attempts to prevent chronic GVHD including the use of IV IgG and thalidomide have been unsuccessful.37,38 A more recent trial of prolonged administration of CSA found no difference in chronic GVHD or mortality when CSA was given for 24 months rather then six months.39 Current transplantation practices, including the use of DLI and peripheral blood stem cells, older patient age, and the increasing use of unrelated and mismatched marrow donors make it likely that chronic GVHD is going to be a progressively more common problem and more frequently in patient populations that are going to be very difficulty to treat. Ongoing research to further characterize the pathogenesis of this disease is crucial to the development of new therapeutic approaches. Several new therapies are currently under evaluation, including the antineoplastic and immunosuppressive drug pentostatin; daclizumab, a soluble IL-2 receptor antagonist; and infliximab, an anti-TNF-α monoclonal antibody.
III. Malignancies After Hematopoietic Stem Cell Transplantation: Why Do They Occur? Can We Predict Them? Can We Prevent Them?
H. Joachim Deeg, MD*
Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue, N., D1-100, P.O. Box 19024, Seattle, WA 98109-1024
HSCT provides effective therapy for patients with lymphohemopoietic, immunologic, metabolic and other disorders. Many patients have now been followed for two or three decades post-transplant and are presumably cured. Some patients, however, develop long-term complications, including new malignancies, which may not be unexpected. Secondary malignancies occur in patients with Hodgkin disease or non-Hodgkin lymphoma (NHL) treated with chemotherapy or radiochemotherapy,1,2 and in recipients of solid organ transplants treated with immunosuppressive therapy.
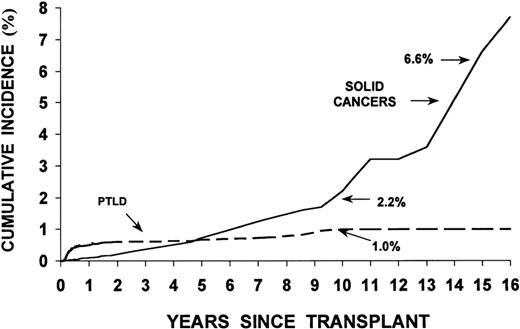
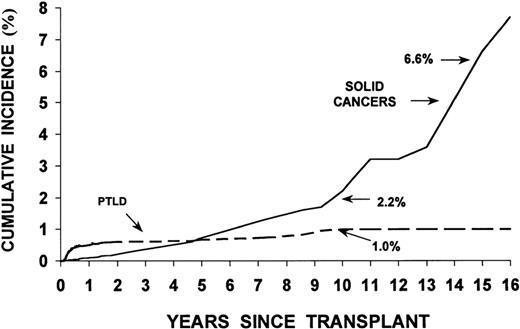
Studies in the 1970s and 1980s showed a significant increase in the incidence of malignancies relative to controls in rhesus monkeys and dogs irradiated with lethal doses of TBI and infused with autologous or allogeneic marrow cells (radiation chimeras). Patients who undergo HSCT, in addition, may have genetic defects associated with their primary disease predisposing them to the development of new malignancies. Finally, viruses such as Epstein-Barr virus (EBV), used to immortalize cell lines in vitro, can transform cells in vivo, particularly in immunodeficient patients.3,4Table 4 lists factors that are thought to contribute to the development of new malignancies in HSCT recipients. Major categories of malignancies are described in Table 5 . Figure 3 shows the incidence of PTLD and solid cancers over time post-transplant.
Lymphoid Malignancies
B-cell PTLD
Incidence.
Results in 18,014 allogeneic transplant recipients followed for up to 25 years revealed 78 cases of post-transplant lymphoid malignancies, a rather low incidence compared to solid organ transplant patients.6 In agreement with an earlier review by Cohen,3 82% were diagnosed within 1 year of transplantation, with peak occurrence (120 cases/10,000 patients/year) at 2–5 months, and a decline to < 5 cases/10,000/year among 1-year survivors. The incidence at 4–10 years was 1–2%, although figures of 10% have been reported in patients transplanted for immunodeficiency disorders.
Clinical and pathologic features.
B-cell PTLD are clinically and morphologically heterogeneous, usually associated with EBV and developing in a milieu of T-cell dysfunction.3,7 Patients frequently present with fever, even a sepsis-like syndrome, and lymphadenopathy. Intra-abdominal lymphadenopathy, splenomegaly, hepatomegaly, or bowel involvement may cause abdominal pain, vomiting, and diarrhea. Often lungs, kidneys, and the central nervous system (CNS) are involved. B-cell PTLD after allogeneic HSCT are almost always of donor origin. Few studies have examined in detail the histologic features of PTLD in HSC recipients.8,9 Some are similar to the monomorphic or polymorphic, diffuse large-cell lymphomas of B-cell origin observed after solid organ transplantation, while at least half show aggressive features of immunoblastic lymphoma.5,10 Most PTLD after HSCT are oligoclonal or monoclonal, as determined by analysis of immunoglobulin gene rearrangement or fused termini of episomal EBV DNA.5,7,11,12
Risk factors.
Risk factors5,6,8,14 are listed in Table 6 : Use of antithymocyte globulin (ATG) (relative risk [RR] 6.4) or anti-CD3 MAB (RR 43.2) for acute GVHD prophylaxis (RR 5.9) or in the preparative regimen (RR 3.1), use of TBI in the conditioning regimen (RR 2.9), T-cell depletion of donor marrow (RR 11.9;12.7),15 unrelated donor or HLA non-identical related donor (RR 4.1; 8.9), and primary immune deficiency disease (RR 2.5), occurrence of acute GVHD (grades III-IV) (RR 1.9), and treatment of acute GVHD with ATG or monoclonal anti-T cell antibody. However, while in patients transplanted with marrow depleted of T-cells with specific anti-CD3 monoclonal antibodies, the incidence of EBV-positive PTLD was 11% to 25%, the incidence was < 1% with techniques removing both T and B lymphocytes (e.g., soybean agglutinin or Campath-1).5,16 The risk of PTLD is particularly high in EBV-negative patients transplanted from EBV-positive donors.
The impact of risk factors is additive (or synergistic). In one analysis the risk of B-cell PTLD in patients with primary immune deficiency given T-cell–depleted HLA non-identical transplants was 64.8% ± 17.7% at 4 years, versus 0.9 ± 0.2% (P < 0.001) in patients given unmanipulated HLA identical marrow. In one large study the incidence of PTLD was 8% ± 2.9% with one or two risk factors, and 22% ± 17.9% with three or more risk factors present. The role of HLA-mismatching in the pathogenesis of B-cell PTLD is not clear but may consist in chronic antigenic stimulation, or delayed immune reconstitution. There is a direct correlation between viral load in peripheral blood and the development of PTLD.17,18
Pathogenesis.
EBV, a herpes virus, is present as a latent virus (in B lymphocytes and certain epithelial cells) in 95% of individuals by adulthood. EBV type A (distinguished from type B by sequence divergence in the EBNA-2 gene) is present in most cases of PTLD after solid organ transplantation;19 several strains have been identified. Whether the same applies to HSCT recipients remains to be determined.
The oncogenic potential of EBV is due to its ability to immortalize B cells. The same viral genes that drive proliferation in vitro, EBNA-1 and 2, LMP-1, and EBNA-3A, -3B, and -3C, are expressed in PTLD.4,5 LMP-1 acts as a constitutively activated TNF receptor-like protein, induces gene expression through NFκB, and acts as a transforming oncoprotein in certain experimental systems.20 Deletions near the 3′ end of the LMP-1 gene appear to correlate with EBV malignancy.21 Other viral proteins (ENV-2A, -3A, -3B, and -3C) control transcription of viral and cellular genes. The result is expression of IL-1β, IL-6, IL-10, and TNFβ, which act as growth factors for EBV-infected cells, as well as soluble CD23 and low affinity IgE receptor.
Prophylaxis and therapy.
Investigators at Memorial Sloan-Kettering Cancer Center22 used limiting dilution analysis to quantify anti-EBV specific cytotoxic T-lymphocyte precursor (CTLp) frequencies in recipients of unmodified or T-cell depleted grafts from EBV-positive donors. At 3 months (interval of peak incidence of B-cell PTLD) only 20% of patients had EBV CTLp frequencies in the range of seropositive controls, while at 6 months 70% were normal. Based on studies in xenografted SCID mice23 the same investigators12 used unirradiated donor leukocytes (1.0 × 106 CD3+ T cells/kg) to treat PTLD. The initial 5 patients had complete responses but 3 developed chronic GVHD, and 2 died of respiratory failure with no evidence of PTLD. An update showed 14 of 15 patients responded and 6 of 12 evaluable patients developed GVHD. Gene-marked EBV-specific cytotoxic T-lymphocytes persisted in vivo and restored cellular immunity against EBV.
The St. Jude’s team used rising titers of EBV DNA in patient plasma as a criterion to institute pre-emptive therapy with EBV-specific T-cell clones in 25 high-risk patients. None developed PTLD. Among 6 patients who refused CTL therapy or were ineligible, 2 developed PTLDs that were successfully treated with CTL.24 Bonini et al showed that HSV-TK gene-modified donor lymphocytes can be used effectively and can be inactivated by ganciclovir if GVHD develops.25 Rooney et al26 and Heslop et al24 confirmed the efficacy of gene-marked EBV-specific T lymphocytes and showed long-term restoration of anti-EBV immunity.
While EBV-transformed B cells contain a circular viral DNA that is not susceptible to inhibition by thymidine kinase (TK) antagonists, tumor regression with acyclovir or ganciclovir therapy was noted in anecdotal reports (reviewed in ref. 27). The proliferative program of EBV-infected cells is restricted to latently infected cells. That treatment directed at the lytic cycle should be effective may depend on productive infection in other tissues that contribute to PTLD.
Anti-CD21 and anti-CD24 antibodies were tested in a multicenter trial.28 Among 19 marrow transplant recipients, 10 achieved complete remissions, and 6, all with oligoclonal disease, survived at a median follow-up of 20 months.27 However, studies in SCID mice29 indicate that residual EBV-positive B cells persist and can provoke a second tumor in the absence of efficient cytotoxic T cells.
Based on in vitro data showing antitumor effects of anti-IL-6 antibody,30 neutralization of IL-6 may also be a strategy worth exploring.
Chemotherapy, irradiation, and surgical resection are useful in selected cases in solid organ recipients.3 The best approach in patients after HSCT is close monitoring of plasma EBV DNA and pre-emptive therapy in patients with rising EBV DNA titers.
T-cell lymphoproliferative disorders
Rare T-cell proliferative disorders with or without EBV association occur, usually more than 1 or 2 years after transplantation. After HSCT only few such cases have been reported,31 none associated with HTLV1, HIV, or HHV6 infection.
Hodgkin disease and other late-onset lymphomas
Several late occurring lymphomas have been reported,32 some linked to EBV (just as early onset PTLD), and others associated with T-cell depletion of the graft. Clinical presentation was like ordinary non-Hodgkin lymphoma with lymph node enlargement with or without generalized symptoms.
A recent collaborative study of 18,531 transplant recipients (covering more than 42,000 patient years), found 8 cases of Hodgkin disease 2.9–9.1 years after HSCT (observed/expected ratio 6.2).33 Five cases (67%) showed mixed cellularity subtype, and 5 of 6 cases studied contained the EBV genome. Two patients were also positive for HIV. Risk factors identified for early PTLDs were generally absent. Patients with Hodgkin disease were more likely to have acute GVHD and require therapy for chronic GVHD (RR in one study 4.0). These data add support to the theory that links overstimulation of cellular immunity and exposure to EBV to various subtypes of Hodgkin disease.34
Hematologic Malignancies
After allogeneic HSCT
Incidence.
Lymphoblastic leukemias in donor cells were first recognized 30 years ago.35,36 The incidence was estimated at 3–5%. More recent data suggest a lower incidence.37 In addition, new leukemias in patient cells, i.e., leukemias of a different morphology or lineage than the patient’s primary disease, have also been described (reviewed in ref. 38).
Pathogenesis.
The mechanism remains unclear. Transformation of donor cells via antigenic stimulation by host tissue, a leukemogenic host environment, fusion of normal cells with leukemic cells still residing in the recipient, or transfection with a host oncogene or virus have been proposed. Studies using molecular techniques (e.g., microsatellite analysis) to determine host-versus-donor origin of abnormal cells post-transplant indicate that recurrence in donor-derived cells is infrequent.37 However, cases of leukemia or MDS transplanted from the donor into the patient have been reported.39,40
Recent work suggests that “replicative stress” after transplantation may result in accelerated telomere shortening of donor HSC.41,42 This in turn might lead to chromosomal instability and increased probability of MDS or leukemia. While telomere shortening occurs in HSCT recipients, the concept has remained controversial. Nevertheless, several cases of MDS/AML in donor cells presenting 5, 10 years or even later after HSCT have been observed (unpublished).
Therapy.
There is no known prophylaxis, and no therapeutic standards have been established. Some patients have recently been treated with second transplants using myeloablative or non-myeloablative protocols.
After Autologous HSCT
Incidence.
In studies involving more than 1200 patients the incidence of MDS was 4–18% at 3–6 years, with the post-transplant interval ranging from 2.5 to 8.5 years.51–,54 A case control study revealed 12 cases of MDS/AML in 511 patients after autologous transplants for Hodgkin disease (n = 249) or NHL (n = 262) for a cumulative incidence of 4% at 5 years. Another report showed clonal chromosomal abnormalities in 10 of 275 patients 1.8–6.5 years after chemotherapy, and 0.5–3.1 years after autologous transplant for Hodgkin disease or NHL,54 but only 5 patients had morphological evidence of MDS or AML. The cumulative probability of developing clonal chromosomal abnormalities reached 9% ± 4.7% at 3 years after transplantation.
Risk factors.
In one analysis a prolonged pretransplant interval, and use of radiotherapy, especially pelvic irradiation, were significant pretransplant variables. In several studies the risk was higher with peripheral blood cell transplants (e.g., 31% ± 33% versus 10.5% ± 12% with marrow; RR 5.8; similar in another study55), in patients more than 35 (40) years of age at transplantation (RR 3.5), and with the use of TBI.51
A case control study under the auspices of the NCI analyzed data on 56 patients who developed myelodysplastic syndrome (MDS)/leukemia, and 168 controls within a cohort of 2739 patients with Hodgkin disease or NHL transplanted at 12 institutions (Metayer, Curtis et al, unpublished data). MDS/AML was significantly correlated with the intensity of pre-transplant chemotherapy, specifically mechlorethamine (RR 2.0; 4.3 for doses of < 50; ≥ 50 mg/m2), and chlorambucil (RR 3.8; 8.4 for duration < 10; ≥ 10 months; P = 0.0009) compared to cyclophosphamide. Also, higher doses of TBI (> 1200 cGy) used for transplant conditioning tended to carry a higher risk (RR 4.7). The difference between marrow and peripheral blood stem cells was not significant in this analysis.
Pathogenesis.
Whether MDS/AML arises from infused HSC or from residual cells in the patient is controversial. If the former were true, then the type of conditioning therapy given for transplant should not be a risk factor—unless we postulate that conditioning (TBI) modifies the microenvironment and enhances the risk of leukemogenesis. If MDS/AML is related to transplantation, then the culprit could be the procedure itself or the status of immunoincompetence after transplantation (Table 4). In some patients, cells harvested pre-transplant contain cytogenetic abnormalities, while in others they don’t. However, posttransplant MDS/AML is a clonal disorder, and in most patients cytogenetic abnormalities (e.g., -7, +8, 7q-, 20q-, 11q23) are present, even if they are not detected in cells used for transplantation. In some female patients without cytogenetic abnormalities clonality has been documented by X-inactivation based clonality assays,56 and mutations of RAS, FLT3, AML 1, CBF among others, have been recognized (reviewed in ref. 57).
More than one mutagenic/leukemogenic event is required for MDS or AML to develop. Gene fusion products recognized as leukemogenic (e.g., BCR/ABL, TEL/AML 1) are present even in normal individuals with the use of sensitive PCR technology. Thus, such clones may exist in patients pre-transplant, and a “second hit” might occur during or after transplantation. It has also been argued that many patients may not have MDS but rather “disordered engraftment.”49
Prophylaxis and therapy.
These data suggest that reducing pretransplant exposure to alkylating agents, topoisomerase inhibitors, and irradiation and shortening the duration of therapy should reduce the risk of MDS.58 Alkylators and topoisomerase inhibitors should probably not be used for stem cell mobilization.53 If cytogenetics are abnormal at the time of stem cell harvest, an allogeneic transplant should be considered. In addition to standard cytogenetics, interphase FISH, determination of loss of heterozygosity or point mutations, and X-inactivation-based clonality assays are useful. Once MDS/AML has evolved, the options are limited. Chemotherapy is often not well tolerated, and remissions are of short duration. Allogeneic HSCT with standard or reduced intensity conditioning is a realistic option for some patients.59
Solid Tumors
In irradiated and transplanted rhesus monkeys solid tumors developed 7.5 to 15 (median 11.5) years after x-irradiation, and 4 to 15 (median 8) years after fission neutrons. The time interval in gamma-irradiated dogs was 1.6 to 10.5 (median 8) years. Extrapolation to humans suggests that post-transplant solid tumors might develop after a decade or more.38
After allogeneic HSCT
Incidence and spectrum of tumors.
Among 2145 patients transplanted from 1970-1987 13 solid tumors were observed, including glioblastoma, melanoma, squamous cell carcinoma, adenocarcinoma, hepatoma, and basal cell carcinoma.14 In another cohort of 2150 patients, 15 developed solid tumors (8 of 1400 allogeneic, and 7 of 750 autologous transplant recipients)15 for an incidence of 5.6% at 13 years. In a third study of 1211 patients who had survived at least 5 years after HSCT,60 47 developed malignancies, including squamous cell carcinoma, breast cancer, glioblastoma, and lymphoma. In comparison to normal controls, the incidence was increased significantly for malignancies of the oral cavity, skin, esophagus, uterine cervix, and brain.
A collaborative study analyzed results in 19,220 patients (97.2% allogeneic, 2.8% syngeneic recipients) transplanted between 1964 and 1992.61 There were 80 solid tumors for an observed/expected (O/E) ratio of 2.7 (P < 0.001). In 10-year survivors, the risk was increased 8-fold. The tumor incidence was 2.2% at 10, and 6.7% at 15 years. The risk was increased significantly for melanoma (O/E 5.0), cancers of the oral cavity (11.1), liver (7.5), CNS (7.6), thyroid (6.6), bone (13.4), and connective tissue (8.0). The risk was highest for the youngest patients and declined with age. This study, comprising now 28,874 patients (< 1–72 years of age, 74% with leukemia, 76% transplanted from an HLA identical sibling, 59% given TBI as part of the conditioning regimen) transplanted from 1964–1996, was recently updated. Among 5-year survivors there were 161 solid tumors for an O/E ratio of 2.2. The highest ratios, ranging from 10.0 to 4.1, were observed for bone, buccal cavity, connective tissue, liver, brain, thyroid, and melanoma (in that order). Among 10-year survivors, the O/E ratios were 26.5 for buccal cavity, 32.3 for liver, 18.3 for thyroid, 6.0 for melanoma, and 3.3 for breast. Table 7 illustrates the spectrum of malignancies observed in patients transplanted at FHCRC.
Risk factors.
Risk factors are summarized in Table 5. In the above collaborative study the rates of excess cancers/10,000/year were highest in patients < 17 years (16.06), and lowest for patients > 40 years of age (2.42). Results in a study of 700 patients with aplastic anemia suggested that irradiation (RR 3.9), treatment of chronic GVHD with azathioprine (RR 7.5) and older age (RR 1.1) increased the risk of a posttransplant malignancy.62 The incidence was highest in patients transplanted for Fanconi anemia (Kaplan-Meier estimate at 15 years 40%). Irradiation, in particular limited field irradiation, also is a significant risk factor for the development of solid tumors,62 with RRs of 2 to 6. In addition, donor age, chronic GVHD, treatment of GVHD with cyclosporine or azathioprine, and the number of agents used for therapy were found to be significant risk factors. There was a strong link between chronic GVHD (and male gender) and squamous cell carcinoma. Preliminary data from an ongoing nested case control study in a cohort of 29,737 patients suggest that duration of chronic GVHD > 2 years and prolonged therapy are risk factors, in particular for the development of squamous cell carcinoma.
After autologous HSCT
Incidence and spectrum.
French study in 4322 patients with Hodgkin disease found 18 new malignancies in the 467 patients who had received autologous HSCT (8.9% at 5 years; P = 0.039 in comparison to non-transplanted patients).55 Another study found 7 solid tumors in 750 autotransplant recipients for an incidence of 5.6% at 13 years.15 In a third analysis among 625 autologous transplant recipients who survived at least 3 years after HSCT, 14 developed second neoplasms at 4–116 months; 10 of these had been given TBI (unpublished). The types of tumors observed were similar to those seen with allogeneic transplants. As with MDS/AML, the incidence was particularly increased in patients more than 35 years of age and in recipients of peripheral blood (rather than marrow) HSC.
Pathogenesis of solid tumors posttransplant.
Interactions of various factors (Table 4) contribute to the pathogenesis of secondary solid tumors. Socié et al63 identified human papilloma viruses (HPV) 13, 15, or 16 in 3 of 8 squamous cell carcinomas; HHV8 was present in 1. Further, the pattern of p53 expression suggested mutations of this gene in all 8 tumors studied. Mutations might be induced by cytotoxic therapy, and suppressed immunity would interfere with normal surveillance. There is also evidence that polymorphism at position 72 in p53 (in particular homozygosity for arginine) may confer unusual susceptibility of p53 to inactivation by HPV. Chronic inflammation and impaired DNA repair are other factors. However, considerable work is needed for a better understanding of those questions.
Observations in autologous patients will be of great interest because etiologic factors, such as chronic alloantigenic stimulation and GVHD are absent, thus allowing to focus on cytotoxic therapy and genetic pre-disposition (xenobiotic polymorphism).
Prophylaxis and therapy
Risk factors varied, dependent upon the type of tumor. Omission of (high dose) irradiation from the conditioning regimen should be beneficial, in particular for the prevention of melanomas, thyroid carcinomas, carcinomas of the buccal cavity, and breast cancer. Prevention (or different therapy) of chronic GVHD should have an effect on the development of buccal carcinomas.
Surgical resection, whenever possible, is the front-line therapy for solid tumors. Selective immunostimulation and measures aimed at scavenging free radicals have yielded some promising results in experimental studies.
Conclusions
HSCT offers curative therapy for many patients. Currently about 30,000 transplants are carried out annually, and most patients who do not relapse within a year or two of transplantation do well and lead productive lives. However, complications occur, one of them being the development of new malignancies. While the overall incidence is low, high-risk situations such as an underlying diagnosis of immunodeficiency or other genetic defects, high-dose irradiation for conditioning, T-cell depletion of the marrow, HLA nonidentity of the donor, and chronic GVHD have been identified. We have begun to develop an understanding of the mechanism involved in PTLD. Insights into the development of hemopoietic disorders and solid tumors is more limited. Other potential factors, e.g., smoking before or after HSCT, need to be examined further. Also, longer observation is required before the full extent of the risk of solid tumors in particular can be assessed. While many questions remain, available data provide a basis on which to develop pre-emptive approaches.
Multifactorial etiology of post-transplantation complications.
Reprinted with permission from Deeg HJ. Chapter 66. Delayed complications after hematopoietic cell transplantation. In Forman SJ, Blume KG, Thomas ED, eds: Hematopoietic Cell Transplantation, 3rd Ed. Boston: Blackwell Scientific Publications, Inc; 1999: 776-806.
Abbreviations: TBI, total body irradiation; GVHD, graft-versus-host disease.
Multifactorial etiology of post-transplantation complications.
Reprinted with permission from Deeg HJ. Chapter 66. Delayed complications after hematopoietic cell transplantation. In Forman SJ, Blume KG, Thomas ED, eds: Hematopoietic Cell Transplantation, 3rd Ed. Boston: Blackwell Scientific Publications, Inc; 1999: 776-806.
Abbreviations: TBI, total body irradiation; GVHD, graft-versus-host disease.
Potential mechanism of chronic graft-versus-host disease (GVHD) induction.
Autoreactive T cells are generated by prior damage to the immune system from acute GVHD, host immune system factors such as age, and/or T helper cell population administered with the transplant. Effector cells help generate a graft versus tumor effect as well as chronic GVHD. (Figure from Dr. A. Hess, Johns Hopkins Oncology).
Potential mechanism of chronic graft-versus-host disease (GVHD) induction.
Autoreactive T cells are generated by prior damage to the immune system from acute GVHD, host immune system factors such as age, and/or T helper cell population administered with the transplant. Effector cells help generate a graft versus tumor effect as well as chronic GVHD. (Figure from Dr. A. Hess, Johns Hopkins Oncology).
Cumulative incidence (%) of posttransplant lymphoproliferative disorders (PTLD) and invasive solid cancers following allogeneic marrow transplantation at 235 centers worldwide.
Reproduced with permission from Curtis RE, Travis LB, Rowlings PA, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999;94:2208-2216.
Cumulative incidence (%) of posttransplant lymphoproliferative disorders (PTLD) and invasive solid cancers following allogeneic marrow transplantation at 235 centers worldwide.
Reproduced with permission from Curtis RE, Travis LB, Rowlings PA, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999;94:2208-2216.
This work was supported by PHS grants CA18029, CA15704, HL36444, and CA87948.
Acknowledgments: I thank R. Curtis, G. Gilliland, W. Schubach, H.J. Kolb, G. Socié, and D. Rizzo for their comments and contributions, and B. Larson and H. Crawford for help with manuscript preparation.